Abstract
Aging leads to a decline in function and structural changes. To mimic these processes, D-galactose (D-Gal) administration is widely used as a pro-aging model in animal studies due to its reproducibility and practicality. In this study, D-Gal was administered via intraperitoneal injection to induce aging in rats. This study aimed to investigate age-related changes in colon function and structure in an animal model through histological analysis and immunohistochemical assessment of aging biomarkers. Twelve-week-old male Sprague Dawley rats were divided into control and GAL groups. The functional test was performed by measuring the colon transit time duration and faecal output amount. Structural analysis was performed by histological examination using hematoxyllin-eosin staining and immunohistochemistry against p16Ink4A and lamin B1 antibodies. Colon transit time in the GAL group was significantly faster, but fecal pellet output and body weight in the GAL group had lower values. Microscopic examination revealed significant differences (p = 0.00) between the GAL and control groups. The GAL group exhibited mild epithelial (score 1) and muscle tissue damage (score 2), along with leukocyte infiltration (score 3). The immunohistochemistry results showed a significant difference in the GAL group (p < 0.05) for p16Ink4A and lamin B1 antibodies, which confirmed the ongoing aging process. Mild structural and functional changes, accompanied by significant inflammation, suggest that the D-Gal-induced aging model accelerates colon aging.
Similar content being viewed by others
Introduction
The gastrointestinal tract plays a crucial role in absorbing nutrients and drugs while providing protection against pathogens, this is primarily attributed to the presence of mucous membranes within the gastrointestinal system. In the context of aging, a distinctive decline occurs in the gastrointestinal tract’s structural integrity (such as decreased epithelial regeneration, mucosal and smooth muscle layer’ defect), significantly affecting intake, digestion, and nutrient absorption and utilization, leading to functional impairment1,2. Consequently, this condition causes motility disorders, including fecal incontinence and constipation3,4. Constipation is a common problem among the elderly population, affecting 20–50% of elderly individuals5.
The motility disorders associated with aging are also influenced by the contractility of smooth muscles, which play a role in gut transit time. Previous studies reported that, as a result of colonic neuromuscular elements influence, aged rats display a decrease in fecal output and an increase in transit time compared with young rats, this suggests that transit duration and fecal output can be indicators of disturbances in the gastrointestinal tract, especially the colon, in constipation cases3,6.
Research that focuses on age-related gastrointestinal tract disorders is important for enhancing the quality of life for affected patients1. Experimental animals with artificially induced accelerated aging have gained popularity owing to their shorter time requirements, ease of application, and high survival rate7. D-galactose (D-Gal), a reducing sugar found in dietary and natural sources, is frequently used to induce aging in experimental animals. When present in excess, D-Gal is converted into aldose and hydrogen peroxide, subsequently generating reactive oxygen species (ROS) that induce oxidative stress in cells and tissues, thereby hastening the aging process1,8.
Cellular senescence is marked by senescent cell accumulation and leads to structural changes and chronic inflammation. Aging cells secrete growth factors, extracellular matrix components, and inflammatory cytokines while resisting apoptosis and maintaining a long lifespan. These cells exhibit the Senescence-Associated Secretory Phenotype. Senescence results from irreversible cell cycle arrest driven by molecular factors, including lamin B1 and the cyclin-dependent kinase inhibitor p16Ink4a. Lamin B1, crucial for nuclear integrity and cell survival, can trigger senescence when p53 is inactivated or under hypoxic conditions. p16Ink4a regulates the cell cycle and promotes senescence, which, while protective against malignancy, also drives chronic inflammation and tissue remodeling, thereby contributing to dysregulated motility and reduced regenerative capacity9,10,11.
A previous study examined the aging effects of the D-Gal animal model on the digestive tract, whereas another study used the D-Gal-induced (GAL) mouse model as an aging animal model to observe the gut microbiome in the intestine after functional food administration. Consequently, D-Gal use has been proven effective in inducing aging characterized by a decreased body weight, thymus index, and muscle mass, organ atrophy to a certain degree, and other histopathological damages1,8.
