Abstract
Increasing evidence confirms that obesity, inflammation, and uric acid (UA) are important hypertension risk factors. However, the interplay among these factors in hypertension remains unclear. We explored this intricate relationship in young and middle-aged adults using data from the National Health and Nutrition Examination Survey (2011–2018). We analyzed the associations between obesity indicators, including weight-adjusted waist index (WWI), fat mass index (FMI), and visceral adiposity index (VAI) and hypertension, considering mediating roles of the systemic immune-inflammation index (SII) and UA. We included 5,166 participants aged 18–59 years, and 1,154 (22.34%) experienced hypertension. Patients with hypertension exhibited significantly elevated SII, UA, and obesity indicators than did those without hypertension (p < 0.001). Weighted logistic regression analysis revealed a positive correlation between WWI, FMI, VAI, and hypertension risk, with significant dose-response and nonlinear associations. SII and UA partially mediated the association between WWI and FMI with hypertension, with UA also mediating the VAI–hypertension link. Subgroup analyses indicated that the association between WWI, FMI, VAI, and hypertension was significant in most subgroups. Our findings highlight the strong association between obesity indicators and hypertension and underscore the importance of targeting inflammation and UA levels in managing hypertension risk among young and middle-aged adults.
Similar content being viewed by others
Introduction
Hypertension (HT) is the leading cause of premature death from cardiovascular diseases, affecting over one billion people globally. Its prevalence continues to rise, posing a significant public health challenge. HT is also acknowledged as a key modifiable risk factor for both cardiovascular diseases and overall mortality1,2. Previous research has identified obesity, dyslipidemia, physical inactivity, inflammation, and high uric acid (UA) levels as independent risk factors contributing to an elevated risk of HT3,4,5,6,7. However, existing studies have primarily focused on the independent effects of each factor, while the potential mediating roles of inflammation and UA in the relationship between obesity and HT are less understood.
Obesity, a chronic metabolic condition, is closely linked to HT development. Various indicators are used to measure obesity, such as body mass index (BMI) for overall obesity and waist circumference (WC) for abdominal or central obesity8. Recently, the weight-adjusted waist index (WWI), fat mass index (FMI), and visceral adiposity index (VAI) have emerged as valuable measures of obesity, reflecting different aspects of this condition9,10. Despite their increasing use, the impact of WWI, FMI, and VAI on HT in young and middle-aged adults has not yet been thoroughly investigated using data from the National Health and Nutrition Examination Survey (NHANES).
The systemic immune-inflammation index (SII) is a novel inflammation marker that combines neutrophil, lymphocyte, and platelet counts into a single index11, offering insights into inflammation. The SII has gained attention for its predictive value in cardiovascular diseases, often outperforming traditional inflammation indicators in predicting cardiovascular events12.
Given the complex relationships among obesity, SII, UA, and HT, this study aimed to explore these associations in young and middle-aged adults using NHANES data. Specifically, we evaluated the associations between obesity indicators (WWI, FMI, and VAI) and HT and conducted mediation analyses to determine whether SII and UA mediate the relationship between these obesity indicators and HT.
Methods
Participants and study design
The NHANES is a thorough cross-sectional study organized by the National Center for Health Statistics. Launched in 1999, this biennial survey aims to evaluate the health and nutritional status of the U.S. population. It utilizes complex multistage probability sampling techniques to ensure a representative sample. All participants provided informed consent. More detailed information regarding the survey methodology can be found at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
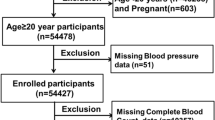
For this study, participants were drawn from the NHANES cycles conducted between 2011 and 2018. Initially, 39,156 individuals were considered; however, after excluding those under 18 or over 59 years of age, as well as individuals with missing data on HT status, SII, UA, WWI, FMI, and VAI, a total of 5,166 participants were ultimately included. A flowchart of the study population screening process is provided in Fig. 1.
