Abstract
Based on our previous results obtained on the coordination of bis-pyrazolyl bis-acetate pincer ligand towards heavy metals, mesoporous silica (pore size: 60 Å) was functionalized with a new NNN pincer ligand prepared through three surface modification steps to obtain a novel mesoporous material @SiA3. This study reports the synthesis and the application of @SiA3 for the removal of heavy metal ions (Pb2+, Cu2+, and Cd2+) from aqueous media. The obtained material @SiA3 was characterized by a series of analytical techniques including FT-IR, solid-state NMR 13C and 29Si, BET, EA, SEM and BJH which all confirmed the successful grafting. Following the characterization, batch adsorption experiments were conducted to investigate the influence and the effect of various parameters: initial ions concentration, pH solution, equilibrium time, kinetics, temperature, thermodynamic properties and selectivity toward Pb2+, Cu2+ and Cd2+. The main findings from batch adsorption experiments demonstrated that @SiA3 has a remarkable affinity and high selectivity toward Cu2+ achieving a maximum adsorption capacity of 127 mg/g in less than 15 min with stable efficiency after 5 cycles of regeneration/reusability maintaining over 98% of its initial efficiency. The adsorption kinetics were best described by the pseudo second order (PSO) model, while the equilibrium data fitted the Langmuir isotherm model, confirming a chemisorption mechanism involving monolayer adsorption onto a homogenous surface. Furthermore, thermodynamic studies revealed the adsorption to be spontaneous and endothermic with increased efficiency at higher temperatures. @SiA3 practical applicability could be used for the adsorption of copper in real river water sample from the Oued Za river in Morocco without interferences of other transition metal ions. To further understand this performance, the mechanism of metal ion adsorption is discussed in detail, highlighting the role of ligand structure in driving selectivity. The proposed removal mechanism is chelation, where the nitrogen cavity NNN of the rigid pincer ligand selectively coordinate with Cu2+ ions, as supported by Hard-Soft-Acid-Base (HSAB) theory.
Similar content being viewed by others
Introduction
In recent years, extensive coordination chemistry investigations have been carried out on compounds containing N-heterocyclic ligands (pyrazole1, pyridine2, imidazole3, etc.) and their derivatives because of their good binding abilities. Particularly, tridentate pincer ligands have gained considerable attention due to their ability to form stable supramolecular complexes with metal ions4,5,6. The preorganized and rigid tridentate binding pocket of NNN pincers not only enhances binding affinity but also dictates selectivity for specific metal geometries for applications requiring targeted removal7. Furthermore, the ability to adjust the properties and functions of the organic backbone by varying substituents allows precise modulation of the electronic properties of the ligand framework, influencing the binding characteristics of the resulting metal complex8. In our recent work9, we have explored the effect of ligating topologies, counter anions and the metal ions nodes on the supramolecular structure obtained from a conformationally flexible bis-pyrazol-bis-acetate (L) ligand having a pyridine backbone. This demonstrated excellent complexation properties with heavy metals, due to its pincer geometry and nitrogen − rich environment. Building on this foundation, we hypothesized that immobilizing this pincer ligand NNN (L) onto silica matrix would create a new hybrid material (@SiA3) for environmental applications that retains at the same time the ligand’s high affinity and selectivity for heavy metals with the mechanical stability and reusability of silica support .
The use of silica as a solid support, offers several advantages, including enhanced mechanical stability, easy separation, and cost-effectiveness10,11,12,13,14. Silica-based materials are particularly attractive due to their high surface area, porosity, and chemical inertness making them ideal candidates for environmental applications15,16. Recent studies have explored the functionalization of silica with various organic ligands for heavy metal adsorption, demonstrating promising results. For example, porphyrins have been reported to condense with the surface amino groups on mesoporous silica17. In similar way, other compounds containing nitrogen Schiff bases18, EDTA19, triethylenetetramine20 and 2-phenylimidazo[1,2-a]pyridine-3-carbaldehyde21 have also been reported to form various nitrogen platforms grafted on the mesoporous silica substrate for adsorption application.
However, to our knowledge the integration of NNN pyrazolyl pyridine pincer ligands into silica matrices for heavy metal removal remains underexplored, particularly in real-world water treatment applications.
In this study, we present the synthesis, characterization, and application of @SiA3, a silica-based material functionalized with the L pincer ligand, for the removal of Pb2+, Cd2+, and Cu2+ from aqueous solutions. Those metals were chosen because they are common and highly toxic heavy metal pollutants frequently found in industrial wastewater, originating from industries such as mining, electroplating, and battery manufacturing. They are known for their high toxicity to humans and aquatic life, even at low concentrations, and their tendency to bioaccumulate. Following this, the effects of several factors including equilibrium time, kinetics, temperature, thermodynamic properties, initial ions concentration, pH, selectivity and regeneration were all investigated. Furthermore, we evaluate the performance of @SiA3 in real water samples collected from the Oued Za river in Morocco, demonstrating its effectiveness in removing trace amounts of heavy metals under real-world conditions. The results highlight the potential of @SiA3 as a sustainable and efficient adsorbent for environmental remediation, particularly in regions affected by heavy metal pollution.
Experimental section
Materials
All solvents and chemicals (Acros Organics and Sigma-Aldrich purity 99.5%) were of analytical grade and used without further purification. Initial mesoporous silica gel spherical (0.03–0.2 mm) with a median pore diameter of 60 Å was purchased from Acros Organics. 1H NMR spectroscopic data were recorded with an Avance 300 MHz Bruker spectrometer. Surfaces were characterized by Fourier-transformed infrared (FT-IR) spectroscopy using an Equinox 55 (Bruker) equipped with an ATR modulus and a MCT detector in the range 400–4000 cm− 1. The Bruker software OPUS was used for data treatment. Thermogravimetric analyses (TGA) were performed in O2:N2 (90:10) atmosphere at a heating rate of 10 °C min− 1 from 25 to 850 °C using 3–4 mg with a Mettler Toledo TGA/SDTA 851e analyzer. CHN analysis was performed by MEDAC Ltd (UK). N2 adsorption–desorption analyses were performed at 77 K using a volumetric adsorption analyser (Micromeritics ASAP 2020). Before the analysis, the samples were pretreated at 150 °C for 16 h under reduced pressure (0.1 mbar). The BET method was applied in the P/P0 = 0–1 range to calculate the specific surface area, and the pore size distributions were calculated from the adsorption isotherm using the BJH method. Solid state 13C and 29Si CP-MAS NMR spectra were recorded at r.t. using a JEOL ECZ-R spectrometer operating at 14.1 T (119.2 MHz for 29Si and 150.9 MHz for 13C). Samples were spun at 10 kHz using zirconia rotors and a 3.2 mm AUTOMAS probe. The chemical shift scale was calibrated at r.t. with respect to a 3-(trimethylsilyl)-1 propane sulfonic acid sodium salt (DSS; δ = 0.0 ppm) and solid adamantane (38.48 and 29.45 ppm) for 29Si and 13C NMR-solid state, respectively. Scanning electron microscopy was performed on a FEI Nova Nano SEM 450 instrument.
Batch adsorption experiments
Batch experiments were conducted under standard conditions. The effects of various parameters on the adsorption, such as initial concentration, the pH of the solution, contact time and temperature were investigated. After adsorption, the solid phase was separated by filtration (0.45 μm). Experiments were carried out three times in each case, and only the mean data were reported. The concentration of each metal ion was determined by atomic absorption measurements using Spectra Varian A.A. 400 spectrophotometer and the amount of anions retained in the adsorbent phase (mg/g) was calculated by:
where Ci and Ce are the initial and final (equilibrium) concentrations of the anions in solution (mg/L), V is the solution volume (L), and mis the mass of adsorbent (g).
Effect of initial concentration: To assess the influence of initial metal ion concentration (10 mL) of individual solutions containing Cd2+, Cu2+ and Pb2+ at different concentrations (from 10 to 300 mg/L) were transferred into conical flasks containing 10 mg of @SiA3. The mixtures were stirred for 2 h at 25 °C and pH = 6. Metal ion concentrations in the filtrate were then analysed as described previously. Based on this experiment, the metal ion’s optimal concentrations, where adsorption by @SiA3 reaches its maximum, were determined (Table 1).
