Abstract
This study investigates the structure–property relationship in Jin-Yuan period (1115–1368 CE) oil-spot glazes from the archaeological site of Jining Lu to elucidate the scientific principles governing their distinctive coloration patterns. Through multi-scale characterization of 20 ceramic fragments using ultra-depth microscopy, SEM–EDS (Scanning Electron Microscopy-Energy Dispersive Spectrometer), XRD (X-ray Diffraction), WDXRF (Wavelength-dispersive X-ray fluorescence) and XPS (X-ray photoelectron spectrometer) , we establish fundamental correlations between processing conditions, crystalline phases, and optical properties. The ceramic bodies exhibit a characteristic high-alumina composition (Al2O3: 24.5–34.0 wt%), reflecting regional clay sources and manufacturing traditions. Glaze chemistry reveals intentional flux optimization, with high CaO content (4.5–7.2 wt%) and Fe2O3 enrichment facilitating liquid-phase sintering. Most significantly, we demonstrate that the silver/red dichroism is predominantly governed by atmospheric conditions modulating Fe2+/Fe3+ ratios and crystal polymorphism:(i) In a strong oxidizing atmosphere, vapor-phase deposition produces specular α-Fe2O3 (hematite) microcrystals (2–5 μm), yielding metallic luster; (ii) In a weak reducing-weak oxidizing atmosphere, co-precipitation of dendritic α-Fe2O3 and magnetoelectric ε-Fe2O3 (luogufengite) is promoted, generating distinct chromatic contrast. These findings provide mechanistic insights into ancient craftsmen’s empirical control of phase transformations in iron-oxide systems.
Similar content being viewed by others
Introduction
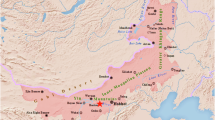
The archaeological site of Jining Lu is located in Tuchengzi Village, Chahar Right Front Banner, Ulanqab City, Inner Mongolia Autonomous Region (Fig. 1). Situated at the convergence zone of steppe and agricultural civilizations, it served as a vital hub for trade between the Central Plains and northern regions during the Jin (1115–1234 CE) and Yuan (1271–1368 CE) dynasties, boasting a strategically significant location. Established as a county during the Jin dynasty and later elevated to a regional administrative center (luzhi) in the early Yuan period, the site was abandoned after being destroyed in warfare by the late Yuan dynasty, resulting in its relatively well-preserved state1. From 2002 to 2005, archaeological excavations were conducted by Inner Mongolian researchers, and the site was listed among China’s “Top Ten Archaeological Discoveries of 2003.” A large number of ceramic fragments from the Jin-Yuan period were unearthed, among which oil-spot glazed wares have attracted significant academic attention due to their distinctive silver and red crystalline patterns and exquisite craftsmanship, offering multidimensional insights into the cultural exchange along the Grassland Silk Road during this period.
Location map and site plan of the The Archaeological Site of Jining Lu. (a) The archaeological site of Jining Lu is situated in Chahar Right Front Banner, Ulanqab City, Inner Mongolia Autonomous Region, China. (b) Site plan of the archaeological site of Jining Lu showing concentration of excavated ceramics in the marketplace remains.
Oil-spot glaze is a rare category within ancient Chinese black-glazed ceramics, representing the pinnacle of iron-based crystalline glaze technology. It during the Song Dynasty (960–1279 CE), named for their surfaces densely dispersed with silvery or russet spots. During the Song-Yuan period, kilns across northern and southern China competed to produce oil-spot glazed ceramics, with representative kilns including Jian Kiln (Fujian Province) in the south and Ding Kiln (Hebei Province), Hebiji Kiln (Henan Province), Linfen Kiln (Shanxi Province), and Zibo Kiln (Shandong Province) in the north2. However, existing research has predominantly focused on Jian Kiln products3,4,5,6, leaving a notable gap in the study of the technological characteristics and formation mechanisms of oil-spot glazes from northern kilns. The morphological differences between the crystalline patterns of oil-spot glazes excavated at the archaeological site of Jining Lu and those from Jian Kiln provide critical specimens for investigating iron-based crystalline glaze technology in northern kilns during the Jin-Yuan period.
Current research indicates that the formation of oil-spot glaze is closely linked to the decomposition of iron oxides, bubble migration, and crystal precipitation within the glaze under high temperatures. These iron oxide crystals primarily include hematite7, magnetite (Fe3O4)8,9, and ε-Fe2O310,11. The silvery luster of Jian Kiln oil-spot glaze originates from ε-Fe2O3 crystals, which measure several micrometers in size and are arranged in periodic two-dimensional patterns12. Scholars have experimentally reconstructed the glaze recipes and firing conditions of Jian Kiln, analyzing the effects of different firing parameters on the glaze’s spot patterns13,14,15,16. Nevertheless, compared to the extensive studies on Jian Kiln oil-spot glazes, those produced in northern kilns or unearthed at northern sites have rarely been examined in depth. Whether the formation mechanisms of these northern oil-spot glazes differ significantly from Jian Kiln products remains unresolved.
To address the critical gaps in understanding the formation mechanisms and technological characteristics of ancient oil-spot glazes, this study systematically examines 20 fragments (10 silver-spot and 10 red-spot glazes) excavated from the Jining Lu archaeological site. By integrating multiscale characterization from macro-morphology to nanoscale crystallography, including ultra-depth microscopy, metallographic microscopy, XRF, XRD, SEM–EDS, XPS and Raman spectroscopy, we investigate the microstructural evolution, chemical composition, and phase assemblage of both the ceramic body and glaze. This study deciphers the composition-structure–property relationships in oil-spot glazes, offering new insights into the materials science of ancient high-temperature crystallization glazes.