Exposure variables
Licensed health technicians collected height, weight, and WC measurements at a mobile examination center (MEC) following standardized procedures. Participants were required to remove shoes and outer clothing to ensure accurate weight measurements. WC was assessed by placing a horizontal line at the narrowest point between the lowest rib and the iliac crest using a tape measure. Fat mass was evaluated through dual-energy X-ray absorptiometry, while the VAI was calculated based on BMI, WC, triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C). In this study, WWI, FMI, and VAI were used as exposure variables and calculated as follows:
In these formulas, WC was measured in cm, height in m, weight in kg, fat mass in kg, and TG and HDL-C levels in mmol/L.
Assessment of mediators
SII was calculated from complete blood count results, including platelet, neutrophil, and lymphocyte counts, using a Beckman Coulter HMX Hematology Analyzer at the MEC, and the results were documented as ×103 cells/µL. Detailed laboratory procedures for the complete blood count test can be found on the official NHANES website. The SII was calculated as:
Serum UA levels were measured as part of routine serum biochemical profiles from blood samples obtained at the MEC. UA concentrations were determined using the time-endpoint method, which monitors the change in absorbance of the colored product generated by the reaction of hydrogen peroxide, produced by the oxidation of UA, with 4-aminoantipyrine using 3,5-dichloro-2-hydroxybenzenesulfonate as a catalyst.
Outcome variable
The outcome variable in this study was HT, defined by meeting any one of the following criteria13: (1) a self-reported answer of “yes” to the questionnaire question “Ever told you had high blood pressure”; (2) confirmed use of oral antihypertensive medications, including calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-receptor antagonists, and diuretics; (3) a mean systolic blood pressure ≥ 140 mmHg or mean diastolic blood pressure ≥ 90 mmHg, based on three consecutive readings by trained and licensed personnel after participants had rested for five min. A fourth measurement was performed if any of the initial three readings were unsuccessful.
Study covariates
Covariates potentially influencing the association between obesity indicators (WWI, FMI, and VAI) and HT were selected based on clinical relevance and previous studies14. These covariates included age (years), sex (female, male), race and ethnicity (Mexican–American, other Hispanic, non-Hispanic white, non-Hispanic black, other race), education level (less than 11th grade, high school graduate, some college or above), marital status (living alone, married/living with a partner), annual household income (<$20,000, ≥$20,000), smoking behavior (never, former smoking, current smoking), drinking behavior, physical activity (inactive, moderate, vigorous, moderate and vigorous), diabetes, heart failure, coronary heart disease (CHD), angina pectoris (AP), myocardial infarction (MI), stroke, hepatopathy, kidney failure, and cancer.
Definitions for heart failure, CHD, AP, MI, stroke, hepatopathy, kidney failure, and cancer were based on self-reported questionnaire data. Diabetes was defined by meeting at least one of the following criteria15: glycosylated hemoglobin ≥ 6.5%, fasting blood glucose ≥ 7.0 mmol/L, self-reported physician diagnosis of diabetes, or self-reported use of insulin or hypoglycemic medications. Smoking behavior was described as having consumed at least 100 cigarettes during one’s Lifetime. Drinking behavior was assessed through two 24-hour dietary recalls, with participants classified as drinkers if they reported alcohol consumption in at least one of the recalls. Detailed measurement procedures for these variables are available on the official NHANES website.
Statistical analysis
Considering the study design, which involved complex multistage probability sampling of the NHANES data, this study applied weighting to the data using the sample weight calculation method recommended by the NHANES to enhance the reliability of the findings. The MissForest method based on random forests was performed to address missing values for potential confounders. Baseline data were examined for normal distribution, and due to the non-normal distribution of all continuous variables, they were reported as medians with interquartile ranges (IQRs). Categorical variables were expressed as frequencies (percentages). The Mann–Whitney U test was utilized for comparing continuous variables, whereas the chi-square test was applied to categorical variables.