Therefore, this study used the GAL model to explore the effects of aging on structural changes by observing epithelial damage, cellular infiltration and smooth muscle damage as well as the expression of aging protein markers, p16Ink4a and lamin B1. In addition, distal colon motility and fecal pellet output will be assessed.
Results
This study used experimental animals intraperitoneally induced by D-Gal at 100 mg/ kg bodyweight/ day for 6 weeks. Structural examinations using Hematoxylin-Eosin staining and immunohistochemistry were performed in the treated and control groups. Functional examinations were conducted by measuring the distal colon transit time and by performing fecal analysis (number of pellets and weight and percentage of water content). Additional data, such as colon length, feed weight per day, and body weight per week, were also collected during treatment. The results of the study, as shown in Fig. 1, indicated that body weight, average weekly food intake, fecal water content percentage, and colon length in mice after D-Gal administration were not significantly different compared to the control group. However, distal colon transit time and total pellet output after D-Gal treatment were lower and significantly different compared to the control group. The results in Fig. 1 also showed that colon weight and the ratio of colon weight to body weight were higher and significantly different compared to the control group. These findings indicated that D-Gal administration was able to induce early aging.
Structural examination
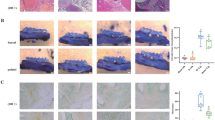
The qualitative histological examination (obj 4×, scale 100 μm and obj 40×, scale 50 μm) identified mild epithelial damage (red arrow) in the treated group. Inflammatory cell infiltration (green arrow) was observed in the mucosal layer, with some infiltration penetrating through all colon layers (Table 1; Fig. 2); the damage to the muscular layer (yellow arrow) affected one-third of the tissue length in the colon.
Hematoxylin-Eosin staining of the colon (obj 4× and 40×, scale 100 μm); (a) Gal-induced group (b) control group. The red arrow indicates epithelial damage manifested as loose epithelial cell arrangements, green arrow indicates infiltration of inflammatory cells in the mucosal layer, yellow arrow indicates smooth muscle damage.
Expression of the aging marker protein
The expression of p16INK41 and lamin B1 was significantly higher in the treated group than in the control group (Table 2). Immunohistochemistry scoring was conducted by multiplying the percentage and staining intensity as shown in Fig. 3 for p16INK4a and Fig. 4 for lamin B1 (objective 40×, scale 50 μm).
Distal colon transit time
The GAL-treated group exhibited a significantly shorter transit time than the control group (p < 0.05) as shown in Fig. 1. The control group transit time was nearly five times longer than that of the GAL-induced aging group. The fecal pellet output has several parameters, including the total pellet count, water percentage in the pellets, and average colon length. The total number of pellets obtained was higher in the control group than in the GAL-treated group (p < 0.05). The water percentage in the pellets and colon length did not significantly differ between the two groups, although both parameters showed an increased tendency in the GAL-treated group (Fig. 1).
Discussion
D-Gal is used at elevated concentrations to induce aging and accumulates intracellularly in the form of galactitol. This transformation yields ROS, which subsequently triggers oxidative stress, mitochondrial dysfunction, and apoptosis, which are hallmarks of aging7,12,13. Observable indicators, such as weight reduction, thymus index, and diminished motor function, corroborate the efficacy of GAL aging in rodent models1,8. In the current study, the GAL group did not exhibit an increase in the total feed compared with the control group in the first week. These results are similar to those in another study, in which the mean of the total feed in the GAL group started to increase and stabilize in the second week after induction12,14. The mean body weight of the GAL group was lower and more stable than that of the control group (Fig. 1). It was assumed that the decrease in food intake influenced the reduction in the body weight of the aged rats in this study, which is consistent with a previous study showing that aging rats tended to experience a decrease in food intake, leading to weight loss and requiring a longer recovery from inflammation. The cause of this condition was age-related reductions in taste and smell caused by olfactory receptor loss and degenerative alterations in the olfactory epithelium. Although the processes behind changes in food intake with age are unknown, a study indicate that the F344BNF1/NIA (FBN) rat may be affected by decreased sensitivity to leptin and β-adrenergic signalin; therefore older rats were less capable of maintaining body weight than younger rat2,15,16,17,18,19. These results indicate the successful induction of the aging model in the experimental animals.