Results
The ceramic assemblage comprises 20 oil-spot glazed shards from the Jininglu Ancient City Site commercial district, Inner Mongolia Autonomous Region, including 10 silver-spotted (labeled YD-1 to YD-10) and 10 red-spotted (labeled HYD-1 to HYD-10) samples. The vessels primarily consist of tea bowls, bowls, and plates, featuring a lustrous black glaze adorned with either silver or red oil-spot patterns. On some fragments, exposed body areas reveal an ocher or grayish-black slip. Representative specimens are illustrated in Fig. 2. Based on archaeological context, these ceramics are dated to the Jin-Yuan period (1115–1368 CE).
Typical specimens of oil-spot black-glazed porcelain unearthed at the archaeological site of Jining Lu. (a) Macroscopic view of specimen YD-5. (b) Magnified view (× 150) of silver oil-spot patterns on YD-5 glaze surface. (c) Cross-sectional interface of YD-5 showing: Glaze layer thickness: 470 μm; Spherical bubbles (30-240 μm diameter); Crystal-rich inclusions; Grayish-yellow body. (d) Macroscopic view of specimen HYD-1. (e) Magnified view (× 150) of red oil-spot patterns on HYD-1 glaze surface with sporadic silver crystalline deposits (arrowed). (f) Cross-sectional interface of HYD-1 showing:Glaze layer thickness: 573 μm; Spherical bubbles (15–40 μm diameter); Crystalline phase concentration; Off-white body.
Microscopic observation results and analysis
Observations were conducted on the natural glaze surfaces and body-glaze cross-sections of the samples using ultra-depth microscopy.
YD series samples (Fig. 2a-c): The glaze surfaces exhibited a black background with silver-white oil-spot patterns measuring 0.4–1.5 mm in diameter, displaying localized purple or brown tones. At 200 × magnification, rust-like brick-red or ochre transition zones were visible at the pattern edges. The glaze layer thickness ranged from 0.4 to 0.8 mm, containing numerous unevenly sized circular bubbles with lustrous crystals inside, presumably resulting from iron oxide migration to the glaze surface. The body color was predominantly matte yellowish-white or grayish-white with relatively coarse particles.
HYD series samples (Fig. 2d-f): The glaze surfaces showed irregular cloud-like reddish-brown oil-spot patterns measuring 0.5–1 mm in diameter, scattered with small silver crystalline spots. The glaze layer thickness ranged from 0.5 to 1.1 mm, containing unevenly distributed bubbles of varying sizes with fine crystals inside. The body color was mostly matte yellowish-white or grayish-white, with a few samples exhibiting finer and whiter body texture.
Combined metallurgical-microscope and scanning electron microscopy (SEM) revealed distinct crystallization characteristics in both sample types (YD and HYD), as shown in Figs. 4, 5, 6 and 7; the corresponding EDS test results are provided in Table 1.
Crystallization characteristics of YD samples: OM observation (Fig. 3a) showed that the silver oil-spot patterns consisted of numerous micron-sized crystals forming snowflake-like clusters. SEM analysis (Fig. 3b) further revealed these crystals were uniformly sized (2-5 μm), densely packed, and predominantly irregular polygonal in shape. EDS analysis (Fig. 3c) indicated these crystals were iron-rich (Fe content 35–45%), significantly higher than in non-crystalline areas (Fig. 3d). The aggregation of these iron-rich crystals was the primary cause of the macroscopically visible silver oil-spot patterns.
The microstructure of body-glaze cross-sections showed relatively pure glaze layers with most impurities concentrated in the body (Fig. 4a). Numerous bubbles existed in the glaze layer (Fig. 4c), with iron-rich substances inside rising and migrating to the glaze surface(Fig. 4d-e), promoting crystal formation and distribution. Polygonal crystals near the upper glaze and bubbles (Fig. 4f) showed similar morphology and high iron content (Fig. 4g-h) to surface iron-rich crystals.
Cross-sectional microstructure of YD-5 body-glaze interfaces. (a) Microstructure of body-glaze cross-sections (SEM-SE) (b) Interface layer (SEM-SE) (c) Bubbles in the glaze layer (SEM-SE) (d) EDS Spectrum3 (e) EDS Spectrum4 (f) Polygonal crystals near the upper glaze and bubbles (SEM-SE) (g) EDS Spectrum5 (h) EDS Spectrum6 (i) Magnified view of anorthite crystals in the glaze layer (SEM-SE) (j) EDS Spectrum7 (k) Comb-like cristobalite (SEM-SE) (l) Flake-like mullite and partially melted quartz grains (SEM-SE) (m) Zircon (SEM-SE) (n) EDS Spectrum8.
Needle-like mullite were distributed at the body-glaze interface (Fig. 4b). Rod-shaped anorthite (CaAl2Si2O8) crystals cluster formation was related to reactions between high-calcium glaze and high-alumina body during initial firing (Fig. 4i-j). Some anorthite was carried into the glaze interior during gas escape . The body contained comb-like cristobalite (Fig. 4k) with residual quartz cores and primary flaky mullite (Fig. 4l). In ceramic bodies, mullite formation initiates at 1130 °C and develops progressively above 1200 °C. Conversely, the transformation of α-quartz to cristobalite requires prolonged exposure above 1200 °C. This indicates that sample YD-5 was fired at temperatures exceeding 1200 °C for a sustained duration, as confirmed by its cristobalite content.