Multivariate logistic regression analysis was conducted to investigate the association between obesity indicators (WWI, FMI, and VAI) and HT. To ensure robust conclusions, three regression models were developed to analyze potentially influential factors in different combinations. Additionally, trend analysis was performed after categorizing WWI, FMI, and VAI into quartiles, and restricted cubic spline (RCS) plots were generated to evaluate linear and nonlinear associations.
To further validate the reliability of findings from the regression models, subgroup analyses and interaction tests were conducted across age (18 to < 40 years, 40 to < 60 years), sex, race and ethnicity, education level, marital status, annual household income, diabetes, smoking behavior, and drinking behavior subgroups.
The study sought to explore both the direct effects of obesity indicators (WWI, FMI, and VAI) on HT and the indirect effects mediated by SII and UA. Correlations between SII and UA were assessed and mediation analyses conducted separately for SII and UA. Statistical significance was determined by a two-tailed p-value of < 0.05.
Results
Characteristics of the study population
The baseline characteristics of the study population are summarized in Table 1. This study included 5,166 participants, with 1,154 (22.34%) that were diagnosed with HT. The median age of the participants was 39.00 years (IQR: 28.00–49.00), with a nearly equal distribution of men (2,582) and women (2,584). Compared to the non-HT group, the HT group displayed higher proportions of participants who were non-Hispanic blacks, married/living with a partner, individuals with annual household incomes <$20,000, smoking behavior (former or current), and those with comorbid conditions such as diabetes, heart failure, CHD, AP, MI, stroke, hepatopathy, kidney failure, and cancer (p < 0.05). Furthermore, the HT group possessed a significantly older median age and exhibited higher levels of neutrophil count, TG, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and obesity-related indices such as body weight, WWI, FMI, and VAI, compared to these factors in the non-HT group (p < 0.05). Levels of UA and SII were also significantly elevated in the HT group (p < 0.05).
Association between obesity indicators and HT
As presented in Table 2, weighted multivariate logistic regression analysis demonstrated a robust positive association between all three obesity indicators (WWI, FMI, and VAI) and the risk of HT. These associations remained statistically significant across all three models, even after adjusting for potential confounders such as demographics, socioeconomic factors, lifestyle behaviors, and comorbidities.
When analyzed as continuous variables, increases in WWI, FMI, and VAI were all associated with elevated odds of hypertension. Notably, WWI exhibited the strongest association, followed by FMI and VAI.
To further explore these associations, each obesity indicator was divided into quartiles. Participants in the highest quartile (Q4) of WWI exhibited almost a three-fold increased risk of HT compared to those in the lowest quartile, even after full adjustment. Similar patterns were observed for FMI and VAI, with progressively higher quartiles corresponding to significantly increased HT risk. These findings suggest a strong, graded association between excess adiposity (particularly central and visceral fat) and hypertension.
A significant dose-response association was observed for all models (p for trend < 0.05). As presented in Fig. 2, the RCS plots further confirmed the nonlinear association between the obesity indicators (WWI, FMI, and VAI) and HT (p < 0.05).
The restricted cubic spline curve is used to model the relationship between obesity indicators and the risk of HT (A–C), adjusted for age, sex, race and ethnicity, education level, marital status, annual household income, smoking behavior, drinking behavior, physical activity, diabetes, heart failure, CHD, AP, MI, stroke, hepatopathy, kidney failure, and cancer. OR, odds ratio; CI, confidence interval; WWI, weight-adjusted waist index; FMI, fat mass index; VAI, visceral adiposity index; HT, hypertension; CHD, coronary heart disease; AP, angina pectoris; MI, myocardial infarction.
Correlation of SII and UA and their association with obesity indicators and HT
Pearson’s product-moment correlation analysis confirmed no significant correlation between SII and UA (r =−0.013, p = 0.360). As presented in Supplementary Tables S1–S3, weighted linear regression analyses revealed significant correlations between all obesity indicators (WWI, FMI, and VAI) and SII (p for trend < 0.05). Regarding UA, only FMI in Model 1 was not statistically significant (p for trend = 0.065), while the other models showed significant associations (p for trend < 0.05).
