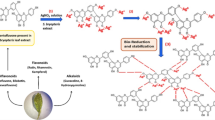
Abstract
Multidrug-resistant Candida infections demand urgent therapeutic solutions. This study demonstrated the green synthesis of silver nanoparticles designated At-AgNPs using Alkanna tinctoria root extract, showing enhanced antifungal activity against Candida species including resistant C. auris. Characterization confirmed spherical At-AgNPs with 19.91 nm diameter by TEM and 120.6 nm hydrodynamic size, exhibiting a UV-Vis peak at 406.27 nm. EDX analysis revealed 79.24% silver content, while XRD patterns verified face-centered cubic crystallinity. The nanoparticles maintained excellent stability with zeta potential measuring − 23.34 mV and PDI 0.202. At-AgNPs displayed strong antifungal effects, particularly against C. parapsilosis showing 15.46 mm inhibition zone with MIC and MFC values of 1.0 and 2.0 mg/L respectively. Notable synergy emerged when combining At-AgNPs with conventional antifungals, especially clotrimazole against C. albicans demonstrating 37.28 mm inhibition compared to 33.84 mm for clotrimazole alone. SEM imaging revealed extensive morphological damage to fungal cells following combination treatment. These results position At-AgNPs as a viable adjunct therapy capable of boosting conventional treatment effectiveness while potentially lowering required drug doses and minimizing side effects in resistant Candida infections.
Similar content being viewed by others
Introduction
Nosocomial infections, or hospital-acquired infections (HAIs), are a major global public health issue, increasing mortality, morbidity, and healthcare costs. Candida species, including C. albicans, C. tropicalis, C. glabrata, C. parapsilosis, and the multidrug-resistant C. auris, are key opportunistic fungi in healthcare settings1. These pathogens disproportionately affect immunocompromised patients, such as those undergoing transplants, chemotherapy, or prolonged antibiotic therapy, as well as individuals with indwelling medical devices2. The rise in candidal infections, particularly candidemia and invasive candidiasis, is alarming, with mortality rates exceeding 40% despite antifungal advances3. Growing antifungal resistance, especially in non-albicans species, complicates treatment, prolongs hospital stays, and raises costs4.
The impact of nosocomial Candida infections extends beyond acute illness, often causing long-term disability. Survivors may face chronic organ dysfunction, prolonged immobility, and neurological complications like cognitive impairment or stroke, severely affecting their quality of life. These disabilities can reduce independence, increase caregiver reliance, and diminish well-being. For individuals with pre-existing disabilities, such as immunocompromised or chronically ill patients, HAIs exacerbate challenges, creating additional barriers to recovery. Addressing these infections is critical to mitigating their disproportionate burden on vulnerable populations, including those supported by disability-focused initiatives5. A previous retrospective study analyzed Candida auris outbreaks across 45 Saudi Ministry of Health (MOH) hospitals (2020–2022), identifying 511 cases (56.9% infected, 43.1% colonized) with a high mortality rate of 41.5%, particularly among ICU patients (95.5%) and those with invasive devices or COVID-19 coinfection6. Moreover, a previous report analyzed anonymized data to characterize the epidemiological profile of C. auris infections, examining age distribution, gender prevalence, risk factors, and specimen sources. Among 53 confirmed cases (62% male, median age 64 years), the majority (83%) occurred in patients aged ≥ 50 years, with all cases presenting comorbidities - predominantly diabetes and chronic kidney disease. Notably, only one case was hospital-acquired, while urine specimens accounted for the highest proportion of positive samples (30.2%)7. A previous study aimed to evaluate the antifungal minimum inhibitory concentration (MIC) values of Candida auris isolates collected between November 2020 and July 2024. The findings revealed concerning resistance rates, with 84.4% of isolates resistant to fluconazole and 16.6% resistant to amphotericin B. These results highlight the critical need for rapid antifungal susceptibility testing to guide timely and effective treatment, particularly given C. auris’s association with high morbidity, mortality, and rapid nosocomial spread8. Another study investigated the antifungal susceptibility patterns of Candida auris clinical strains at Bahrain Oncology Center–King Hamad University Hospital (October 2021–November 2022). Among 40 isolates (25% blood, 65% urine, 10% soft tissue), resistance rates were 100% to fluconazole, 2.5% to caspofungin, and 0% to amphotericin B, with only one bloodstream isolate showing dual resistance (fluconazole and caspofungin). Voriconazole exhibited low MICs (median 0.015 µg/mL), highlighting the urgent need for antifungal stewardship amid rising C. auris resistance9.