Plate-like crystals were also found on the glaze surface and cross-sections (Fig. 4m). EDS analysis (Fig. 4n) showed they were rich in Si, Zr and O, possibly resulting from precipitation of special glaze components under high temperatures.
The HYD samples exhibited multi-layer crystallization phenomena on glaze surfaces: Reddish-brown oil-spot patterns composed of densely packed dendritic crystals with size variations. Small silver spots scattered above the dendritic crystal layer, consisting of leaf-like and pyramidal shape. Specific characteristics: OM observation (Fig. 5a) showed a few leaf-shaped (30-35 μm) and pyramidal (30-45 μm) crystals precipitated on reddish-brown spots, forming complex morphologies that created the macroscopically visible silver spots. Figure 5b-c (SEM image of the red rectangle in 5a) showed these densely packed dendrites measured 2-4 μm with regular arrangement, appearing as reddish-brown oil-spot patterns macroscopically. Figures 5d and 5f showed SEM images of leaf-shaped and pyramidal shape respectively which are similar to the ε-Fe2O3 phase crystals in the previous study12, with EDS-measured iron contents of 34.3% (Fig. 5e)and 32.5%(Fig. 5g). The precipitation of these iron-rich crystals was closely related to firing atmosphere effects, creating unique glaze colors and luster.
Further SEM observation of body-glaze cross-sections revealed extensive crystallization in both glaze and body (Fig. 6a). Figure 6b showed needle-like mullite and rod-shaped anorthite crystal clusters at the intermediate layer, accompanied by submicron droplets from liquid phase separation (Fig. 6c), while Fig. 6e displayed polygonal granular crystals near glaze bubbles with 33.9% iron content by EDS (Fig. 6f). Figure 6g-h showed oval crystals rich in Si, Zr and O in the glaze. The body composition resembled silver oil-spot samples from the archaeological site of Jining Lu, mainly containing comb-like cristobalite and primary flaky mullite, with minor needle-like mullite in some samples (Fig. 6d).
Cross-sectional microstructure of HYD-1 body-glaze interfaces. (a) Microstructure of body-glaze cross-sections (SEM-SE) (b) Interface layer (SEM-SE) (c) Liquid phase separation (SEM-SE). (d) Needle-like mullite (SEM-SE) (e) Polygonal granular crystals (SEM-SE) f) EDS Spectrum12 (g) Oval crystals (SEM-SE). (h)EDS Spectrum13.
Chemical composition analysis
The chemical compositions of the bodies and glazes of silver oil-spot (YD) and red oil-spot (HYD) samples excavated from the archaeological site of Jining Lu were analyzed using X-ray fluorescence spectroscopy (XRF). The results of the body and glaze compositions are presented in Table 2.
Both types of samples exhibited a characteristic "high-alumina, low-silica" composition in their ceramic bodies. The silver oil-spot bodies showed an average SiO2 content of 60.9% (range: 59.5–64.9%) and Al2O3 content of 29.7% (range: 27.4–30.8%). The red oil-spot bodies had a slightly higher average SiO2 content of 61.2% (range: 59.1–66.0%) and Al2O3 content of 30.0% (range: 24.5–34.0%). The Al2O3 content of both types (24.5–34.0%) closely matches the typical high-alumina ceramic body formula used in northern China from the Tang to Yuan dynasties17 and is significantly higher than that of contemporary southern kilns.
The major elements (SiO2 and Al2O3) in both glaze types were highly similar, with mean differences of less than 1.5%, indicating a shared recipe system primarily composed of clay, quartz, and feldspar. Notably, the glazes exhibited significantly high CaO content (mean: 5.5% and 5.6%). The coefficient b(RO) for the alkaline-earth metal oxides in the samples was calculated using the formula: b = RO/(RO + R2O). According to the classification criteria for calcium-based glazes in ancient Chinese ceramics, most silver oil-spot glazes had a glaze formula coefficient b(RO) ≥ 0.76 (mean: 0.77), classifying them as typical lime glazes. The red oil-spot glazes had a mean b(RO) of 0.76, with some samples (e.g., HYD-5, HYD-7) showing calcium-alkali glaze characteristics (b(RO) = 0.74–0.75), but overall, they still leaned toward the lime glaze system. This indicates that while the two glaze types underwent minor recipe adjustments, their core technical objective remained consistent: optimizing glaze fluidity and promoting iron oxide crystallization through high-calcium formulations.
To systematically characterize the compositional variations between ceramic bodies and glazes, scatter plot analyses were performed on four key oxide pairs (SiO2-Al2O3, CaO-K2O, MgO-Na2O and Fe2O3-TiO2 ), which were constructed, as shown in Fig. 7.
Figure 7a shows the distribution of the major components SiO2 and Al2O3 in the sample body and glaze. While the SiO2 and Al2O3 contents of both types of ceramic bodies were similar, certain red oil-spot samples (e.g., HYD-1, HYD-7, HYD-10) exhibited notably higher Al2O3 content, accompanied by increased CaO and significantly reduced Fe2O3 and TiO2 contents. This resulted in whiter bodies with fewer impurities and a finer, harder texture, suggesting the use of low-iron clay. The glazes of both YD and HYD groups exhibited SiO2 contents within 63–71% and Al2O3 contents within 13–17% (ED-XRF verified). Certain HYD specimens (e.g., HYD-5) showed significantly elevated SiO2 (70.3%), likely attributable to a higher quartz proportion in the raw materials. The marginally higher Al2O3 content in YD glazes compared to HYD suggests differential utilization of clay resources in their respective formulations, potentially reflecting intentional selection of kaolinitic versus illitic clays for viscosity modulation.