Effect of contact time: To determine the optimal contact time, 10 mg of @SiA3 was introduced into a conical flask, followed by 10 mL of metal ion’s optimal concentration solution. The concentration of remaining metal ions in the filtrate was measured at different time intervals: 2, 5, 10, 15, 20, 25 and 30 min. All tests were conducted at 25 °C and pH 6.0.
Thermodynamic study: To evaluate the thermodynamic behaviour of adsorption, 10 mg of each adsorbent was mixed with 10 mL of the metal ion’s optimal concentration solution. The experiment was conducted at four different temperatures (30 °C, 35 °C, 40 °C and 45 °C) while keeping all other parameters constant (contact time: 30 min, pH: 6.0).
Effect of pH variation: To study the influence of pH, 10 mg of @SiA3 adsorbent was added to a fixed volume of 10 mL of metal ion solution at the optimal concentration with pH adjusted to 1, 2, 3, 4, 5, 6, and 7. The samples were stirred for 30 min at 25 °C, and metal ion concentrations were measured in the filtrate. pH adjustments were made using diluted HCl and NaOH solutions.
Selectivity study: To investigate selectivity, 10 mg of @SiA3 was introduced into a conical flask containing a mixture of Cu(NO3)2·3H2O, Cd(NO3)2·6H2O and Pb(NO3)2·6H2O in 10 mL of solution each at 150 mg/L. The samples were stirred for 30 min. at 298 K and pH 6.0, and the concentrations of metal ions in the filtrate were analysed to determine the selectivity of each material.
Reusability assessment: To evaluate the reusability of the adsorbents, 10 mg of @SiA3 was reused for adsorption 5 times. The metal-loaded material was washed in 1 M HCl (10 mL) solution and stirred for 2 h. After filtration, the solid was washed with water until the filtrate’s pH was neutral, then dried.
Real water decontamination: 10 mL of water samples from the from Oued Za river were introduced into conical flasks containing 10 mg of adsorbent. After 30 min. of contact at 25 °C, metal ion concentrations in the filtrate were analysed.
Material synthesis and analyses
Material synthesis
Preparation of A3: The ligand 2,2’-{pyridine-2,6-diylbis[(5-methyl-1H-pyrazole-3,1 diyl)]}di(ethan-1-ol) (A3) was obtained by the reduction of diethyl 2,2’-(pyridine-2,6-diylbis(5-methyl-1 H-pyrazole-3,1 (1) diyl)) diacetate (L) prepared and discussed following the guidelines described in our previous work9. The reduction of ligand L (3 g, 7.2 mmol) to the corresponding alcohol was achieved using NaBH4 (3 g, 72 mmol) in absolute ethanol. The reaction was conducted at 60 °C for 24 h. After completion, the ethanol was evaporated, and the reaction mixture was cooled to room temperature. Subsequently, water (30 mL) was added to the mixture, and the solution was neutralized using 2 M HCl. The aqueous layer was extracted with dichloromethane (2 × 20 mL). The combined organic phases were washed with a saturated NaCl solution, dried over magnesium sulfate (MgSO4), filtered, and concentrated. The desired product A3 was obtained as a white powder (0.85 g, 34% yield) after precipitation in hexane, with a melting point of 168(1) °C.
1H NMR [DMSO; δ (ppm)]: 2.33 (s, 6 H, H10-16); 3.75(q, J = 5.6 Hz, 4 H, H20-22); 4.12 (t, J = 5.7 Hz, 4 H, H19-21); 4.90 (t, J = 5.4 Hz, 2 H, H23-24); 6.65(s, 2 H, H8-14); 7.67–7.83 (m, J = 0.9 Hz, 3 H, H1-5-6) RMN 13C [DMSO; δ(ppm)]: 11.54 (2 C, C10-16); 51.85 (2 C, C19-21); 61.02 (2 C, C20-22); 104.18 (2 C, C8-14); 117.85 (2 C, C1-5); 137.63 (1 C, C6) ; 141.07 (2 C, C9-15); 150.35 (2 C, C2 4);152.27 (2 C, C7-13). FT-IR (cm− 1): ν(alcohol): 3180; ν(C = C); 1465; ν(C = N): 1601. Fig. S1-S3.
Preparation of @SiCl: Initially, silica gel (particle size: 0.03–0.2 mm; pore size: 60 Å) was activated by heating at 120 °C for 48 h. Then, 11 g of activated silica gel was suspended in dried toluene (100 mL) in a two-necked round bottom flask, then (3-Chloropropyl)trimethoxysilane (CPTMS) (5 mL) is gradually added into the suspension. Then, the mixture was stirred at 110 °C for 24 h under Ar(g). After that, a filtration system was used to collect the solid, which was then washed extensively with hot toluene (40 mL), methanol (40 mL) and purified by Soxhlet extraction with a methanol: dichloromethane (1:1) mixture for 10 h to remove the remaining silylating agent. The obtained material @SiCl was dried under vacuum at 60 °C for 6 h22.
Preparation of @SiA3 (Fig. 1): @SiA3 was synthesized through the reaction of 4 g of A3 with 4 g of @SiCl and an appropriate amount of NaOH in anhydrous toluene (50 mL) under Ar(g) during 24 h at 110 °C. Finally, centrifugation was used to collect @SiA3, and then it was washed, with Soxhlet extraction apparatus for sequential reflux extraction using methanol: dichloromethane (1:1), and vacuum dried at 60 °C for 6 h.
Material analyses
The chemical environment of the attached ligands was thoroughly investigated using solid-state 13C and 29Si CP-MAS NMR. In detail, the 13C CP NMR spectrum Fig. 2a of @SiCl, shows distinct peaks at 50.0, 46.9, 26.6 and 10.9 ppm, which are characteristic of carbon atoms in four chemically inequivalent carbon sites. 50.0 and 46.9 ppm shifts are consistent with carbon atoms adjacent to electronegative groups (3.5 for oxygen and 3.1 for chlorine on the Pauling scale)23 results in a stronger electron-withdrawing effect, leading to increased deshielding of the adjacent carbon in OMe (50.0 ppm) and C-Cl (46.9 ppm). Additionally, the highly polarized nature of the Si-O bond, combined with potential hyperconjugation effects involving silicon, enhances this deshielding compared to C-Cl group where the effect is less pronounced. In comparison, Fig. 2(b) shows similar but slightly shifted peaks, indicating variations in the local electronic environment, together with several new peaks indicating the successful immobilization of the pincer ligand on the silica framework. The presence of the pyridine and pyrazole rings was further validated by signals between 100 and 160 ppm. The carbon peaks at 61.6 and 57.7 ppm are assigned to the carbon atoms of the linker O-CH2-CH2-N.
29Si NMR spectra of the silica samples reveal significant changes upon functionalization (Fig. 3). Indeed, 29Si CP-MAS experiments enable us to estimate the relative trends of NMR signals present in the different materials semi-quantitatively. Based on deconvolution analysis of NMR signals (Figures S4-S6, Table S1), it was observed that the pure dehydrated silica is dominated by the Q3 signal, which is observed at -100 ppm. Its intensity indicates the presence of silanol (Si-OH) groups, which are the primary reactive sites for grafting24. The abundance of Q3 sites provides more opportunities for organosilane attachment, potentially leading to a higher degree of functionalization and improved grafting efficiency. The Q4 and Q2 signals are observed at – 110 ppm and − 90ppm: They represent fully condensed Si(OSi)4 and partially condensed Si(OSi)2(OH)2 groups, respectively, and have approximately the same intensity in the CP-MAS spectra. Upon functionalization, the intensities of 29Si signals changed significantly: in the @SiCl sample the Q4 increased significantly, with respect to Q3 and Q2 signals, indicating the reactivity of silanol groups (Si-OH) to form Si-O-Si25. Also, the appearance of additional T1 and T2 peaks at − 56 ppm and − 48 ppm suggests the formation of new RSi(OSi)(OH)2 and RSi(OSi)2(OH) species respectively (R = organic moiety), confirming successful grafting. Finally, the 29Si spectrum of @SiA3 material (see Fig. 3) revealed that the relative amount of Q3 signal further decreased, while the Q4 increased, as expected in the case of progressive condensation triggered by the reaction conditions. Besides, two distinct peaks at -65 ppm and − 56 ppm, corresponding to T3 environments (R-Si(OSi)3) and T2 environment (R-Si(OSi)2(OH)), were observed.