Nanotechnology represents a groundbreaking frontier for developing potent antifungal therapies, offering a powerful solution to combat the escalating threat of multidrug-resistant fungal pathogens. Green synthesis offers a promising strategy in various areas of modern nanobiotechnology. This approach utilizes natural compounds such as proteins, alkaloids, flavonoids, and polyphenols to act as both reducing and stabilizing agents during nanoparticle production. Additionally, green synthesis is an eco-friendly and economically viable method, making it well-suited for large-scale industrial applications in nanoparticle manufacturing10. Metal nanoparticles synthesized by green processes and coated with organic molecules may exhibit enhanced biological activity and less toxicity. Metallic nanoparticles have been identified as viable alternatives to traditional antimicrobial agents, capable of overcoming prevalent microbial resistance mechanisms such as target site modification, enhanced drug efflux due to efflux pump overexpression, enzyme inactivation, and reduced cell membrane permeability11. Metallic nanoparticles have many benefits such as diminutive particle size (1–100 nm), low cytotoxicity, exceptional chemical stability, and potential antimycotic effectiveness12. The use of silver nanoparticles (AgNPs) into antifungal treatment is an innovative strategy for alleviating the problems linked to candidal infections. In this regard, AgNPs have significant antimycotic efficiency, especially against drug-resistant Candida species, and are proficient in inhibiting biofilm development on medical devices, including catheters and prosthetic implants13. Recently, a previous study developed the eco-friendly synthesis of AgNPs using Trillium govanianum and Bergenia ligulata extracts, demonstrating potent anticandidal and antibiofilm activity14. Moreover, other study reported the biosynthesis of AgNPs using Cleome rutidosperma leaf extract, demonstrating strong anti-Candida activity with biofilm inhibition at 30–50 µg/mL15. Furthermore, the green-synthesized AgNPs using A. tinctoria aqueous extract exhibited stronger antifungal activity against Aspergillus niger (18 mm inhibition zone) compared to A. flavus (9 mm)16. Accordingly, the present study breaks new ground by evaluating A. tinctoria-mediated AgNPs against multidrug-resistant C. auris, unlike previous research limited to Aspergillus species. Additionally, it systematically explores synergistic interactions between bioengineered AgNPs and conventional antifungals, presenting a novel strategy to antifungal resistance issue. These findings not only reinforce the therapeutic potential of phytogenic nanoparticles but also establish a combinatorial framework to combat high-priority fungal pathogens in clinical settings.
The incidence of resistant fungal pathogens, such as C. auris, highlights the urgent need for innovative antimicrobial strategies. These approaches aim to improve the efficacy of existing antifungal therapies while minimizing the toxicity linked to high-dose regimens. In this context, the present study focuses on the green phytophabrication of At-AgNPs using A. tinctoria root extract. The synthesized nanoparticles were characterized through various techniques, and their antifungal potential were assessed against multidrug-resistant Candida species, including C. albicans, C. tropicalis, C. glabrata, C. auris, C. parapsilosis, and C. krusei. Furthermore, the study seeks to explore the combined antifungal effects of AgNPs with standard antifungal drugs, such as fluconazole, nystatin, clotrimazole, and terbinafine, to boost their antifungal performance.
Results
UV spectroscopy of AgNPs
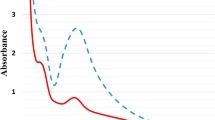
The aqueous extract of A. tinctoria roots (Fig. 1a), when added to the colorless silver nitrate (AgNO₃) solution (Fig. 1b), induces a color change to brown, indicating the formation of AgNPs. The change in colour serves as a visual indicator for At-AgNPs synthesis. Ultraviolet (UV) investigation was performed to inspect the surface plasmon resonance (SPR) effect of At-AgNPs. The SPR of AgNPs is a fundamental optical characteristic resulting from the coordinated oscillation of conduction electrons when exposed to incident light. Figure 1 illustrated the UV peak of A. tinctoria extract and AgNPs, demonstrating a peak formation at 406.27 nm, attributable to SPR of AgNPs.
TEM analysis of the bioprepared AgNPs
TEM was used to detect the morphology, dimensions, and size distribution of At-AgNPs. The synthesized NPs were observed to be spherical in shape and present in aggregates, surrounded by an extracellular matrix attributed to plant phytochemicals, which function as stabilizing agents for At-AgNPs (Fig. 2a). Additionally, the mean size of At-AgNPs was calculated to be 19.91 nm, with the majority of particles distributed within a range of 5 to 30 nm, as illustrated in Fig. 2b. The TEM images also revealed that the At-AgNPs were coated with a thin organic layer originating from the water extract of A. tinctoria.
EDX analysis of At-AgNPs
EDX analysis of At-AgNPs revealed a composition of 79.24% silver (Ag) (Fig. 3), confirming AgNP formation. Additional elements included carbon (C; 6.03%), oxygen (O; 8.44%), and chlorine (Cl⁻; 6.29%), with a total mass percentage of 100.00%.