Figures 7b–c reveal the distribution of the primary fluxing agents CaO-K2O and MgO-Na2O in the sample body and glaze. The HYD bodies exhibited clustered CaO-K2O distributions, contrasting with the dispersed patterns in YD bodies, suggesting differential flux incorporation strategies during clay preparation. Both groups showed low MgO-Na2O contents with insignificant intergroup variations. Both glaze groups exhibited elevated CaO content (YD: 5.5%, HYD: 5.6%) but minimal P2O5 concentrations (mean:0.2% and mean 0.1%). These P2O5 values fall significantly below the typical range for plant ash-glazed ceramics (0.3–3% P2O5), strongly suggesting the absence of ash-derived fluxes. Instead, calcareous fluxing agents such as limestone were likely employed, leveraging their thermal decomposition (CaCO3 → CaO + CO2↑) to lower the glaze maturing temperature18. Sodium oxide (Na2O) showed narrow distributions in both groups (range: 0.7–1.5% and range: 0.8–1.3%), while magnesium oxide (MgO) displayed notably high concentrations (range: 1.8–3.1%). The elevated MgO content, attributable to dolomite or talc additives, functioned synergistically with CaO as a potent flux. This combination enhanced glaze fluidity and promoted the formation of anorthite-rich intermediate layers, which critically mitigated thermal expansion mismatch between the body and glaze.
Figure 7d further presents the distribution of the main colorant elements Fe2O3 and TiO2 in the body and glaze. Both ceramic body types contained moderate Fe2O3 and TiO2, whose synergistic interaction produced grayish-white to yellowish-gray hues. Notably, the HYD group exhibited significantly lower Fe2O3-TiO2 levels compared to YD, corroborating higher refinement of raw materials through iron–titanium oxide removal. While glaze Fe2O3 ranges overlapped substantially, divergent TiO2 concentrations were observed. Recent studies demonstrate that titanium content modulates hematite (α-Fe2O3) crystal coloration by altering charge-transfer transitions in the 450–550 nm wavelength range19.
Phase characterization
For XRD and Raman spectroscopy analysis, representative samples were selected: YD-5 from the YD group and HYD-1 from the HYD group. For YD sample YD-5, XRD patterns (Fig. 8a) showed the glaze was primarily amorphous glass phase, with characteristic peaks of ZrSiO4 and α-Fe2O3. Raman analysis of different glaze surface areas showed oil-spot patterns and polygonal crystals on cross-sections exhibited characteristic α-Fe2O3 peaks at 297, 413, and 1324 cm-1 (Fig. 9a-b)20. Figure 9c showed ZrSiO4 characteristic peaks at 226, 356, 450, and 1005 cm-1(Fig. 9d), consistent with XRD results21. The rod-shaped crystal clusters located in the intermediate layer between the body and glaze in Fig. 4i exhibit Raman peaks at 180, 284, 482, 511, and 570 cm-1 (Fig. 9e-f), consistent with anorthite. Adjacent to these, near the body, acicular crystals show Raman peaks at 270, 422, 601, and 963 cm-1 (Fig. 9g-h), which are indicative of mullite. Figure 4l depicts quartz particles present in the body, with characteristic peaks at 209 and 470 cm-1(Fig. 9i-j). Meanwhile, Fig. 4k shows comb-like cristobalite, identified by Raman peaks at 230, 420, and 1340 cm-1(Fig. 9k-l).
Raman spectra of YD-5. (a) Hematite (Fig. 3b) (b) Reference Raman spectrum of hematite (Hematite, RRUFF ID: R040024). (c) Zircon (Fig. 4m) (d) Reference Raman spectrum of zircon (Zircon,RRUFF ID: R050034). (e) Anorthite (Fig. 4i) (f) Reference Raman spectrum of anorthite (Anorthite,RRUFF ID:R050104). (g) Mullite(Fig. 4l) (h) Reference Raman spectrum of mullite (Mullite,RRUFF ID:R141101). (i) Quartz(Fig. 4l) (j) Reference Raman spectrum of quartz (Quartz,RRUFF ID:R040031). (k) Comb-like cristobalite (Fig. 4k) (l) Reference Raman spectrum of cristobalite (Cristobalite,RRUFF ID:X050046).
For HYD sample HYD-1, XRD patterns (Fig. 8b) showed mainly glass phase matrix with additional α-Fe2O3, ε-Fe2O310,22 and ZrSiO4 diffraction peaks. Raman analysis of multi-layer surface crystallization showed densely packed dendrites, scattered snowflake-like crystals, and leaf-shaped crystals. Results indicated both dendrites and leaf-shaped crystals had similar Raman peaks belonging to α-Fe2O320 (Figs. 10a-c), while pyramidal shape showed characteristic ε-Fe2O3 peaks at 363, 455, 698, 761, and 1365 cm-1 (Fig. 10d)12,13,14,15,16,17,18,19,20,21,22,23. Figure 10e showed characteristic ZrSiO4 peaks(Fig. 10f)21. In Fig. 6b, the rod-shaped crystals observed in the intermediate layer between the body and glaze are well-developed anorthite, as confirmed by Raman peaks at 180, 287, 510, and 570 cm-1 (Fig. 10g-h). Adjacent to these, massive particles near the body are identified as quartz, showing a characteristic peak at 464 cm-1(Fig. 10g, i). Figure 6d displays densely distributed acicular crystals within the body, exhibiting Raman peaks at 186, 269, 421, 600, and 962 cm-1 (Fig. 10j-k), which are indicative of mullite.