In conclusion, the combined 13C CP and 29Si CP MAS NMR analyses confirm the successful grafting of the ligand A3 and further condensation of the silanol moieties during the modification step. These observations are in agreement with recent literature26.
The FTIR spectrum of @SiCl (Fig. 4) exhibits characteristic intense bands broad absorptions at 1062 cm−1 indicative of asymmetric Si-O-Si stretching within the silica network and vibrations at 797 cm−1 associated with symmetric stretching Si-O-Si. Bending vibration approximately at 2961 cm−1, attributed to C-H stretching of CH2 are also observed27. Following the grafting process, @SiA3 (Fig. 4) retains the mentioned Si-O-Si feature, demonstrating preservation of the silica framework. However, notable alterations were evident in the spectrum of @SiA3. The appearance of a shoulder at 1400–1500 cm−1, which was absent in the @SiCl spectrum, strongly suggests the introduction of C = N and C = C bonds stemming from the ligand’s pyazole pyridine rings. Furthermore, an increase in the intensity of the bands within the 2950–3546 cm−1 region, corresponding to C-H stretching modes, confirms the incorporation of alkyl groups from the ligand onto the silica. Characteristic band of C-O deformation associated to the ether function at 1215 cm− 128 was however not observed. These results are in line with other studies employing similar approaches to achieve functionalization of porous materials29.
Upon heating in O2:N2 (90:10) atmosphere from 25 to 850 °C, TGA analysis of SiO2, @SiCl and @SiA3 was conducted. Figure 5 shows that the weight loss of all samples can be divided into two distinct stages. For SiO2, a 4% mass loss was observed in the temperature range of 25–150 °C, which was reduced to 25–125 °C in the presence of grafting agents @SiCl and @SiA3. This initial weight loss is attributed to the evaporation of physically adsorbed water molecules30. Following the step-by-step immobilization process, the organic content in the silica increased, leading to an extended decomposition temperature range from 180 to 700 °C for both functionalized materials. This is primarily due to the combustion of the immobilized organic moieties in the silica31. The second weight loss for @SiCl and @SiA3 was measured at 10.84% and 25.82%, respectively.
Additionally, a slight weight loss was observed in SiO2 and @SiCl at temperatures above 350 °C, which can be attributed to the self-condensation of silanol groups into siloxanes32. This phenomenon is a common thermal behaviour in functionalized silica materials analysis33. Thus, the extent of silanol condensation depends on the thermal stability of the grafted organic groups and the degree of functionalization, which will be calculated in the next section using elemental analysis.
Elemental analysis (EA) provides quantitative information about the composition of the materials, including the degree of functionalization. Table 2 summarizes the results obtained for the synthesized hybrid materials, @SiCl and @SiA3. @SiCl exhibited a carbon content of 4.47%, while @SiA3 showed a carbon content of 11.95% along with 2.97% nitrogen, confirming the successful functionalization with chloropropyltrimethoxysilane and the pyridine-pyrazole pincer ligand, respectively.
The grafting densities (τ), calculated from EA were determined to be 0.93 mmol/g for @SiCl and 0.42 mmol/g for @SiA3. This difference can be attributed to the steric hindrance caused by the bulkier NNN pincer ligands in @SiA3, which limit access to reactive sites on the silica surface, as well as the higher complexity of grafting the A3 ligand compared to the smaller chloropropyl linker molecules. These grafting densities are consistent with values reported in the literature for the functionalization of mesoporous silica. For instance, chloropropylsilanes typically exhibit grafting densities ranging from 0.5 to 1.5 mmol/g34, which are influenced by factors such as reaction conditions, silane concentration, and the porosity of the silica material35,36,37,38.
To further validate the quantification of the grafted organic matrix, we have calculated the grafting densities (τ) from TGA analysis following the formula below37 and compared them with the results obtained from elemental analysis (EA):
With.
Δm1: dehydration mass loss.
Δm2: mass loss due to organic matter decomposition.
m spl: weight of the sample.
Morg: molecular weight of the organic matrix.
This calculation yielded grafting densities of 0.80 mmol/g for @SiCl and 0.50 mmol/g for @SiA3 (Table 3). These values are in good agreement with those obtained from EA, indicating successful and quantifiable grafting of both the chloropropyl linker and the pincer ligand onto the silica surface. The slight differences observed between the two methods can be attributed to divergences in measurement techniques and underlying theoretical constructs. TGA measures the total weight loss upon heating, which may include not only the combustion of the grafted organic matrix at high temperatures but also the dehydroxylation of silanol groups and the loss of physically adsorbed water on silica. In contrast, EA provides a direct measurement of elemental composition, assuming that all detected carbon originates from the grafted matrix. Despite these minor differences, the strong correlation between the grafting densities (τ) obtained from TGA and EA confirm the consistency of our quantification and supports the effective surface functionalization of silica using the NNN pincer ligand.
The BET analysis of SiO2, @SiCl and @SiA3, reveals a Type IV isotherm with H1 hysteresis, characteristic of mesoporous materials (Fig. 6). The specific surface area decreases from 579m2/g for SiO2 to 494 m2/g for @SiCl and 261 m2/g for @SiA3, indicating that surface modifications reduce accessibility due tosurface coverage (Table 4). Pore volume follows a similar trend, declining from 0.56 cm3/g (SiO2) to 0.41 cm3/g (@SiCl) and 0.26 cm3/g (@SiA3) due to pore blocking, while the average pore size remains relatively stable (38.57 Å for SiO2, 35.26 Å for @SiCl, and 35.40 Å for @SiA3) (Fig. 7).
The observed decrease in adsorption capacity, particularly for @SiA3, combined with the pronounced reductions in surface area and pore volume despite the preservation of average pore size and the persistence of the H1 hysteresis loop after functionalization, suggests that the grafted organic NNN pincer ligand primarily coats the pore walls or partially blocks pore entrances, rather than causing a uniform narrowing or collapse of the mesopores. This partial pore blocking reduces the accessible porosity of both mesopores and potentially some micropores, without significantly altering the overall pore diameter or mesostructure. These results also demonstrate that the two-step modification procedure effectively controls silica porosity. These findings are consistent with recent studies on functionalized mesoporous silica, which show that grafting ligands can significantly modify surface properties and adsorption behavior39,40.
Scanning electron microscopy (SEM) analysis highlights the morphological evolution and surface structural changes occurring during silica modification. Figure 8a-c presents a comparative view of SEM images for non-functionalized silica (a), chloro-functionalized silica @SiCl (b), and the pyridino-pyrazole silica-hybrid material @SiA3 (c). Image (a) depicts larger, irregularly shaped particles with relatively smooth and homogeneous surfaces compared to @SiA3 (c). Image (b) shows a noticeably disordered texture with particle fragmentation, resulting in a more heterogeneous morphology and an increased tendency for particles to aggregate, suggesting a modification of the silica surface. Compared to (a) and (b), @SiA3 in image (c) exhibits the most significant transformation: a further reduction in particle size, the formation of dense aggregates, and a significant increase in surface roughness, presumably hinting at the creation of interparticle connections through the grafted molecules. These progressive morphological changes indicate a successful surface modification process, with the resulting smaller particle size and increased aggregation having the potential to influence the material’s overall properties. EDX analysis and elemental mapping of the pure silica, @SiCl, and @SiA3 samples are reported in Figure S7.
These observations are consistent with results obtained from complementary characterization techniques, including FT-IR, TGA, BET surface area analysis, and solid-state 13C and 29Si NMR from Sect. 3.2 material analysis, which collectively confirm the coexistence of organic and inorganic networks due to the incorporation of organic molecules within the silica pores, leading to a homogeneous phase.