Histological structure observations showed that colonic damage in the GAL rats involved epithelial damage, smooth muscle damage, and cellular infiltration (Table 1), which was significantly higher in the GAL rats than in the controls. Epithelial tissue damage was less than one-third the length of the colon tissue or included mild damage. Previous studies have reported loose epithelial cell arrangements and incomplete tight junctions in GAL rats. Damage to the tight junction structure that correlates with age-related intestinal barrier dysfunction is also associated with atrophy, increased gut bacteria levels, and decreased expression of epithelial tight junction-related proteins20. These histological changes likely underlie the observed functional impairments, including faster transit and reduced stool output. The structural changes were different from those observed in diabetic-induced rats, which showed a thickening of the mucous and muscular layer14. Despite these differences, the physiological outcomes in both studies were similar, suggesting that D-Gal and diabetes induce oxidative stress, accelerating the aging process through ROS generation1,8.
Smooth muscle damage occurred in more than one-third of the thickness of the colonic tissue. This damage has implications for decreased colonic contractility, slowing transit time and contributing to age-related gastrointestinal motility disorders, such as constipation. It has also been reported that smooth muscle contractility is lower in old tissue than in young tissue, this disrupts excitatory mechanisms of the colonic myenteric plexus, such as neurotransmission21. The mild damage observed in the epithelium and smooth muscle in this study supports the finding of a shorter transit time in the GAL group, aside from a longer colon length. The low damage level indicates an early stage of the aging process; hence, the physiological effects on the colon are not yet significantly pronounced.
The highest scoring value was observed for cellular infiltration occurring in over two-thirds of the network. The high cellular infiltration in GAL rats indicates that an aging process is occurring in these animals. The lymphocyte infiltration shown in the GAL group can be used as an inflammatory marker during the ongoing aging process. Inflammatory cell infiltration contributes to the early stages of aging. The aging process is a complex biological phenomenon characterized by a gradual decline in the structure and function of multiple organs, with common features including vascular remodeling, endothelial dysfunction, and reduced vascular compliance. Among various molecular changes, a notable aspect of aging is the presence of chronic low-grade inflammation that is now referred to as “inflammaging,” signifying persistent low level chronic inflammation as a characteristic feature of the aging process22. The process of inflammaging was observed in this study, although results were obtained at a relatively early stage. Overall, the inflammatory process occurring in the histological tissue indicates structural damage and the initial stages of aging in the GAL animal model.
The aging process can also be observed by measuring the expression of several marker proteins for aging such as p16Ink4a and lamin B1. p16Ink4a plays a role in regulating cell aging, detecting and maintaining DNA damage, and inhibiting cell proliferation10,23. In this study, the immunohistochemical results showed a higher expression of p16Ink4a in the GAL rats (Table 2), similar to a study that showed that with increasing age, the expression of p16Ink4a significantly increases24. These results support the evidence that p16Ink4a is a proaging protein whose expression is directly proportional to the aging process. The elimination of cells responsible for p16Ink4a expression indicates a favorable decline in aging progression rather than aging reversal23. Increased expression of p16INK4A causes negative cell cycle regulation because this protein is recognized as a cell cycle kinase inhibitor24.
In line with p16Ink4a expression, lamin B1 expression as another marker was higher in GAL rats than in control rats, consistent with a study showing that in aging rats there was a telomere instability, leading to DNA damage25,26,27. Lamin B1 is a marker protein for aging that maintains the integrity and function of the nuclear lamina. Increased lamin B1 expression, may indicated stress-induced nuclear remodelling, that inhibited proliferation contributing to the structural instability observed in our histological analyses results. This result also can be correlated with increased aggressiveness and poor prognosis in certain cancer types, which are also caused by cellular aging25. Changes in lamin B1 levels, both depletion and overexpression, are reported to inhibit proliferation. However, the induction of aging conditions is only achieved through the overexpression of lamin B1, which can be prevented by telomerase expression or p53 inactivation9.