Raman spectra of HYD-1. (a) Hematite (Fig. 5b) (b) Hematite (Fig. 5d). (c) Reference Raman spectrum of hematite (Hematite, RRUFF ID: R040024). (d) ε-Fe2O3 (Fig. 5f) e) Zircon (Fig. 6g). (f) Reference Raman spectrum of zircon (Zircon, RRUFF ID: R050034). (g) Anorthite and quartz ( Fig. 6b) (h) Reference Raman spectrum of anorthite (Anorthite,RRUFF ID:R050104). (i) Reference Raman spectrum of quartz (Quartz,RRUFF ID:R040031). (j) Mullite (Fig. 6d) (k) Reference Raman spectrum of mullite (Mullite,RRUFF ID:R141101).
Both YD and HYD samples showed a significant peak near 674 cm-1 in Raman spectra, previously attributed to γ-Fe2O3 (maghemite)3, Ti/Al-substituted α-Fe2O324, or possibly Fe3O425, requiring further verification.
Surface chemical state analysis
To elucidate the influence of firing atmosphere on oil-spot morphology, we performed X-ray photoelectron spectroscopy (XPS) analysis on both silvery-spot (YD-5, YD-7) and reddish-spot (HYD-1, HYD-8) samples excavated from the Jining Road ancient city site. Peak deconvolution of Fe 2p3/2 spectra (Fig. 11) using Avantage software revealed that the characteristic binding energies at ~ 710eV (Fe2+) and ~ 712eV (Fe3+). Particularly, the Fe2+/Fe3+ area ratios are 2.75 (YD-5) and 2.85 (YD-7), whereas the Fe2+/Fe3+ area ratios are 0.76 (HYD-1) and 0.49 (HYD-8). These results demonstrate that the silvery spots formed under strongly oxidizing conditions , and the reddish spots developed in weakly reducing-to-oxidizing transitional atmospheres.
XPS fitting spectra of silvery and russet spots in oil-spot glazes. (a) XPS fitting spectrum of a silvery spot in Sample YD-5. (b) XPS fitting spectrum of a silvery spot in Sample YD-7. (c) XPS fitting spectrum of a russet spot in Sample HYD-1. (d) XPS fitting spectrum of a russet spot in Sample HYD-8.
Discussions
Oil-spot glaze is a type of iron-rich glaze fired under high-temperature conditions. When the glaze melts at high temperatures, iron oxides undergo thermal decomposition and release gas, leading to the formation of iron-enriched zones around them. As these large bubbles rise to the glaze surface and burst, the residual iron-rich melt precipitates a large number of iron oxide crystals during the cooling process, which aggregate to form "oil-spot" patterns. Simulation experiments have shown that the following process conditions must be met8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29: (i) The iron content in the glaze should be moderate. If the iron content is less than 4 wt%, iron crystallization cannot form; if it is too high, the viscosity of the glaze increases significantly, inhibiting bubble elimination. (ii) The glaze layer must be sufficiently thick. If the glaze layer is too thin, the melt cannot maintain the supersaturation required for crystal growth. (iii) The firing temperature and holding time must be strictly controlled. The optimal firing temperature range is 1240–1280℃. If the temperature is too low, bubbles cannot escape and oil spots will not form; if the temperature is too high, the enriched iron minerals will be disrupted due to glaze flow. The optimal holding time is 20–30 min. If it is too long, the oil spots will lose their regular shape due to glaze flow.
In the HYD series samples, the oil-spot patterns on the glaze surface appear as cloud-like clusters. This may be due to excessively high firing temperatures or prolonged holding times, which disrupt iron enrichment through glaze flow. Alternatively, it could result from a higher total flux content in the glaze, reducing its viscosity. From a chemical composition perspective, most YD and HYD series samples exhibit similar body and glaze compositions, indicating no significant difference in raw material selection or formulation. The variations in glaze surface effects show strong correlation with firing atmosphere under the experimental conditions. Under strong oxidation, polygonal α-Fe2O3 crystals form, while under a weakly reducing or oxidizing atmosphere, hematite crystals often precipitate in dendritic forms, typically resulting in yellowish-brown spots or streaks on the glaze surface28. Additionally, a reducing atmosphere is more conducive to the formation and stabilization of larger ε-Fe2O3 crystals13.
Microstructural analysis reveals that the YD series samples primarily consist of hematite crystals on the surface, forming highly reflective silver oil-spot patterns. However, microscopic observations show that certain areas exhibit brick-red or ochre tones resembling oil-immersed rust. This phenomenon may occur when the kiln door is opened near the end of firing, allowing ambient air to rush in. This sudden change in atmosphere at the surface and subsurface of the molten phase promotes the rapid formation of a hematite variant—specularite—as a thin film on the micron-sized hematite crystals, creating highly reflective silver spots29.
In contrast, the HYD series samples feature red oil-spot patterns composed of densely packed dendritic α-Fe2O3, while the small silver spots consist of dendritic α-Fe2O3 and conical ε-Fe2O3. Differences in the alignment of Fe2O3 crystals affect light reflection. Well-aligned Fe2O3 crystals reflect light across the full spectrum, suppressing their inherent brown color and producing a shiny silver appearance. In contrast, randomly distributed and misaligned Fe2O3 crystals exhibit the chemical color of Fe3+, appearing as reddish-brown or yellowish-brown macroscopically30.