As presented in Supplementary Tables S4 and S5, weighted logistic regression analyses indicated that participants with HT had significantly higher levels of SII and UA across all three models (p for overall < 0.05).
The mediating role of SII and UA
The results of the mediation analysis, presented in Fig. 3, indicate that SII and UA partially mediated the association between WWI and FMI with HT. Specifically, SII mediated 4.42% (p < 0.001) of the association for WWI and 3.70% (p < 0.001) for FMI. UA mediated 14.90% (p < 0.001) of the association for WWI and 10.11% (p < 0.001) for FMI. Furthermore, UA mediated 33.05% (p < 0.001) of the association between VAI and HT, whereas SII did not show a significant mediating effect in this context (p = 0.410).
Mediating effects of SII and UA on the association between obesity indicators and HT (A–F). In all mediation analyses, adjustments are made for the following covariates: age, sex, race and ethnicity, education level, marital status, annual household income, smoking behavior, drinking behavior, physical activity, diabetes, heart failure, CHD, AP, MI, stroke, hepatopathy, kidney failure, and cancer. HT, hypertension; SII, systemic immune-inflammation index; UA, uric acid; WWI, weight-adjusted waist index; FMI, fat mass index; VAI, visceral adiposity index; CHD, coronary heart disease; AP, angina pectoris; MI, myocardial infarction.
Subgroup analysis of obesity indicators and HT
Supplementary Figures S1-S3 present the results of the subgroup analyses exploring the association between obesity indicators (WWI, FMI, and VAI) and HT, stratified by age, sex, race and ethnicity, education level, marital status, annual household income, diabetes, smoking behavior, and drinking behavior. The analysis revealed significant interactions between WWI and smoking behavior (p for interaction = 0.016), with the effects of WWI on HT varying by smoking behavior, although all effects remained significant. Interactions between diabetes and both FMI and VAI were also observed, with FMI showing a significant association with HT in both diabetic and non-diabetic patients (p < 0.05), while the VAI-HT association was significant only in non-diabetic patients (p < 0.001). There was also an interaction between FMI and smoking behavior (p for interaction = 0.001), but smoking behavior did not affect the significant correlation. Additionally, there was an interaction between VAI and sex (p for interaction = 0.026), but the VAI-HT association was significant for both sexes.
These findings indicate that the association between VAI and HT may be particularly relevant in non-diabetic individuals. Importantly, no significant interactions were observed for age, race and ethnicity, education level, marital status, annual household income, or drinking behavior, indicating that these factors do not substantially influence the relationship between obesity indicators and HT risk.
Discussion
In this study, we analyzed data from 5,166 participants across four cycles of NHANES (2011–2018), including 2,584 women and 2,582 men, where 1,154 (22.34%) participants were hypertensive. Our logistic regression analysis identified obesity indicators—WWI, FMI, and VAI—as independent risk factors for HT. Trend analysis revealed a significant dose-response association between these obesity indicators and HT, and RCS plots confirmed the presence of a nonlinear association. Mediation analyses further indicated that the SII and UA mediated some of the associations between obesity indicators and HT, except for the association between VAI and HT, where SII did not play a mediating role. Notably, our preliminary analysis confirmed no significant correlation between SII and UA. Subgroup analyses also highlighted that VAI was significantly associated with HT only in participants without diabetes, while other obesity indicators were consistently associated with HT across different subgroups.
Obesity is well recognized as a significant risk factor for various cardiovascular diseases, particularly HT, with approximately 70% of patients with HT being clinically comorbid with obesity16. While BMI is commonly used to assess overall obesity, it does not account for fat distribution, which is crucial since the biological impact of adipose tissue is location-dependent17. Visceral fat, for instance, has been shown to have more detrimental effects on metabolic processes than subcutaneous fat, even in individuals with a normal BMI18. In this context, our study is the first to establish the associations of WWI, FMI, and VAI with HT in a young-to-middle-aged population using NHANES data. Our findings revealed that these obesity indicators were significantly elevated in HT patients compared to levels in non-HT individuals and exhibited a significant nonlinear dose-response relationship with HT, independent of confounders. The mechanisms underlying the obesity-HT link are multifaceted, involving factors such as excessive activation of the sympathetic nervous system, overactivation of the renin-angiotensin-aldosterone system, insulin resistance, endothelial dysfunction, elevated leptin levels, and adiposopathy19,20. These findings underscore the importance of using effective obesity indices to identify high-risk individuals for early intervention.