Azman & Zakaria (2019) explained that D-galactose can induce aging through several mechanisms. D-galactose is reduced by galactose reductase into galactitol, which induces osmotic stress and mitochondrial dysfunction, resulting in decreased ATP levels7. D-galactose can also be oxidized by galactose oxidase into H₂O₂, leading to a reduction in SOD levels and impaired redox homeostasis. Additionally, D-galactose can react with amines to form Schiff base compounds and Amadori products, which increase the formation of AGEs, activation of RAGE, and NADPH oxidase. These changes contribute to increased oxidative stress (ROS). Previous research mentioned that increasing ROS can lead to the demethylation of the p16^INK4A gene, resulting in its elevated expression26. Increased p16^INK4A then bind to CDK4/6, preventing Rb phosphorylation, thereby allowing Rb to remain bound to E2F and blocking the G1 to S phase transition, leading to cellular senescence27. Senescent cells subsequently increase the expression of Lamin B1 and cause telomere instability28,29,30. Therefore, in this study, D-galactose administration was assumed to cause an increase in ROS, which may lead to p16^INK4A demethylation. Elevated p16^INK4A expression may cause cells to arrest in the G1 phase and enter senescence. These senescent cells then lose nuclear and telomeric stability, resulting in increased Lamin B1 expression. However, the molecular mechanisms by which D-galactose induces p16^INK4A upregulation still require further investigation.
The increase of p16Ink4a expression suggests senescent cell accumulation, which may contribute to the release of proinflammatory cytokines. Concurrently, lamin B1 disruption may activate NF-κB and the NLRP3 inflammasome, further amplifying the inflammatory response. These molecular events likely contributed to the observed histological features of leukocyte infiltration and tissue damage, mediated by downstream cytokines such as IL-6 and TNF-α10,11,31.
Slow transit time sometimes becomes an aging symptom as well as leads to a decrease in fecal output. Aging typically induces several functional changes such as a slowed transit time leading to decreased fecal output3,15,32. The colon transit time was shorter in the GAL group than in the control group. This finding contradicts existing theories and may be attributable to an early-stage inflammatory process in the GAL group, which is one of the hallmark processes of aging at an early stage, or disruption of enteric nervous system regulation14,15,33. Additionally, another possibility stems from the disparity between natural aging processes and artificially induced aging, such as through D-GAL, resulting in transit time not exhibiting the expected increase7. This finding differs from previous research, which reported enhanced motility in D-Gal-treated mice owing to inflammatory cytokine activity, such as TNF-α and IL-6, which can affect intestinal smooth muscle contractions6,15,20. A lower research quality or the presence of other factors that affect the model process may also explain the misalignment of the aging process in animal models34. Another study mentioned that age progression does not have a clinically significant effect on colon transit time and constipation unless other influencing factors are present, such as anticholinergic medication use, decreased motility, changes in dietary patterns, and underlying medical conditions35,36. Additionally, diurnal rhythm is another factor affecting transit time, with longer transit times reported in mice exhibiting significant gastrointestinal activity after 12:00 pm35,36,37.
In contrast to the transit time, the total pellet output results were consistent with the indicators of disruptions caused by aging. The total pellet count was significantly lower in the GAL group than in the control group, although the water percentage in the stool was not significantly different. This finding suggests a tendency toward diarrhea, likely resulting from epithelial damage and muscle injury, impairing peristalsis and stool consolidation. However, the low total pellet count in this study can be attributed to a longer colon length in the GAL group and not associated with the shorter transit time in this study, which is consistent with previous findings that showed increased fecal weight owing to propulsive contraction in aging mice and heightened inflammatory activity in diabetic rats14. Other factors contributing to the decrease in total pellet output include pellet propulsion and decreased tissue stimulation. Previous studies have reported that a decrease in the number and water content of pellets occurs with age and slower transit times in older animals15. The water content did not significantly differ between the two groups, although a tendency toward higher water content was observed in the GAL group; however, pellet consistency and size did not significantly differ between the groups.