Heavy metal removal under different operational conditions
Effect of pH
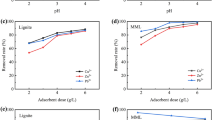
In the adsorption process, solution pH is crucial since it influences both the adsorbate and the adsorbent’s surface charge41. Therefore, the effect of pH on @SiA3 adsorption process was investigated over the range 1–7 for Pb2+, Cu2+ and Cd2+ (Fig. 9). Given that a considerable removal efficiency was recorded at pH 6, it was selected as the optimum pH for the adsorption of such metals onto @SiA3. Such an optimum pH value matches the one of an earlier study working on same metals using nanoparticles42. Extremely strong acidic conditions damage the material, corrode surfaces, and alter the chemical composition of the material43. This could hinder the intended interactions between the adsorbent and adsorbate, decreasing the efficiency of the adsorption process. On the other hand, under basic conditions (pH > 7), metal ions easily form insoluble hydroxide precipitates (e.g. M(OH)+, M(OH)2 and M(OH)3)44.
Kinetic study
The effect of contact time on Pb2+, Cd2+ and Cu2+ adsorption was studied using 10 mg of @SiA3 up 30 min. while keeping concentration, temperature and pH as constant (Fig. 10).
Initially, rapid adsorption was observed between 2 and 10 min due to the abundance of available binding sites on the adsorbent surface. As time progressed, the rate of adsorption showed no significant change in removal, eventually reaching equilibrium. This plateau occurred between 15 and 20 min. for all metal ions, indicating optimal contact time for efficient removal. In comparing those results with other materials documented in the literature, notable differences emerge. For instance, a bioinspired grafted mesoporous silica material demonstrated significant competitive adsorption efficiencies for these metals, but required longer contact time to reach optimal capacities, over 120 min. for effective removal of Pb2+, Cu2+, and Cd2+45. Another recent study on oak-activated carbon, found optimal contact times of 60 min. for Pb2+ and 80 min. for Cu2+. In comparison, @SiA3 exhibited much faster adsorption, making it a significant advantage in practical applications where time efficiency is critical46.
Effect of contact time on the adsorption of Cu2+, Cd2+ and Pb2+ onto @SiA3. Pseudo-second-order non-linear fitting was applied. Adsorption conditions: 10 mg of @SiA3 in 10 mL of an aqueous solution containing each metal ion at its optimal concentration, at 25 °C, pH 6, and periods varying between 2 and 30 min.
The kinetics of adsorption were further analyzed using pseudo-first-order and pseudo-second-order models to understand the mechanism of metal uptake47. The linear form of pseudo-first-order kinetic equation is expressed by the following equation:
where qe and qt are the amount of metal ions adsorbed on the adsorbent in mg.g− 1 at equilibrium and at time t, respectively, and k1 is the constant of first-order adsorption (min− 1). The pseudo-second-order kinetic rate equation is linearly expressed as follows:
where k2 is the pseudo-second-order rate constant at the equilibrium (g mg− 1 min− 1) that can be determined experimentally. The kinetics parameters and correlation coefficients were calculated from the linear plots (Table 5).
Clearly, the pseudo second order model give a better fit to experimental data (Fig. 11). This high degree of correlation suggests that the adsorption kinetics are predominantly controlled by chemisorption, involving valency forces through sharing or exchange of electrons between the adsorbent and adsorbate48.
To support this theory, we have calculated ∆qe, representing the difference between the experimental and theoretical values obtained from the linear plots. For instance, in the adsorption of Cu2+ ions, the qe calculated values from the pseudo-second-order model 129.87 mg/g closely matched the experimental values (127.64 mg/g). This negligible difference between theoretical and experimental capacities highlights the model’s accuracy in predicting the adsorption behaviour. Similar trends were observed for Cd2+ and Pb2+ ions, further validating the model’s applicability across different studied metal ions.
We have also calculated residual root mean square error (RMSE) values for the PSO kinetic model to @SiA3’s adsorption data for Cu2+, Cd2+, and Pb2+. This quantitative assessment of model fit is not commonly reported for heavy metal ion adsorption using silica materials49,50.
where qt, exp (mg/g) is the experimental adsorption capacity at time t, qt, cal is the theoretical adsorption capacity calculated using the PSO model, n is the number of data points.
Recent literature on silica adsorbents often reports strong correlations between experimental data and kinetic models, while explicit RMSE values are rarely provided. For instance, Tighadouini et al. reported an excellent fit of the PSO model to Cu2+ adsorption data for their silica hybrid material21, and El Abiad et al. demonstrated good fits for Porphyrin-silica gel hybrids in Cu2+ adsorption17, but neither study included specific RMSE values.
In our study, the calculated values were found to be 0.54,1.22 and 1.04 for Cu2+, Cd2+ and Pb2+ metal ions, respectively. The smaller RMSE indicates that the PSO model predictions are in perfect agreement with the experimental data. By incorporating this quantitative measure, our work not only confirms the excellent performance of @SiA3 according to PSO model but also sets a new standard for evaluation in the field of silica-based adsorbents.
In conclusion, the kinetic studies highlight the potential of the synthesized silica material @SiA3 as an effective adsorbent for heavy metal ions, with the PSO model providing a robust framework for understanding the adsorption kinetics. The high R2, negligible Δqe, and zero RMSE values collectively affirm the material’s suitability for environmental remediation applications.
Temperature effect
Temperature is a critical factor in optimizing the efficiency of an adsorbent and to evaluate the adsorption performance. To investigate the effect of temperature on the adsorption capacity of@SiA3, adsorption was performed over the range 30–45 °C. 10 mg of @SiA3 were added into 10 mL Pb2+, Cu2+ and Cd2+ metal solution separately and stirred for 30 min at pH 6. The increase in temperature significantly enhances the adsorption capability of @SiA3, for the studied heavy metal ions. As the temperature rises from 30 to 45 °C, the adsorption of these metal ions consistently increases. This implies that both kinetic energy and the probability of successful adsorption increase. This phenomenon can be attributed to several factors. First, higher temperatures provide greater kinetic energy to both metal ions and adsorbent, facilitating more frequent and effective collisions between them51,52. Additionally, higher temperatures may strengthen the interactions between the metal ions and the adsorbent, potentially due to changes in the physical properties of the silica hybrid material that enhance accessibility to internal binding sites by widening pores53,54.
Thermodynamic study
Thermodynamic parameters (Gibbs free energy changes ΔG; entropy ΔS and enthalpy change ΔH) were determined to assess the spontaneity of the adsorption process as follows55:
where R is the ideal gas constant (8.314 J/mol K), T is the temperature in Kelvin, Kd is the thermodynamic equilibrium constant, C0 (mg L− 1 ) is the initial concentration of metal ion, and Ce (mg L− 1) is the equilibrium concentration of metal ion, V (L) is the volume of solution and m (g) is the dosage of sorbents (Table 6).
Positive ΔH values suggest that the interactions between the adsorbate Cu2+, Cd2+ and Pb2+ and the adsorbent @SiA3 are more dynamic and favourable at higher temperatures. This can indicate that the adsorption of the metal ions by @SiA3 is of endothermic nature and higher temperatures are more favourable for sorption.
The positive ∆S values for @SiA3 exhibited the increasing randomness at the solid–liquid interfaces during the adsorption of metal ions on the adsorbent and could be due to some structural changes in the adsorbent. Furthermore, with the increase of temperature, the negative ΔG values of Cu2+, Cd2+ and Pb2+ indicate that the adsorption efficiency is higher at higher temperature and the adsorption is thermodynamically favourable and spontaneous (Fig. 12).
Isotherm adsorption
The isotherm adsorption describes the relationship between the adsorbent and its concentration in solution56. To clarify the mechanism of Cu2+, Cd2+ and Pb2+ adsorption by @SiA3, the Langmuir and Freundlich model was used to fit the experimental data.
The Langmuir model assumes that adsorption occurs as a single layer on a homogeneous adsorbent surface, without any interactions between the adsorbed molecules57. The Langmuir model is expressed in the following form:
where qe and q are the maximum amount of the adsorbed solute on the adsorbent surface and q the adsorption capacity at equilibrium (mg/g) respectively, Ce is the equilibrium concentration of the ions in the solution (mg/L), KL is the Langmuir adsorption constant (L/mg). The values of q and KL can be calculated by a linear relationship.
The equilibrium constant RL of the Langmuir model can also be used to describe the adsorption effect of the adsorption process. Depending on the value of RL, the isotherm’s shape can be classified as favorable (0 < RL < 1), irreversible (RL = 0), linear (RL = 1), or unfavorable (RL > 1)58. The equation RL is calculated following the form:
where C0 is the initial concentration of metal ions.