Before conducting mediation analysis, we examined the individual associations of SII and UA with HT. Both the SII and UA levels were found to be significantly associated with HT development. SII, a novel marker combining neutrophil, lymphocyte, and platelet counts, was introduced by Hu et al. in 2014 to assess systemic inflammation and immune responses11.Previous studies have linked elevated SII levels with worse cardiovascular outcomes, including higher blood pressure and asymptomatic organ damage in patients with HT21,22. Moreover, a study by Xu et al. further emphasized that compared to the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio, SII may serve as a more effective systemic inflammation warning marker for hypertension23. Furthermore, Shi et al. provided additional evidence supporting a nonlinear relationship between SII and hypertension. Their findings demonstrated that when SII was below the threshold of 501.2, it was not significantly associated with hypertension prevalence. However, among individuals with SII levels equal to or above 501.2, the prevalence of hypertension increased markedly with rising SII levels24.
UA, recognized as a cardiovascular risk factor, has also been implicated in HT development25. Research has consistently demonstrated that elevated UA levels remain an independent risk factor for HT, even after adjusting for confounders26,27. For instance, Bombelli et al. reported that every 1 mg/dL increase in UA levels was associated with a 22% increase in the risk of cardiovascular death28, and some studies indicate that lowering UA levels may help to reverse HT29,30. Our findings are consistent with the existing literature regarding the roles of inflammation and UA in HT.
To further validate our findings, we performed subgroup analyses across various demographic and clinical characteristics, including age, sex, race and ethnicity, education level, marital status, annual household income, diabetes, smoking behavior, and drinking behavior. The results indicated that these factors did not significantly alter the associations between obesity indicators and HT, except for diabetes status. Specifically, while WWI and FMI were consistently associated with HT regardless of diabetes status, the association between VAI and HT was significant only in non-diabetic individuals. This may be due to the inherent metabolic abnormalities associated with diabetes, particularly dyslipidemia, that could influence VAI levels and their relationship with HT31,32.
Our study focused on the mediating roles of SII and UA in the relationship between obesity indicators (WWI, FMI, and VAI) with HT. The mediation analyses confirmed that SII and UA mediated part of the association between WWI, FMI, and HT, with SII mediating 4.42% and 3.70%, and UA mediating 14.90% and 10.11%, respectively. For VAI, UA mediated 33.05% of its association with HT, while SII did not play a significant mediating role. These findings highlight the importance of systemic inflammation (as reflected by SII) and UA as potential biological pathways linking obesity, particularly central and visceral adiposity, to hypertension. The observed mediating effects suggest that these biomarkers may represent intermediate mechanisms through which excess adiposity contributes to elevated blood pressure. Notably, the greater mediating effect of UA, especially in the VAI–HT pathway, indicates a stronger role of metabolic dysfunction in the development of hypertension among individuals with visceral obesity, whereas the modest mediating role of SII underscores the contribution of chronic low-grade inflammation to obesity-related hypertension. From a clinical perspective, SII is a simple, cost-effective, and easily accessible index derived from routine complete blood counts, which makes it a practical tool for early identification of hypertensive risk, particularly in obese individuals. It may also serve as a valuable parameter for monitoring inflammatory status and assessing the potential benefit of interventions targeting systemic inflammation. Understanding these mediators not only provides mechanistic insight into the obesity–hypertension relationship but may also help inform more targeted prevention and treatment strategies that address both inflammation and metabolic abnormalities in at-risk populations.