The aging process involves a complex series of events. D-Gal induction results in this study demonstrate the acceleration of the aging process in experimental animals. The initial stages of GAL aging are characterized by inflammation, followed by structural changes, and functional disturbances. Functional changes occur when structural damage is at a certain stage in the later phases of the process. The early-stage inflammation observed in this study may not adequately reflect specific functional disruptions, especially in the gastrointestinal tract, such as colon transit time and total fecal output. As shown in Table 3, these alterations appear less pronounced in the D-galactose-induced model compared to alternative induction methods and natural aging. However, the implications of this early inflammation were evident in the structural changes observed in the histological preparations; the higher expression of these aging marker proteins also supported the acceleration of the aging process in these experimental animals.
Materials and methods
Experimental animals
Twelve male Sprague Dawley rats (Rattus norvegicus) aged 12 weeks, based on the Mead’s resource equation (E = N-B-T)35 were used in this research to find minimal sampling. All experimental animal weighing approximately 277 ± 1.99 g procured from Unit IV of the Integrated Research and Testing Laboratory at Universitas Gadjah Mada, Yogyakarta. Acclimatization was performed for 7 days in individual cages under controlled room temperature (25 –27 °C), with a 12/12-h light/dark cycle. All actively moving rats were provided with Rat BioTM pellets, according to their body weight, and ad libitum access to water was ensured. All methods were performed in accordance with the relevant guidelines and regulations, and were approved by the Ethics Commission for Research at the Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University (KE/FK/0676/EC/2023). All methods and results of this pure animal laboratory experiment were reported according to the ARRIVE guidelines. The exclusion criteria of this research were death of the animal subject during the research.
Experimental design
Following acclimatization, rats were fasted for 12 h and then divided randomly into two groups, each comprising six rats: the negative control group and the induction group (GAL). Subsequently, body weights were recorded, and glucose levels were measured. The induction group was administered 100 mg/kg bodyweight/ day of D-Gal (Sigma Aldrich G0750-10G, CAS number #59-23-4) via intraperitoneal injection to induce aging38.
Distal colon transit time
This method aims to assess distal colon motility, which can be used as a marker of colon functional. All rats were fasted overnight before the procedure. Rats were sedated with 20 mg/kg bodyweight of ketamine. Red beads with a 5-mm diameter were placed in the anus and pushed by a rubber elastic stick until approximately 3-cm deep into the rectum. After treatment, each rat was placed in a new cage cleaned with 70% alcohol. The duration from when the beads are inserted until they exit the anus is measured using a timer.
Fecal pellet output
This method calculated the percentage of water in the stool and the total weight of the stool. These data were used to determine intestinal motility state. Each rat was placed in a new cage cleaned using 70% alcohol and covered with paper. Every 15 min in the first hour and then every hour for the next 5 h, the stools were collected and weighed in pots as the wet weight of the stool. Subsequently, all pots were incubated at 60 °C overnight and then reweighed the next day for the dry weight of the stool. The percentage of water was calculated using the following formula: (wet weight – dry weight) / wet weight * 100%.
Necropsy
At the end of the study, rats were euthanized by administration of toxic doses of ketamine (100 mg/kg body weight) and Xylazine (30 mg/kg body weight). Subsequently, transcardial perfusion with cold phosphate-buffered saline (PBS) was performed to remove circulating blood. Vertical incisions were made in the abdomen and thorax exposed organs in the respective cavities. Then, the colon was identified and cleaned for further processing.
Colon collection
The colon was collected, measured, and divided into 1-cm segments. About 8–10 segments were randomly selected from 20 to 21 total obtained segments. Each selected segment was cut open on a random side, placed on paper with the mucosal side facing up and fixed in 10% neutral buffered formalin.