The conical ε-Fe2O3 crystals (30–45 μm) observed on the surface of HYD series samples exhibit significant ferromagnetic resonance and excellent magnetoelectric coupling properties, displaying strong coercivity at room temperature. These crystals have become a focus of extensive research31. Similar crystals have been identified in various historical black or brown glazes, including: Jian Kiln’s (Fujian, Southern Song) oil-spot and hare’s fur glazes13, Yaozhou Kiln’s brown glaze (Shaanxi, Northern Song)32, Jingdezhen Kiln’s purplish-gold glaze (Jiangsu, Qing)33, Linfen Kiln’s (Shanxi, Yuan) glazes34.
The ZrSiO4 observed in the glaze layers of both YD and HYD series is a highly refractory (> 2430 °C) and stable mineral. It likely occurs naturally in the clay or quartz sand used for glaze preparation. Similar ZrSiO4 inclusions have been reported in Yaozhou hare’s fur glaze (Shaanxi)35 and Linfen Kiln’s oil-spot glaze (Shanxi)25.
Through chemical composition analysis, microstructure characterization, and phase identification of the oil-spot black-glazed porcelain excavated from the Jining Road ancient city site, the following conclusions were drawn:
(1) Chemical analysis results demonstrate that the body and glaze compositions of the silver oil-spot and red oil-spot samples from the archaeological site of Jining Lu are similar. The ceramic bodies exhibit a typical "high-alumina, low-silica" characteristic, consistent with traditional ceramic production techniques in northern China. This indicates the use of local aluminum-rich clay, classifying them as high-alumina porcelain. The relatively high iron content results in grayish-yellow body coloration. The glaze composition is characterized by high Fe2O3 and CaO content, predominantly lime glaze, suggesting the addition of limestone to promote glaze sintering.
(2) Integrating experimental observations with archaeological excavation data, we propose the following hypothesis regarding the formation mechanism of the oil-spot black-glazed porcelain at the archaeological site of Jining Lu: During the Jin-Yuan period, most kilns in northern China employed mantou kilns (dome-shaped kilns), which exhibited uneven internal temperature and energy distribution, creating complex firing environments. In the initial firing stage, as temperature increased, anorthite crystallized at the body-glaze interface while iron minerals released oxygen. Under the combined effects of anorthite formation and oxygen bubble generation, iron-rich glaze gradually accumulated near the glaze surface along with rising bubbles. At higher temperatures, the precipitation and growth of iron oxide crystals on the glaze surface were influenced by high iron concentration. The bubble formation and boiling process caused iron redistribution in the glaze, ultimately forming distinct iron-enriched zones near the surface.
(3) The fundamental distinction between silver and red spot patterns is largely attributed to the firing atmosphere’s regulation of Fe2+/Fe3+ equilibrium and crystal orientation. Silver oil spots formed under strong oxidation consist of densely arranged hematite microcrystals. The abrupt atmospheric changes during the final firing stage promoted rapid deposition of Fe2O3 molecules on pre-existing micron-sized hematite crystals, forming a hematite variant—specularite with thin-film crystallization—resulting in highly reflective silver spots. Red oil spots developed under weakly reducing or oxidizing conditions, precipitating densely packed dendritic or branch-like α-Fe2O3 crystals along with conical ε-Fe2O3 crystals. These randomly distributed and variably oriented crystals exhibit the inherent chemical color of Fe3+, appearing as reddish-brown or yellowish-brown macroscopically. By contrast, well-aligned α-Fe2O3 and ε-Fe2O3 crystals demonstrate metallic silver effects.
Conclusions
In Summary, this study demonstrates that the Jin-Yuan period oil-spot glazes from Jining Lu were crafted using distinctive high-alumina clay bodies and lime-alkali glazes, with their characteristic silver and red spots arising from precise atmospheric control during firing. The metallic luster of silver spots originates from aligned α-Fe2O3 microcrystals (2–5 μm) formed under strong oxidation, whereas red spots arise from randomly oriented dendritic α-Fe2O3 and ε-Fe2O3 crystals grown in a weakly reducing-oxidizing atmosphere. The findings highlight northern Chinese potters’ sophisticated mastery of phase separation and crystallization kinetics in iron-rich systems. This study unveils the unique craftsmanship of Jin-Yuan period northern oil-spot glazes, providing critical evidence for understanding regional innovation and transmission of ancient high-temperature glaze technologies. Future research should combine experimental archaeology with advanced materials characterization to: (i) reconstruct the exact temperature/redox profiles of mantou kilns, (ii) investigate potential technological exchanges between northern and southern oil-spot glaze traditions, and (iii) explore how trace elements (e.g., P, Mg) influence crystallization pathways. Such work would provide deeper insights into the innovation and diffusion of medieval Chinese high-temperature glaze technologies.
Methods
Ultra-depth microscopy
A Keyence VHX-6000 ultra-depth microscope (Japan), equipped with a progressive scan system, DVD-ROM drive, and CMOS image sensor, was used for high-resolution surface morphology observation. The sherds were cut with a diamond saw and the resulting sections were mounted in epoxy and mechanically polished. The surface patterns of the samples were also studied directly without any sample preparation.