Freundlich’s model is based on the adsorbate forming multiple layers on the heterogeneous solid surface of the adsorbent, and the binding strength decreases with increasing site occupation59. The empirical Freundlich equation is expressed as follows:
where qe is the adsorption capacity (mg/g), Ce is the equilibrium concentration (mg/L), KF is the Freundlich constant (mg g−1), and n is the Freundlich constant indicating the adsorption intensity.
The adsorption isotherms and corresponding parameters for Cu2+, Cd2+, and Pb2+ adsorption onto @SiA3 are summarized in Table 7. The correlation coefficients R2 from these isotherms indicate the fit of each model to the experimental data. The Langmuir model shows a higher R2 value than the Freundlich model, suggesting that the adsorption of Cu2+, Cd2+, and Pb2+ onto @SiA3 aligns more closely with the Langmuir model, which describes monolayer adsorption on a uniform surface without interactions between the adsorbed heavy metals ions. This implies that once the surface is saturated and the monolayer is formed, no additional adsorption occurs, likely due to the uniform and specific adsorption sites present on @SiA360,61.
Moreover, the RL values calculated from the Langmuir model range between 0.06 and 0.10, indicating a highly favourable adsorption process for these metal ions onto @SiA3. Further validation is provided by comparing the experimental adsorption capacities qe with the theoretical q values from the Langmuir model: experimental capacities of 127, 35 and 45 mg/g for Cu2+, Pb2+ and Cd2+ respectively closely match the calculated values of 128, 37 and 48 mg/g, respectively.
In contrast, the Freundlich constant n, which reflects the degree of surface heterogeneity, provides additional insight into the adsorption type. Values of n between 1 and 10 indicate favorable chemical adsorption, while n < 1 suggests a physical adsorption process. The n values in Table 7 confirm that the adsorption of metal ions onto @SiA3 is dominated by favourable chemical interactions, supporting the effectiveness of @SiA3 as an adsorbent.
Concentration effect
The effect of initial concentration on the adsorption capacity of @SiA3 has been carefully studied, revealing significant insights into the adsorption mechanisms and efficiency. Figure 13 demonstrate that the adsorption capacity of @SiA3 increases with the increases of initial concentration of Pb2+, Cu2+ and Cd2+ in solution which is attributed to the to the presence of abundant NNN functional groups within the pyrazolyl-pyridine pincer ligand incorporated into the silica, which serve as active sites capable of effectively coordinating with metal ions based on their affinity62. As the concentration of metal ions increases, these active sites become progressively occupied, resulting in higher adsorption capacity. However, once all accessible sites are saturated, adsorption reaches a maximum, as reflected by the constant qe values (Fig. 13).
The adsorption data fitted well with the Langmuir isotherm discussed in Sect. 4.5 indicating monolayer adsorption on a homogeneous surface, which is reflected by the formation of a plateau indicating saturation beyond a 127 mg/g for Cu2+, 35 mg/g for Pb2+ and 45 mg/g for Cd2+.
Moreover, concentration effect highlights the importance of the initial metal concentration in achieving optimal adsorption performance63: @SiA3 shows effective binding to Cu2+ (127 mg/g) compared to Cd2+ and Pb2+ (45 and 35 mg/g) at higher concentrations, and the maximum adsorption capacity was highly relative to Cu2+ suggesting that the pincer NNN on the modified silica has a higher affinity for Cu2+ (see Sect. 4.8).
In summary, the adsorption capacity of modified silica material @SiA3 for Cu2+, Cd2+ and Pb2+ is strongly influenced by the initial metal concentration. Higher concentrations generally enhance the adsorption efficiency due to increased availability of metal ions for interaction with the functional groups on the silica surface, leading to more effective and rapid adsorption until the active sites are saturated.
Relationship between equilibrium concentration Ce [mg/L] of heavy metal ions and adsorption effect qe [mg/g] using non-linear Langmuir model fit. Adsorption conditions: 10 mg of @SiA3 in 10 mL of an aqueous solution containing each metal from 10 to 300 mg/L at 25 °C, pH 6, and a contact time of 2 h.
Regeneration/reusability
The reusability and regeneration potential of @SiA3 are crucial factors in its practical application for environmental remediation. To evaluate its regeneration capacity, multiple adsorption-desorption cycles were conducted involving a 2 h wash with a 1 M HCl solution. The material demonstrated excellent regeneration efficiency, maintaining over 98% of its initial adsorption capacity after five consecutive cycles. Figure 14 represents elimination percentages of @SiA3 towards copper, as it is selective to it, compared to other used metals. This high regeneration performance can be attributed to its robust structure, which combines the mechanical stability of the silica and the functional properties of the organic component. These characteristics make @SiA3 a promising candidate for sustainable and cost-effective treatment processes, given it significantly reduces waste generation and material costs compared to single-use adsorbents. For instance, single-use materials such as TiO2 photocatalysts require UV light within a spectrum of 290–390 nm and achieve only 45.56% removal efficiency for copper ions under optimal conditions64. Other examples include snail shell powder and activated carbon prepared from hazelnut shells, which demonstrate relatively high adsorption capacities of 63.5 mg/g and 82.9 mg Cu2+/g, respectively. However, these single-use materials require longer equilibrium times and exhibit lower adsorption capacities compared to @SiA365,66. Thermogravimetric analysis (TGA) conducted after each adsorption-desorption cycle between 25 and 850 °C further confirmed that the material remains stable with no significant change, underscoring the robust nature of the chemical bonding within @SiA3 (Figure S8).
Mechanism
The process of heavy metal ion adsorption onto the silica hybrid material@SiA3 is largely influenced by the structural and electronic properties of the bispyrazolyl pyridine ligand attached to the silica matrix. This study builds on the evolution of ligand design9, starting with the free bispyrazolyl pyridine ligand, known for its excellent ability to form complexes due to its nitrogen-rich, pincer structure. The ligand consists of two pyrazolyl groups and a central pyridine, with nitrogen atoms that serve as coordination sites, forming stable bonds with metal ions. In our previous research9, X-ray diffraction (XRD) confirmed the creation of octahedral metal-ligand complexes, showcasing the importance of the pincer design in enabling strong and selective binding. Building on the success of the free ligand, it was grafted onto silica to develop @SiA3, which combines the selectivity of bispyrazolyl pyridine with the stability and reusability of a silica matrix. Notably, @SiA3 showed high selectivity for Cu2+ ions, thanks to the rigid pincer geometry of the ligand, which favours smaller cations like Cu2+ (0.73 Å) over larger ones like Pb2+(1.19 Å) or Cd2+(0.95 Å). To further understand how ligand structure affects performance, a simpler pyrazolylpyridine ligand was also grafted onto silica22. This version showed strong selectivity for Pb2+ ions, likely due to its smaller size and more flexible coordination environment, which better suits the larger ionic radius of Pb2+. These results highlight that even when the active sites (pyridine and pyrazole) are the same, the ligand’s size, rigidity, and spatial arrangement play a key role in determining metal ion selectivity. Comparison of the two silica-based materials revealed that the smaller pyrazolyl pyridine ligand creates a less crowded environment, favoring Pb2+, while the bulkier bispyrazolyl pyridine ligand preferentially binds Cu2+ due to steric and electronic factors. The adsorption process for @SiA3 works through chelation, where the nitrogen atoms of the pyrazole and pyridine groups coordinate with metal ions. This interaction stabilizes the metal-ligand complex, and the rigid pincer geometry ensures selectivity. The octahedral geometry of these complexes, confirmed in earlier XRD studies, supports the idea that multidentate interactions play a key role in stabilizing the metal ions.
To complement the discussion on affinity, it is important to consider the effects of hydrated ionic radii and hydration enthalpy on the adsorption mechanism. In aqueous solution, metal ions are stabilized by surrounding water molecules, which must be displaced to allow coordination with the ligand. This dehydration step introduces an energetic barrier, quantified by the hydration enthalpy. Notably, Cu2+ exhibits a high hydration enthalpy (approximately − 2100 kJ/mol), reflecting its small ionic radius and high charge density, compared to Cd2+ (− 1807 kJ/mol) and Pb2+ (− 1481 kJ/mol)67.