This study had several strengths. First, we leveraged a large, nationally representative sample to explore the associations between multiple obesity indicators and HT. By using a combination of obesity indicators, we performed a comprehensive assessment of obesity from different perspectives. Second, we examined the potential mediating roles of two uncorrelated markers, SII and UA, in the obesity-HT relationship. Finally, we adjusted for several key confounders and conducted subgroup analyses to ensure the reliability of our findings.
However, this study had some limitations. The cross-sectional design restricted our capacity to determine causality between obesity indicators and HT. Additionally, the findings are based on a U.S. adult population, which may not be fully generalizable to other populations. Finally, although we adjusted for several confounders, the potential for residual confounding cannot be completely ruled out.
In conclusion, our study highlights the complex interactions between obesity indicators (WWI, FMI, and VAI), SII, and UA in relation to HT. We found that elevated levels of these obesity indicators, as well as increased SII and UA levels, were significantly associated with a higher risk of HT. Notably, SII and UA partially mediated the relationship between WWI and FMI with HT, although SII did not mediate the association between VAI and HT. These findings suggest that strategies focused on weight loss, reducing inflammation, and lowering UA levels may be effective in preventing and managing HT, particularly in young and middle-aged populations.
Data availability
The datasets generated and analyzed during the current study are available in the NHANES repository, (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx).
References
GBD, Risk Factor Collaborators. Global, regional, and National comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 392, 1923–1994 (2018).
Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 76, 2982–3021 (2020).
Mouton, A. J., Li, X., Hall, M. E. & Hall, J. E. Obesity, hypertension, and cardiac dysfunction: novel roles of immunometabolism in macrophage activation and inflammation. Circ. Res. 126, 789–806 (2020).
Laaksonen, D. E. et al. Dyslipidaemia as a predictor of hypertension in middle-aged men. Eur. Heart J. 29, 2561–2568 (2008).
Diaz, K. M. & Shimbo, D. Physical activity and the prevention of hypertension. Curr. Hypertens. Rep. 15, 659–668 (2013).
Zhang, Z., Zhao, L., Zhou, X., Meng, X. & Zhou, X. Role of inflammation, immunity, and oxidative stress in hypertension: new insights and potential therapeutic targets. Front. Immunol. 13, 1098725 (2022).
Lanaspa, M. A., Andres-Hernando, A. & Kuwabara, M. Uric acid and hypertension. Hypertens. Res. 43, 832–834 (2020).
Wang, S. et al. A novel quantitative body shape score for detecting association between obesity and hypertension in China. BMC Public. Health. 15, 7 (2015).
Kim, K. J., Son, S., Kim, K. J., Kim, S. G. & Kim, N. H. Weight-adjusted waist as an integrated index for fat, muscle and bone health in adults. J. Cachexia Sarcopenia Muscle. 14, 2196–2203 (2023).
Jabłonowska-Lietz, B., Wrzosek, M., Włodarczyk, M. & Nowicka, G. New indexes of body fat distribution, visceral adiposity index, body adiposity index, waist-to-height ratio, and metabolic disturbances in the obese. Kardiol Pol. 75, 1185–1191 (2017).
Hu, B. et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 20, 6212–6222 (2014).
Yang, Y. L. et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur. J. Clin. Investig. 50, e13230 (2020).
Li, G. et al. Association between triglyceride-glucose index and hypertension in adults with cancer from NHANES 2005–2018: a cross-sectional study. BMC Cancer. 25, 993 (2025).
Zheng, H. et al. Associations between systemic immunity-inflammation index and heart failure: evidence from the NHANES 1999–2018. Int. J. Cardiol. 395, 131400 (2024).
Guo, D. et al. Association between the triglyceride-glucose index and impaired cardiovascular fitness in non-diabetic young population. Cardiovasc. Diabetol. 23, 39 (2024).
Faulkner, J. L. & de Belin, E. J. Sex differences in mechanisms of hypertension associated with obesity. Hypertension 71, 15–21 (2018).