Histological examination
Tissue embedding and sectioning with a 5-µm thickness were performed in the horizontal orientation. Hematoxylin-eosin and immunohistochemistry staining were applied to the tissue sections, and observation was conducted under a light microscope (Olympus CX 21) with 400× magnification and photographed using Optilab advance V2 with Optilab viewer 2.2 software. Colon samples were examined using a scoring system provided by Nguyen39 based on epithelial damage, inflammation, and smooth muscle damage (score 0 - normal, score 1- damage/ cellular infiltration ≤ 1/3 of tissue length, score 2 − 1/3- - ⅔ of tissue length, score 3 - >⅔ tissue length).
Immunohistochemistry
Immunohistochemistry was performed in formalin-fixed paraffin-embedded colon. Immunohistochemistry was performed using anti-rat p16INK4a antibody (Invitrogen Cat.#:PA5-20379, dilution 1:400) and anti-rat Lamin B1 antibody (Invitrogen Cat.#:PA5-19468, dilution 1:200).
Immunodetection was performed using micropolymer anti-mouse and rabbit-specific HRP/DAB IHC Detection Kit-Micro-polymer (ABCAM, #Ab236466) rabbit immunoglobulin, and diaminobenzidine chromogen and counterstained with hematoxylin Mayer.
Positive control slides were prepared from colon tissue (p16INK4a) and lung (Lamin B1). The negative control was obtained by replacing the primary antibody with PBS. The semiquantitative (H-score) IHC results were assessed by two independent observers.
Data analysis
All quantitative data are reported as means ± standard of deviation. Statistical analysis for comparison between the control and aging induction groups was conducted using the Levene test for data homogeneity and the Shapiro–Wilk test for data normality using Graphpad Prism version 10. Further group comparisons were performed using independent t-tests at a 95% confidence level.
Data availability
Regarding raw data and other additional data, the datasets studied during the current investigation are not publically available because they are still being analyzed for another study. However, they are available from the corresponding author upon reasonable request.
References
Meng, J. et al. Integration of LncRNA and mRNA profiles to reveal the protective effects of Codonopsis pilosula extract on the Gastrointestinal tract of mice subjected to D-galactose-induced aging. Int J. Mol. Med 47, (2021).
Drozdowski, L. BR thomson, A. aging and intestine. World J. Gastroenterol. 7578–7584. https://doi.org/10.3748/wjg.v12.i47.7578 (2006).
Soenen, S., Rayner, C. K., Jones, K. L. & Horowitz, M. The ageing gastrointestinal tract. Current Opinion in Clinical Nutrition and Metabolic Care vol. 19 12–18 Preprint at (2016). https://doi.org/10.1097/MCO.0000000000000238
Deb, B., Prichard, D. O. & Bharucha, A. E. Constipation and Fecal Incontinence in the Elderly. Current Gastroenterology Reports vol. 22 Preprint at (2020). https://doi.org/10.1007/s11894-020-00791-1
Mari, A., Mahamid, M., Amara, H., Baker, F. A. & Yaccob, A. Chronic constipation in the elderly patient: Updates in evaluation and management. Korean Journal of Family Medicine vol. 41 139–145 Preprint at (2020). https://doi.org/10.4082/kjfm.18.0182
Broad, J. et al. Changes in neuromuscular structure and functions of human colon during ageing are region-dependent. Gut 68, 1210–1223 (2019).
Azman, K. F. & Zakaria, R. d-Galactose-induced accelerated aging model: an overview. Biogerontology vol. 20 763–782 Preprint at (2019). https://doi.org/10.1007/s10522-019-09837-y
Xie, D., Jiang, L., Lin, Y. & Liu, Z. Antioxidant activity of selenium-enriched Chrysomyia megacephala (Fabricius) larvae powder and its impact on intestinal microflora in D-galactose induced aging mice. BMC Complement. Med. Ther 20, (2020).