The ideal geometric of Cu2+ ions within the NNN pincer cavity enables the formation of highly stable coordination bonds in the [Cu@SiA3] complex. The energy released upon this complexation is sufficient to overcome the dehydration energy barrier, making the overall process thermodynamically favorable. In contrast, the larger Cd2+ and Pb2+ ions experience a steric mismatch within the ligand cavity, resulting in the formation of weaker complexes ([Cd@SiA3] and [Pb@SiA3]), where the energy released upon binding does not fully compensate for the dehydration enthalpy.
In summary, @SiA3 combines the properties of bispyrazolyl pyridine and silica to create a material with improved adsorption capabilities and a strong preference for Cu2+, achieving an adsorption capacity of 127.64 mg/g. These findings shed light on how ligand design and functionalization influence adsorption performance and offer valuable guidance for developing advanced materials for heavy metal removal.
Selectivity
Selectivity is a crucial aspect to study for removing heavy metal ions from aqueous solutions. An efficient adsorbent should selectively bind the target metal while exhibiting minimal affinity for other co-exisiting ions existing in environmental samples68. For that reason, adsorption of mixed Cu2+, Cd2+ and Pb2+ under constant conditions (pH 6, temperature 25 °C, 10 mg adsorbent) for 30 min was investigated for @SiA3. Figure 15 shows the great affinity toward Cu2+ followed by Cd2+, and then Pb2+, suggesting that the coexistence of different metals does not significantly alter the efficiency of @SiA3. In a mixed-ion system with a concentration of 150 mg/g of each metal, the adsorbed amount of Cu2+ remains the highest, accounting for 68% of its adsorption capacity in a single-ion system. Although the adsorption capacities for Cd2+ (10%) and Pb2+(6%) were slightly lower, the differences were not substantial, suggesting that @SiA3 maintains a relatively high selectivity for Cu2+ even in the presence of competing ions.
The reason maybe presumable in the well-designed dimensions of the N-pyrarole-N-pyridine-N-pyrazole cavity, providing an optimal fit for Cu2+ over the other ions.
Furthermore, this trend can be partially explained using Hard-Soft-Acid-Base (HSAB) theory. Cu2+ is considered a borderline acid, Cd2+ a soft acid, and Pb2+ also leans towards being a soft acid69. The N-containing ligands, possessing intermediate hardness, may exhibit a stronger interaction with Cu2+ based on the HSAB principle, alongside considerations such as ionic radii and coordination preferences influencing the overall selectivity70.
Environmental experiences
In order to demonstrate the validity of @SiA3 under real experimental conditions, a river water was collected 100 km west of Oujda, Morocco from Oued Za river at 34°25’13.6’’N2°52’53.5’’W. The adsorption efficiency of @SiA3 was investigated under optimal conditions by the batch method using 10 mL of the river water. The initial concentrations of Cd2+, Cu2+ and Pb2+ in the untreated river water, measured using flame atomic absorption spectrometry (FAAS) were significantly lower than instrument’s detection range: 1–12 ppm for Pb2+, 0.1–0.6 ppm for Cd2+ and 1–4 ppm for Cu2+ indication the absence or only trace presence of these harmful heavy metals in the selected river water. To assess the material’s performance under more challenging and realistic environmental conditions, and in light of its reported affinity for Cu2+ ions, the river water sample was doped with 150 mg/g Cu2+.
Following treatment with 10 mg of @SiA3, analysis of the Cu-spiked sample revealed a 63% reduction in Cu2+ concentration. While this removal efficiency is lower than observed in deionized water, it represents a strong performance in a natural complex matrix. This reduction, can be attributed to the possible interference of organic matter and alkaline ions in real water samples such as Mg2+, Ca2+, Na+, K+, HCO3−, SO42−,
Cl−… These natural organic substances present in aquatic environments potentially block access to the ligand pincer sites. Therefore, achieving 63% removal of copper is hypothesized to be highly competitive with other modified adsorbents containing nitrogen materials recently reported. For instance, materials like amine-modified silica achieved 33.33 mg/g Cu2+ removal at similar mg/g rates71. Comparatively, activated carbon derived from Hydrilla Verticillata has been known to be around 44%72. @SiA3 is strategically designed for fast, low-cost synthesis, high stability, and enhanced selectivity towards Cu2+ over other competing ions.
Comparative study of adsorption capacity of Cu(II) to previous studies
Various methods for the removal of heavy metals from aqueous solution have been extensively studied in recent years, such as ion exchange73, chemical precipitation, electrochemical reduction74, and adsorption. By combining the advantages and disadvantages of these different technological approaches, adsorption is generally preferred for the removal of heavy metal ions due to its remarkable simplicity and low environmental impact. In this approach, @SiA3, unlike conventional adsorbents, is a novel silica hybrid material, renewable, highly stable over time and temperature, selective and with a minimal environmental impact. Table 8 shows superior characteristics for @SiA3 compared to selected literature examples.
Conclusion
In summary, we report the preparation of an inorganic-organic hybrid material obtained from mesoporous silica structure (579.15 m2/g) and bispyrazolyl pyridine ligand covalently grafted onto the SiO2 surface through 3-chlorotrimethoxy silane linker. The results on single and multicomponent adsorption show that the adsorption capacities of @SiA3 are in the order of Cu2+ (127 mg/g) > Cd2+ (45 mg/g) > Pb2+ (35 mg/g). The adsorption behaviour aligns more closely with the Langmuir isotherm model with PSO kinetic model. @SiA3 demonstrated high efficiency as an adsorbent for the removal and separation of heavy metals, even in real wastewater, achieving a high removal ratio of 63% in less than 15 min. and reusable for five times without significant loss of performance.
This study highlights the influence of ligand structure on the performance and selectivity of the adsorbent. Not only does this work advance the understanding of ligand design and mechanism in silica functionalization for heavy metal adsorption but also provides valuable insights into the practical application of silica-based materials for water purification.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Wang, Y. et al. Constructing pyrazole-based metal–organic layers for efficient separation of light hydrocarbons. ACS Appl. Mater. Interfaces. 15, 49895–49903 (2023).
Sharma, A., Kumar, A., Kaur, N. & Kumar, S. Synthesis, characterization, and biological activity of novel coordination complexes of Cu(II), Ni(II), and Co(II) with pyridine-based schiff base ligand. J. Coordin Chem. 76, 2061–2077 (2023).
Mrozinski, J. et al. Synthesis, crystal structure, and magnetic properties of new copper(II) complexes with imidazole derivatives. J. Inorg. Org. Polym. Mater. 33, 2955–2966 (2023).
Neuman, T., Gallo, G., Dinnebier, R. E. & Näther, C. Synthesis, crystal structures, and properties of Mn(NCS)2 coordination compounds with 4-picoline as coligand and crystal structure of Mn(NCS)2. Z. Anorg Allg Chem. 646, 88–94 (2020).
Zhou, T. et al. Self-assembly of pincer-type palladium complexes into metallacycles and cages: versatile platforms for supramolecular construction. Chem. Commun. 59, 9367–9370 (2023).
Malek, F., Ramdani, A., Zidane, I., Yahyi, A. & Radi, S. Tetrapyrazolic tripods. Synthesis and preliminary use in metal ion extraction. Tetrahedron 61, 2995–2998 (2005).
Ghosh, S., Thompson, L. K., Patrick, B. O. & Schafer, L. L. Synthesis and characterization of sterically encumbered NNN pincer complexes of group 4 metals: enhanced Lewis acidity and catalytic ethylene polymerization. Dalton Trans. 52, 9368–9377 (2023).
Haik, J., El, Kilner, C. A. & Halcrow, M. A. An iron(ii) complex salt that crystallises in three crystal forms, one of which undergoes a sterically controlled incomplete spin-state transition on cooling. CrystEngComm 7, 151 (2005).
Oulmidi, A., Radi, S., Miras, H. N., Adarsh, N. N. & Garcia, Y. New bis-pyrazole-bis-acetate based coordination complexes: influence of counter-anions and metal ions on the supramolecular structures. Sustainability 13, 288 (2021).
Zhou, T. et al. Mechanical performance and thermal stability of glass fiber reinforced silica aerogel Composites based on Co precursor method by freeze drying. Appl. Surf. Sci. 437, 321–328 (2018).