Karpe, F. & Pinnick, K. E. Biology of upper-body and lower-body adipose tissue–link to whole-body phenotypes. Nat. Rev. Endocrinol. 11, 90–100 (2015).
Dhana, K. et al. Body shape index in comparison with other anthropometric measures in prediction of total and cause-specific mortality. J. Epidemiol. Community Health. 70, 90–96 (2016).
Kotchen, T. A. Obesity-related hypertension: epidemiology, pathophysiology, and clinical management. Am. J. Hypertens. 23, 1170–1178 (2010).
Zheng, F., Mu, S. & Rusch, N. J. Leptin activates Trpm7 channels in the carotid body as a mechanism of obesity-related hypertension. Circ. Res. 125, 1003–1005 (2019).
Saylik, F. & Sarıkaya, R. Can systemic immune-inflammation index detect the presence of exxaggerated morning blood pressure surge in newly diagnosed treatment-naive hypertensive patients? Clin. Exp. Hypertens. 43, 772–779 (2021).
Lee, R. M., Dickhout, J. G. & Sandow, S. L. Vascular structural and functional changes: their association with causality in hypertension: models, remodeling and relevance. Hypertens. Res. 40, 311–323 (2017).
Xu, J. P. et al. Systemic inflammation markers and the prevalence of hypertension: a NHANES cross-sectional study. Hypertens. Res. 46, 1009–1019 (2023).
Shi, Y. & Zhou, W. Threshold effect of systemic immune inflammatory index on hypertension in American adults (NHANES 2017–2020). J. Hypertens. 41, 2107–2112 (2023).
Terawaki, H. et al. Elevated uric acid levels as a risk factor for new-onset hypertension in newly enrolled Japanese university students (JSH46-0100). Hypertens. Res. 48, 780–785 (2025).
Ali, N. et al. Relationship between serum uric acid and hypertension: a cross-sectional study in Bangladeshi adults. Sci. Rep. 9, 9061 (2019).
Perlstein, T. S. et al. Uric acid and the development of hypertension: the normative aging study. Hypertension 48, 1031–1036 (2006).
Bombelli, M. et al. Prognostic value of serum uric acid: new-onset in and out-of-office hypertension and long-term mortality. J. Hypertens. 32, 1237–1244 (2014).
Liu, J. et al. Survey on uric acid in Chinese subjects with essential hypertension (SUCCESS): a nationwide cross-sectional study. Ann. Transl Med. 9, 27 (2021).
Feig, D. I., Soletsky, B. & Johnson, R. J. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA 300, 924–932 (2008).
Yu, J. et al. The visceral adiposity index and risk of type 2 diabetes mellitus in china: A National cohort analysis. Diabetes Metab. Res. Rev. 38, e3507 (2022).
Wang, D. et al. Association between visceral adiposity index and risk of prediabetes: A meta-analysis of observational studies. J. Diabetes Investig. 13, 543–551 (2022).
Acknowledgements
We thank the CDC/NCHS for providing NHANES data from 2011 to 2018, and we also thank the Director of the Department of Cardiology, Baotou Central Hospital.
Author information
Authors and Affiliations
Contributions
C.Z. and Y.L. conceived the study, C.Z. and Z.D.S compiled and analyzed the data, C.Z. drafted the manuscript, Z.D.S revised and edited the manuscript, and all authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zuo, C., Shen, ZD. & Lu, Y. Systemic immune-inflammation index and uric acid mediate the association between obesity and hypertension in young and middleaged adults. Sci Rep 15, 35704 (2025). https://doi.org/10.1038/s41598-025-19468-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-19468-8
Keywords
This article is cited by
-
Association between remnant cholesterol and isolated diastolic hypertension in young adults: a cross-sectional study in China
BMC Cardiovascular Disorders (2026)
-
Neutrophil-to-lymphocyte ratio predicts mortality for sepsis-induced coagulopathy: a retrospective study
European Journal of Medical Research (2025)