Dreesen, O. et al. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J. Cell Biol. 200, 605–617 (2013).
Malvezzi, H. et al. Altered p16Ink4a, IL-1β, and lamin b1 protein expression suggest cellular senescence in deep endometriotic lesions. Int J. Mol. Sci 23, (2022).
Shimi, T. et al. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 25, 2579–2593 (2011).
Han, H. et al. D-Galactose induces chronic oxidative stress and alters gut microbiota in weaned piglets. Front Physiol 12, (2021).
Zhao, J. et al. Effects of probiotics on D-galactose-induced oxidative stress in plasma: A meta-analysis of animal models. Journal of Functional Foods vol. 39 44–49 Preprint at (2017). https://doi.org/10.1016/j.jff.2017.09.055
Sholikah, T., Dian, E. S., Yustina, A. A. S. & Susilowati, R. Prevention of colon enlargement by TNF-α antagonist in a streptozotocin-induced diabetic rat model. Histol. Histopathol. 39, 1443–1455 (2024).
Patel, B. A. et al. Impaired colonic motility and reduction in Tachykinin signalling in the aged mouse. Exp. Gerontol. 53, 24–30 (2014).
Nakayasu, C., Kanemura, F., Hirano, Y., Shimizu, Y. & Tonosaki, K. Sensitivity of the olfactory sense declines with the aging in Senescence-Accelerated mouse (SAM-P1). Physiology & Behavior 70 (2000).
Seiberling, K. A. & Conley, D. B. Aging and olfactory and taste function. Otolaryngologic Clinics of North America vol. 37 1209–1228 Preprint at (2004). https://doi.org/10.1016/j.otc.2004.06.006
Scarpace, P. J., Matheny, M., Moore, R. L. & Tümer, N. Impaired Leptin Responsiveness in Aged Rats. DIABETES vol. 49 (2000). http://diabetesjournals.org/diabetes/article-pdf/49/3/431/365160/10868965.pdf
Kumar, M. V., Moore, R. L. & Scarpace, P. J. β3-Adrenergic regulation of leptin, food intake, and adiposity is impaired with age. Pflügers Archiv. 438, 681–688 (1999).
Wang, Y., Ren, K., Tan, J. & Mao, Y. Alginate oligosaccharide alleviates aging-related intestinal mucosal barrier dysfunction by blocking FGF1-mediated TLR4/NF-κB p65 pathway. Phytomedicine 116, (2023).
Tran, L. & Greenwood-Van Meerveld, B. a non-human primate model, aging disrupts the neural control of intestinal smooth muscle contractility in A region-specific manner. Neurogastroenterol. Motil. 26, 410–418 (2014).
Barbu, E., Popescu, M. R., Popescu, A. C. & Balanescu, S. M. Inflammation as A Precursor of Atherothrombosis, Diabetes and Early Vascular Aging. International Journal of Molecular Sciences vol. 23 Preprint at (2022). https://doi.org/10.3390/ijms23020963
Wagner, K. D. & Wagner, N. The Senescence Markers p16INK4A, p14ARF/p19ARF, and p21 in Organ Development and Homeostasis. Cells vol. 11 Preprint at (2022). https://doi.org/10.3390/cells11121966
Feng, X. et al. P16INK4A mediates age-related changes in mesenchymal stem cells derived from human dental pulp through the DNA damage and stress response. Mech. Ageing Dev. 141–142, 46–55 (2014).
Kim, Y. The impact of altered lamin B1 levels on nuclear lamina structure and function in aging and human diseases. Current Opinion in Cell Biology vol. 85 Preprint at (2023). https://doi.org/10.1016/j.ceb.2023.102257
Sasaki, M., Kajiya, H., Ozeki, S., Okabe, K. & Ikebe, T. Reactive oxygen species promotes cellular senescence in normal human epidermal keratinocytes through epigenetic regulation of p16INK4a. Biochem. Biophys. Res. Commun. 452, 622–628 (2014).