X-ray diffraction (XRD)
A PANalytical Empyrean diffractometer (Netherlands) was employed for crystal structure analysis, operating at 3 kW with a goniometer reproducibility of 0.0001° and a minimum step size of 0.001°. Samples were divided into body and glaze components using a diamond saw. Ultrasonic cleaning in deionized water (3 cycles, 10 min each). Oven-drying at 60 °C for 12 h. Grinding with an agate mortar and sieving through 200-mesh (75 μm).
Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS)
A Hitachi SU8000 field-emission SEM (Japan) coupled with an EDS detector was used for surface morphology and elemental composition characterization at accelerating voltages of 5 kV and 15 kV. Sample Preparation: First, regions with typical glaze characteristics and cross-sections of the glaze-body interface were selected and cut into 1 cm × 1 cm specimens using a diamond saw. The specimens were then cleaned in an ultrasonic cleaner with deionized water three times (10 min each), followed by drying in an oven at 60 °C for 12 h. Next, the samples were mounted in epoxy resin and subjected to grinding and polishing using a polishing machine until a surface finish of 1 μm diamond paste was achieved. After polishing, the specimens were etched with 1% HF solution for 60 s, followed by another round of ultrasonic cleaning and drying. Finally, prior to SEM analysis, the sample surfaces and cross-sections were coated with a thin layer of gold using a sputter coater to enhance conductivity and ensure optimal imaging.
Wavelength-dispersive X-ray fluorescence (WD-XRF)
A PANalytical Zetium WD-XRF spectrometer (Netherlands) was utilized, equipped with a 4000 W Rh-target X-ray tube (bottom-irradiated, 30 mm spot size), a Deplex composite detector, a HiPro high-energy scintillation detector, and a flow-gas proportional detector. Operating conditions included: 60 kV, 40 mA; 50 kV, 48 mA; 25 kV, 96 mA. All measurements were conducted under vacuum conditions for elemental quantification. Specimen preparation followed the identical protocol as for XRD analysis.
Raman spectroscopy
A HORIBA Jobin Yvon RAMARAMIS spectrometer (France) was used with a 532 nm solid-state laser (2–3 μm spot size). Each Raman spectrum was acquired for 100 s to analyze molecular structures on the sample surface and cross-sections. The preparation procedure was consistent with that employed for Ultra-depth microscopy.
Metallographic microscopy
Sample morphology was examined using a motorized metallurgical microscope (Leica DM6000 M) equipped with 6-position objective turret and bright/dark field illumination. Polished cross-sections of glazes were observed at 50 × –250 × magnification.
X-ray photoelectron spectrometer
Surface chemical analysis was performed using an X-ray photoelectron spectrometer (XPS, Thermo Scientific Escalab Xi +) equipped with a monochromatic Al Kα source (hν = 1486.68 eV). Measurements were conducted under an ultrahigh vacuum (analysis chamber pressure: 1 × 10-9_5 × 10-8 mbar). Survey spectra were acquired at a pass energy of 100 eV, while high-resolution regional scans used a pass energy of 20 eV.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Chen, Y. Z. Porcelain Unearthed from Jininglu Ancient City Site in Inner Mongolia 7–13 (Cultural Relics Press, 2004).
Zhang, F. K. The Science of Ancient Chinese Ceramics 84–87 (Shanghai People’s Fine Arts Publishing House, 2000).
Li, F. et al. Phase-separated Tenmoku “Blue” glaze: Microstructure and coloring mechanism. J. Eur. Ceram. Soc. 43, 6581–6589 (2023).
Chen, Q. et al. Micro-structural study of crystals and glassy matrix of black glazed wares from Jian kilns, China (Song Dynasty, AD 960–1279). J. Raman. Spectrosc. 54, 532–539 (2023).
Yong, Q. et al. End-to-end ancient ceramic classification toolkit based on deep learning: A case study of black glazed wares of Jian kilns (Song Dynasty, Fujian province). J. Ceram. Int. 48, 34516–34542 (2022).
Chen, Y. G. et al. Mössbauer study of the black-glazed Jian bowl in the Song dynasty. Int. J. Appl. Ceram. Technol. 20, 3795–3802 (2023).
Xu, Y., Qin, Y. & Ding, F. Characterization of the rare oil spot glazed bowl excavated from the Xiao kiln site of north China. Ceram. Int. 43, 8636–8642 (2017).
Li, X., Lu, J., Yu, X., Zhou, J. & Li, L. Imitation of ancient black-glazed Jian bowls (Yohen Tenmoku): Fabrication and characterization. Ceram. Int. 42, 15269–15273 (2016).
Wang, T. et al. Morphological and structural study of crystals in black-to-brown glazes of Yaozhou ware (Song dynasty) using imaging and spectroscopic techniques. J. Eur. Ceram. So. 41, 6049–6058 (2021).
Tao, S., Liu, S., Yuan, Y., Dong, J. & Li, Q. A microstructural and compositional study of ε-Fe2O3 crystals in the hare’s fur Jian ware. Crystals 12, 367 (2022).
Tao, S., Liu, S., Dong, J., Yuan, Y. & Li, Q. Morphological and structural analysis of iron-rich porcelains excavated from the Jian kiln site of Song dynasty. J. Microsc. 292, 3–18 (2023).
Dejoie, C. et al. Learning from the past: Rare ε-Fe2O3 in the ancient black-glazed Jian (Tenmoku) wares. Sci. Rep. 4, 4941 (2014).
Wen, D. Y. Development and formation mechanism of oil-drop and rabbit-fur glazes. SD. Ceram. 22, 35–37 (1999) (in Chinese).
Jiang, C. S. et al. Formation mechanism of crystal spots in jian kiln oil-spot glaze revealed by simulation experiments. Appl. Sci. 14, 10210 (2024).