Alasti Bonab, S., Moghaddas, J. & Rezaei, M. In-situ synthesis of silica aerogel/polyurethane inorganic-organic hybrid nanocomposite foams: characterization, cell microstructure and mechanical properties. Polymer 172, 27–40 (2019).
Radi, S. et al. An efficient hybrid adsorbent based on silica supported amino penta-carboxylic acid for water purification. J Mater. Chem A. 6, 13096–13109 (2018).
Tighadouini, S. et al. Removal efficiency of Pb(II), Zn(II), Cd(II) and Cu(II) Fro aqueous solution and natural water by ketoenol–pyrazole receptor functionalized silica hybrid adsorbent. Sep. Sci. Tech. 52, 608–6214 (2017).
Radi, S. et al. Efficient extraction of heavy metals from aqueous solution by novel hybrid material based on silica particles bearing new schiff base receptor. J. Mol. Liquids. 223, 112–118 (2016).
Alhokbany, N., Ahamad, T., Naushad, M. & Alshehri, S. Feasibility of toxic metal removal from aqueous medium using Schiff-base based highly porous nanocomposite: adsorption characteristics and post characterization. J. Mol. Liquids. 294, 111598 (2019).
Tighadouini, S. et al. Removal of toxic heavy metals from river water samples using a porous silica surface modified with a new β-ketoenolic host. Beilstein J. Nanotechnol. 10, 262–273 (2019).
El Abiad, C. et al. Porphyrin-silica gel hybrids as effective and selective copper(II) adsorbents from industrial wastewater. J. Envir Chem. Engin. 11, 110097 (2023).
Zhao, J. et al. The adsorption property and mechanism for Hg(II) and Ag(I) by schiff base functionalized magnetic Fe3O4 from aqueous solution. J. Alloys Compd. 825, 154051 (2020).
Bulut, E., Yatmaz, H. C. & Askin, A. Synthesis and characterization of EDTA-modified magnetic nanoparticles for removal of heavy metal ions from aqueous solutions. J. Water Process. Eng. 46, 102604 (2022).
Elwakeel, K. Z., Khairy, M., Ibrahim, H. S. & Nemr, E. Efficient removal of Cd(II) from aqueous solution using triethylenetetramine-functionalized graphene oxide nanocomposite: adsorption mechanism, isotherm, kinetic and thermodynamic studies. J. Mol. Liq. 362, 119674 (2022).
Saddik, R. et al. Mesoporous silica modified with 2-phenylimidazo[1,2-a] pyridine-3-carbaldehyde as an effective adsorbent for Cu(II) from aqueous solutions: a combined experimental and theoretical study. Molecules 27, 5168 (2022).
Oulmidi, A. et al. Rapid and selective separation of heavy metal ions from aquatic medium using a bidentate functionalized hybrid material. Colloids Surf., A. 704, 135462 (2025).
Allred, A. L. Electronegativity values from thermochemical data. J. Inorg. Nucl. Chem. 17, 215–221 (1961).
Morrow, B. A. & Farlen, M. A. J Non-Cryst Solids 120, 61–71 (1990).
Qiao, B., Wang, T. J., Gao, H. & Jin, Y. Surface modification of silica nanoparticles by organosilanes: A review of characterization techniques and applications. J. Mater. Chem. A. 9, 10643–10674 (2021).
Garcia, A., Perez, M., Gomez, C. & Rodriguez, B. Controlling grafting density in silica-based hybrid materials. ACS Appl. Mater. Interfaces. 15, 9876–9885 (2023).
Li, M., Wang, B., Li, J., Dong, L. & Yan, H. Fabrication and characterization of porous silicon-based nanostructures for biosensing applications. Nanomaterials 11, 2788 (2021).
Zhang, Z., Wang, T., Zhang, H., Liu, Y. & Xing, B. Adsorption of Pb(II) and Cd(II) by magnetic activated carbon and its mechanism. Sci. Total Environ. 757, 143910 (2021).
Zou, L. et al. Functionalized porous materials with organosilanes for selective removal of organic pollutants from water. Nanomaterials 12, 3180 (2022).
Samy, M. et al. Heterogeneous activation of persulfate by a novel nano-magnetite/ZnO/activated carbon nanohybrid for Carbofuran degradation: toxicity assessment, water matrices, degradation mechanism and radical and non-radical pathways. J. Process. Saf. Environ. Prot. 169, 337–335 (2023).
Shahbazi, A., Younesi, H. & Badiei, A. Functionalized SBA-15 mesoporous silica by melamine-based dendrimer amines for adsorptive characteristics of Pb(II), Cu (II) and Cd(II) heavy metal ions in batch and fixed bed column. Chem. Eng. J. 168, 505–518 (2011).
Yin, P. et al. Synthesis of functionalized silica gel with poly(diethylenetriamine bis(methylene phosphonic acid)) and its adsorption properties of transition metal ions. Mater. Chem. Phys. 129, 168–175 (2011).
Yin, P. et al. Mater. Chem. Phys. 129,168–175 (2011).
Garcia, A. M. et al. Controlled surface modification of mesoporous silica to produce tailored porous materials with tunable hydrophobicity. Chem. Mater. 31, 5428–5438 (2019).
Knežević, N. et al. Chloropropylsilane and Amino-Functionalization of mesoporous Silica – a comparative study. Materials 16, 4691 (2023).
Zhang, J. et al. Facile construction of surface-supported N-Heterocyclic carbene palladium(II) catalyst for high activity in Suzuki–Miyaura reaction. ChemCatChem 13, 17, 3890–3897 (2021).
Mahtabani, A. et al. Gas Phase Modification of Silica Nanoparticles in a Fluidized Bed: Tailored Deposition of Aminopropylsiloxane. Langmuir 37(15), 4481–4492 (2021).
Ghorbani, H. & Saffar-Teluri, A. One-pot synthesis and characterization of mesoporous silica functionalized with chiral schiff base ligand and its application for enantioselective adsorption of D-/L-tryptophan. J. Mol. Liq. 344, 117768 (2021).
Zhang, Y., Ren, Z., Cao, J. & Du, J. Precisely controlled synthesis of High-Performance polysiloxane elastomers via Thiol-Ene click chemistry. Adv. Funct. Mater. 32, 2109056 (2022).
Sadeghi, S. et al. Enhanced adsorption of heavy metal ions using novel EDTA-functionalized mesoporous silica nanoparticles: synthesis, characterization, and optimization. Envir Res. 246, 118205 (2024).
Kuang, Y., Zhang, X. & Zhou, S. Adsorption of methylene blue in water onto activated carbon by Sur factant modification. Water 12, 587 (2020).
Draoui, Y. et al. Tailoring selectivity and efficiency: pyrazolyl-1H-1,2,4-triazole MCM-41 and silica hybrid materials for efficient cadmium(II) removal from water. Environ. Sci. Pollut Res. 32, 10984–11003 (2025).
Huang, Y. Y. et al. Anion-synergistic adsorption enhances the selective removal of silver ions from complex wastewater by chitosan-coated magnetic silica core-shell nanoparticles. J. Clean. Prod. 339, 130777 (2022).
Li, Y. et al. Super rapid removal of copper, cadmium and lead ions from water by NTA-silica gel. RSC Adv. 9, 397–407 (2019).
Gourmand, C. et al. Competitive adsorption mechanisms of Cd(II), Cu(II) and Pb(II) on bioinspired mesoporous silica revealed by complementary adsorption/isothermal Titration calorimetry studies. Dalton Trans. 53, 3690–3370 (2024).
Khater, D., Alkhabbas, M. & Al-Ma’abreh, M. Adsorption of pb, cu, and Ni ions on activated carbon prepared from oak cupules: kinetics and thermodynamics studies. Molecules 29, 2489 (2024).
Ho, Y. S. & Mekay, G. Kinetic models for the sorption of dye from aqueous solution by wood. Process. Saf. Environ. Prot. 76, 183–191 (1998).
Nayab, S. et al. Silica based inorganic–organic hybrid materials for the adsorptive removal of chromium. RSC Adv. 8, 23963 (2018).