O’Sullivan, E. A., Wallis, R., Mossa, F. & Bishop, C. L. The paradox of senescent-marker positive cancer cells: challenges and opportunities. npj Aging vol. 10 Preprint at (2024). https://doi.org/10.1038/s41514-024-00168-y
Lv, T. et al. Mechanism and role of nuclear laminin B1 in cell senescence and malignant tumors. Cell Death Discovery vol. 10 Preprint at (2024). https://doi.org/10.1038/s41420-024-02045-9
bin Imtiaz, M. K. et al. Declining lamin B1 expression mediates age-dependent decreases of hippocampal stem cell activity. Cell. Stem Cell. 28, 967–977e8 (2021).
Waldherr, A. & Fogtman, A. Radiation symptoms resemble laminopathies and the physical underlying cause May sit at the lamin A C-terminus. Molecular Medicine 31, (2025).
Mowla, S. N., Perkins, N. D. & Jat, P. S. Friend or foe: Emerging role of nuclear factor kappa-light-chain-enhancer of activated b cells in cell senescence. OncoTargets and Therapy vol. 6 1221–1229 Preprint at (2013). https://doi.org/10.2147/OTT.S36160
Parkman, H. P. et al. Constipation in patients with symptoms of gastroparesis: analysis of symptoms and Gastrointestinal transit. Clin. Gastroenterol. Hepatol. 20, 546–558e5 (2022).
Wiskur, B. & Meerveld, G. V. B. The aging colon: The role of enteric neurodegeneration in constipation. Current Gastroenterology Reports vol. 12 507–512 Preprint at (2010). https://doi.org/10.1007/s11894-010-0139-7
Sadigh-Eteghad, S. et al. D-galactose-induced brain ageing model: A systematic review and meta-analysis on cognitive outcomes and oxidative stress indices. PLoS One 12, (2017).
Dumic, I. et al. Gastrointestinal tract disorders in older age. Can J Gastroenterol Hepatol (2019). (2019).
Metcalf, K. J. et al. Immunosuppressive glycoproteins associate with breast tumor fibrosis and aggression. Matrix Biol. Plus 14, (2022).
Bove, G. M. A non-invasive method to evaluate Gastrointestinal transit behavior in rat. J. Pharmacol. Toxicol. Methods. 74, 1–6 (2015).
Jung, W. K. et al. Advanced glycation end products increase salivary gland hypofunction in d-galactose-induced aging rats and its prevention by physical exercise. Curr. Issues Mol. Biol. 43, 2059–2067 (2021).
Nguyen, T. T. et al. Effects of Trang Phuc Linh plus-food supplement on irritable bowel syndrome induced by mustard oil. J. Med. Food. 20, 385–391 (2017).
Acknowledgements
The authors would like to thank Fitrininda Kusumoningrum for the technical assistance.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Ministry of Education, Culture, Research and Technology Republic of Indonesia in the Doctoral Dissertation Research Scheme [122/E5/PG.02.00.PL/2023 and 3099/UNI1/DITLIT/Dit-Lit/PT.01.03/2023].
Author information
Authors and Affiliations
Contributions
All authors participated in the design, conducting the laboratory work, interpretation of the studies and analysis of the data and review of the manuscript; DP created the study design, CA performed animal induction and functional test, CA, RS and YA conducted animal necropsy and tissue collection, YA and CA performed histological examination, DP and YA conducted immunohistochemistry scoring, BZN and CA conducted literature searching, DP, BZN and CA wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Ethical approval for this study was granted by the Ethics Commission for Research at the Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University, under approval number KE/FK/0676/EC/2023.
Animal welfare
The present study followed the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. All efforts were made to minimize potential animal distress in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Azaria, C., Nabila, B.Z., Sumiwi, Y.A.A. et al. Inflammatory and morphological changes in the colon reflect early aging induced by d-galactose in rats. Sci Rep 15, 35726 (2025). https://doi.org/10.1038/s41598-025-19437-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-19437-1