Guan, M. et al. Uncovering the mystery of Al(III) doping of ε-Fe2O3 in the ancient high-iron black-brown glaze. J. Am. Ceram. Soc. 107, 522–533 (2023).
Shi, X. T. Preparation and Mechnism of Fe-series Phase Separation Art Glazes (Doctoral thesis). South China University of Technology, 2020.
Li, G. Z. & Guo, Y. Y. Basic Techniques of Famous Chinese Porcelain 71–74 (Shanghai Scientific and Technical Publishers, 1988).
Wang, E. Y., Xiong, Y. F., Zhu, Y. B. & Wu, J. W. Study on ancient lime glazes and lime alkali glazes-limitations of surface analysis. Microchem. J. 165, 106170 (2021).
Wang, T., Sanchez, C., Groenen, J. & Sciau, P. Raman spectroscopy analysis of terra sigillata: The yellow pigment of marbled sigillata. J. Raman Spectrosc. 47, 1522–1527 (2016).
Marshall, C. P., Dufresne, W. J. B. & Rufledt, C. J. Polarized Raman spectra of hematite and assignment of external modes. J. Raman. Spectrosc. 51, 1522–1529 (2020).
Kock, L. D. et al. The determination of percentage dissociation of zircon (ZrSiO4) to plasma dissociated zircon (ZrO2. SiO2) by Raman spectroscopy. J. Raman. Spectrosc. 43, 769–773 (2012).
Wen, R. et al. The colouring mechanism of the Brown glaze porcelain of the Yaozhou Kiln in the Northern Song Dynasty. Ceram. Int. 45, 10589–10595 (2019).
Hoo, Q. Y. et al. Millimeter-sized flower-like clusters composed of mullite and ε-Fe2O3 on the Hare’s Fur Jian Ware. J. Eur. Ceram. Soc. 40, 4340–4347 (2020).
Wang, T. et al. Morphological and structural study of crystals in black-to-brown glazes of Yaozhou ware (Song dynasty) using imaging and spectroscopic techniques. J. Eur. Ceram. Soc. 41, 6049–6058 (2021).
Huang, R. F., Chen, X. Q., Shui, J. S. & Ruan, M. L. Research on oil-drop black-glaze ware. Jdz. Ceram. 4, 39–46 (1984) (in Chinese).
Xu, L. H. & Li, L. L. Preparation and microstructure analysis of silver oil-spot glaze. Non-Met. Mines. 45, 9–11 (2022) (in Chinese).
Dong, F. W. The development and production of red oil-drop-in-black-glaze. FS. Ceram. 33, 29–32 (2023) (in Chinese).
De Bonis, A. et al. Different shades of red: The complexity of mineralogical and physico-chemical factors influencing the colour of ceramics. Ceram. Int. 43, 8065–8074 (2017).
Xu, Z. Q., Zhao, X. H., Liang, H. D., Li, Z. P. & Tie, S. Characterization of oil spots on black-glazed teabowl made in Song dynasty by time-of-flight secondary ion mass spectrometry. J. Chin. Mass. Spectrom. Soc. 44, 25–33 (2023) (in Chinese).
Li, M., Li, W. D., Lu, X. K. & Xu, C. S. Controllable preparation and decorative effect of iron-based crystalline glazes. J. Chin. Ceram. Soc. 48, 1134–1144 (2020) (in Chinese).
Tuček, J., Zbořil, R., Namai, A. & Ohkoshi, S. ε-Fe2O3: An advanced nanomaterial exhibiting giant coercive field, millimeter-wave ferromagnetic resonance, and magnetoelectric coupling. Chem. Mater. 22, 6483–6505 (2010).
Ren, Z. et al. Study of sauce glazed wares from Yaozhou kilns (Northern Song dynasty, 960–1127 CE): Probing the morphology and structure of crystals in the glazes. J. Eur. Ceram. Soc. 42, 7352–7359 (2022).
Liu, Z. et al. The morphology and structure of crystals in Qing dynasty purple-gold glaze excavated from the Forbidden City. J. Am. Ceram. Soc. 101, 5229–5240 (2018).
Wang, M. et al. Raman study of rusty oil spotted glaze produced in Linfen kilns (Shanxi province, AD 1115–1368). J. Raman. Spectrosc. 53, 582–592 (2022).
Li, W. D., Zhang, W., Lu, X. K., Zheng, N. Z. & Luo, H. J. Chemical compositions and microstructures of hare’s fur black-glazed porcelains from Jian kiln, Jizhou kiln and Yaozhou kiln sites. J. Build. Mater. 14, 329–334 (2011) (in Chinese).
Funding
This work is supported by key projects of Inner Mongolia Autonomous Region Science and Technology Plan Project (Grant No. 2023YFSW0019) and Basic Research Project of Inner Mongolia Autonomous Region Directly Affiliated Universities (Grant No. JY20250063).
Author information
Authors and Affiliations
Contributions
Y.Z.C and X.L.J contributed to sample processing and provision. X.L.J conducted all experimental procedures and result analyses. Y.Z.C, GL.TR, and K.S participated in manuscript refinement and revisions. All authors engaged in discussions and interpretation of the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jia, X., Teri, G., Shi, K. et al. Deciphering the composition and microstructure of Jin-Yuan period oil-spot glazes from the archaeological site of Jining Lu of China. Sci Rep 15, 35504 (2025). https://doi.org/10.1038/s41598-025-19678-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-19678-0