Bagheri, A., Yazdani, A., Abbas, A. & Rafati Selection of better cationic surfactant for zeolite modification using surface studies and its application in the removal of anionic and cationic dyes. J. Mol. Liq. 403, 124881 (2024).
Khaksarfard, Y., Bagheri, A., Abbas, A. & Rafati Synergistic effects of binary surfactant mixtures in the adsorption of diclofenac sodium drug from aqueous solution by modified zeolite. J. Colloid Interface Sci. 644, 186–199 (2023).
Dong, Y. et al. Dynamic experimental study on treatment of acid mine drainage by bacteria supported in natural minerals. Energies 13, 439 (2020).
Aksu, Z. & Kutsal, T. A. A bioseparation process for removing Pb(II) ions from wastewater by using C. vulgaris. J. Chem. Techn Biotech. 52, 108–118 (1991).
Zhang, M. et al. Selective oxidation of organic pollutants based on reactive oxygen species and the molecular structure: degradation behaviour and mechanism analysis. Water Res. 246, 120697 (2023).
Zhang, Y., Zhang, Y. & Zhang, H. Study on Preparation of a novel silica adsorbent and its selective separation applied to genistein. Brazilian J. Chem. Engin. 25, 201–206 (2008).
Kavitha, D. & Namasivayam, C. Experimental and kinetic studies on methylene blue adsorption by Coir pith carbon. Bioresour Technol. 98, 14–21 (2007).
Dehghani, M. H. et al. Process optimization and enhancement of pesticide adsorption by porous adsorbents by regression analysis and parametric modelling. Sci. Rep. 11, 11719 (2021).
Yao, L., Esmaeili, H., Haghani, M. & Roco-Videla, A. Activated carbon/bentonite/Fe3O4 as novel nanobiocomposite for high removal of Cr(VI) ions. Chem. Eng. Technol. 44, 1908–1918 (2021).
Amin, N. K. Removal of reactive dye from aqueous solutions by adsorption onto activated carbons prepared from sugarcane Bagasse pith. Desalination 223, 152–161 (2008).
Khambhaty, Y., Mody, K., Basha, S. & Jha, B. Kinetics, equilibrium and thermodynamic studies on biosorption of hexavalent chromium by dead fungal biomass of marine Aspergillus Niger. Chem. Eng. J. 145, 489–495 (2009).
Hadi Dehghani, M. et al. Statistical modelling of endocrine disrupting compounds adsorption onto activated carbon prepared from wood using CCD-RSM and DE hybrid evolutionary optimization framework: comparison of linear vs non-linear isotherm and kinetic parameters. J. Mol. Liq. 302, 112526 (2020).
Azari, A., Yeganeh, M., Gholami, M. & Salari, M. The superior adsorption capacity of 2,4-Dinitrophenol under ultrasound-assisted magnetic adsorption system: modeling and process optimization by central composite design. J. Hazard. Mater. 418, 126348 (2021).
Al-Saida, B., Sandouqa, A., Shawabkeh, R. & Hussein, I. Synthesis of Nanosilica for the removal of multicomponent Cd2+ and Cu2+ from synthetic water: an experimental and theoretical study. Molecules 27. (2022).
Abuhatab, S., El-Qanni, A., Al-Qalaq, H., Hmoudah, M. & Al-Zerei, W. Effective adsorptive removal of Zn2+, Cu2+, and Cr3+ heavy metals from aqueous solutions using silica-based embedded with NiO and MgO nanoparticles. J. Env Manag. 268, 110713 (2020).
Ab Hamid, N. H. et al. The current State-Of-Art of copper removal from wastewater: A review. Water 14, 3086 (2022).
Ouafi, R., Asri, M., Omor, A., Taleb, M. & Rais, Z. Snail shells adsorbent for copper removal from aqueous solutions and the production of valuable compounds. J Chemistry 9537680 (2021).
Başar, B. & Şayan, E. Optimization of selective Cu2 + adsorption within the multi-ion system by using activated carbon prepared by ultrasound. J. Sci. Tech. – Appl. Sci. Eng. 19, 893–906 (2018).
Marcus, Y. Ion Properties (Marcel Dekker, 1999).
Fu, F. & Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manage. 92, 407–418 (2011).
Ho, Y. S. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 59, 171–177 (2004).
Pearson, R. G. Hard and soft acids and bases, HSAB, part 1: fundamental principles. J. Am. Chem. Soc. 85, 3533–3539 (1963).
Peng, W., Xie, Z., Cheng, G., Shi, L. & Zhang, Y. Amino-functionalized adsorbent prepared by means of Cu(II) imprinted method and its selective removal of copper from aqueous solutions. J. Hazard. Mater. 294, 9–16 (2015).
Rasilta Tarigan, E., Frida, E., Humaidi, S. & Susilawati Adsorption mechanism of heavy metals using activated carbon derived from hydrilla verticillata. Trends Sci. 22, 8732 (2025).
Liu, Y., Wang, H., Cui, Y. & Chen, N. Removal of copper ions from waste water: a review. Int. J. Environ. Res. PublicHeal. 20, 3885 (2023).
Rajasekar, A. et al. Removal of high concentrations of zinc, cadmium, and nickel heavy metals by Bacillus and Comamonas through microbially induced carbonate precipitation. Biodegradation 36, 40 (2025).
Adeyi, A. A. et al. Adsorption of copper(II) from aqueous solution onto Raw and treated rice husks: isotherms, kinetics, and thermodynamics studies. J. Environ. Sci. Pollution Res. 26, 24008–24023 (2019).
Singh, S., Parveen, N. & Gupta, H. Adsorptive removal of lead (Pb (II)) from aqueous solution using prosopis cineraria leaf powder. J. Mol. Liquids. 260, 77–87 (2018).
El-Sayed, G. O., Dessouki, H. A. & Ibrahiem, S. S. Removal of Cd(II) and Pb(II) from aqueous solution by adsorption on Raw rice straw. J. Environ. Chem. Eng. 7, 103303 (2019).
Inglezakis, V. J., Stylianou, M. A., Gkantzou, D. & Loizidou, M. D. Removal of Pb (II) from aqueous solutions by using clinoptilolite and bentonite as adsorbents. Desalination 210, 248–256 (2007).
Erdem, E., Karapinar, N. & Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 280, 309–314 (2004).
Gao, P. et al. Enhanced electrosorptive removal of copper ions from aqueous solution using a novel sulfur-doped microporous carbon. J. Mater. Chem. A. 3, 23366–23376 (2015).
Tarhouchi, S., Tighadouini, S., Hammoudan, I. & Saddik, R. A novel pyrazole-hydrazone modified silica gel as an adsorbent for efficient and selective removal of Pb(II) from aquatic media: kinetic, isotherm, thermodynamic, and functional theory studies. J Clean. Technol. Environ. Policy Clean Technol. Environ. Policy. 7, 103303 (2025).
Deng, H. et al. Efficient removal of lead, cadmium, and zinc from water and soil by MgFe layered double hydroxide: adsorption properties and mechanisms. Sustainability 16, 11037 (2024).
Acknowledgements
This research was supported by CNRST (PPR2-MESRSFC CNRSTP10), the Fonds de la Recherche Scientifique-FNRS (PDR T.0095.21, CDR J.0064.23, EQP U.N027.24). We thank ARES for a ‘mobility fellowship 2022’ allocated to A.O. The authors acknowledge the PC2 platform of the University of Namur for access to solid-state NMR facility, as well as UEFSCDI for the financial support through the project number PNIII-P1-1.1-TE-2019-2194 (Contract No: TE 123/2020).
Author information
Authors and Affiliations
Contributions
Afaf Oulmidi: Writing–review & editing, Writing–original draft, Investigation, Formal analysis, Data curation. Smaail Radi: Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Khalid Karrouchi: Formal analysis, Data curation. Luca Fusaro: review & editing, Validation, Software, Formal analysis, Data curation. Carmela Aprile: review & editing, Supervision. Yann Garcia: Writing–review & editing, Validation, Supervision, Funding acquisition. Aurelian Rotaru: Formal analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Oulmidi, A., Radi, S., Karrouchi, K. et al. Pincer ligand mesoporous material in heavy metal adsorption for environmental purposes. Sci Rep 15, 35546 (2025). https://doi.org/10.1038/s41598-025-19467-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-19467-9