FTIR analysis of B. prattensis root extract and AgNPs
The FTIR spectrometer was utilized to discover the main functional groups of At-AgNPs. In this study, the FTIR analysis of A. tinctoria root extract exhibited nine distinct absorption bands at 3749, 3394, 2939, 2367, 1597, 1419, 1072, 864, and 617 cm⁻¹ (Fig. 4). However, the FTIR spectrum of At-AgNPs displayed slight shifts in certain bands. These shifts suggest that the primary groups of the A. tinctoria root extract likely acted as stabilizing agents for At-AgNPs, thereby contributing to the stabilization and structural integrity of the nanoparticles. The weak band observed at 3749 cm⁻¹ in A. tinctoria spectrum exhibited a shift to 3752 cm⁻¹ in the spectrum of At-AgNPs. This shift can be attributed to the interaction of silver ions with hydroxyl groups, suggesting the presence of polyphenolic compounds. Furthermore, the broad absorption band observed at 3394 cm⁻¹ in the FTIR spectrum of A. tinctoria root extract exhibited a shift to 3425 cm⁻¹ in the spectrum of At-AgNPs. This shift is indicative of O-H stretching vibrations, which might be accredited to phenolic and alcoholic compounds. On the other hand, the weak band found at 2939 cm⁻¹ in A. tinctoria spectrum was shifted to 2924 cm⁻¹ in spectrum of At-AgNPs indicating the C–H stretching of aldehydes. The peaks found at 2367 and 2368 cm⁻¹ in spectrum of both of A. tinctoria root extract and AgNPs might be ascribed to stretching vibration of tertiary amines (C ≡ N). The strong peak found at 1597 cm⁻¹ in both spectrum of A. tinctoria root extract and AgNPs might be allocated to C = C vibration of aromatic compounds. Furthermore, the band found at 1419 cm⁻¹ in A. tinctoria spectrum was shifted to 1381 cm⁻¹ in At-AgNPs spectrum confirming the C–N stretching of amines. The band observed at 864 cm⁻¹ in A. tinctoria spectrum shifted to 833 cm⁻¹ in the At-AgNPs spectrum, likely due to C–H stretching vibrations. The spectral peaks observed between 694 and 540 cm⁻¹ in the At-AgNPs spectrum are likely attributable to the –O–H stretching, indicative of a bond formation between the oxygen atom of Ag₂O and the hydrogen atom of phenolic compounds adsorbed on the nanoparticle surface.
Crystallographic investigation of AgNPs
The crystallographic nature of At-AgNPs was validated using XRD investigation. The XRD spectrum exhibited five different peaks at 2θ angles of 38.515°, 44.540°, 64.566°, 77.508°, and 81.762°, corresponding to the crystallographic planes (111), (200), (220), (311), and (222), respectively (Fig. 5). The observed peaks correspond to the reference data from the Joint Committee on Powder Diffraction Standards (JCPDS) database, card number 04-0782 for face-centered cubic (fcc) silver.
Zeta potential analysis
Zeta potential analysis of At-AgNPs revealed a Z-average size of 120.6 nm with a low polydispersity index (PDI = 0.202), indicating a monodisperse size distribution (Fig. 6a). Dynamic light scattering (DLS) measurements showed a hydrodynamic diameter significantly larger than the particle dimensions obtained from TEM analysis. The nanoparticles exhibited a zeta potential of – 23.34 mV (Fig. 6b), demonstrating moderate colloidal stability in suspension. The physicochemical characteristics of At-AgNPs were summarized in Table 1.
Antifungal sensitivity testing
Figure 7 revealed clear resistance patterns, with C. albicans (10.52 ± 0.71 mm) and C. glabrata (0.00 ± 0.00 mm) showing resistance to terbinafine, while C. tropicalis (23.03 ± 0.55 mm) and C. parapsilosis (32.64 ± 1.11 mm) remain highly susceptible. Fluconazole resistance was striking in C. auris (0.00 ± 0.00 mm) and C. krusei (7.98 ± 0.26 mm), contrasting with the susceptibility of C. albicans (36.09 ± 0.18 mm) and C. tropicalis (30.92 ± 0.22 mm). Notably, nystatin showed consistent efficacy across all species, with inhibition zones ranging from 21.26 ± 0.39 mm (C. auris) to 31.44 ± 0.41 mm (C. parapsilosis), reinforcing its broad-spectrum potential. Intermediate resistance (I) was observed in key species: C. glabrata exhibited intermediate susceptibility to fluconazole (15.35 ± 0.74 mm) and clotrimazole (19.00 ± 0.90 mm), while C. auris showed intermediate responses to itraconazole (20.77 ± 0.39 mm) and clotrimazole (19.07 ± 0.89 mm). C. krusei displayed intrinsic fluconazole resistance (7.98 ± 0.26 mm) but intermediate susceptibility to itraconazole (16.64 ± 0.64 mm). Natamycin and tioconazole performance varied widely, with C. krusei being highly susceptible to natamycin (25.25 ± 0.65 mm) but resistant to tioconazole (8.89 ± 0.27 mm), highlighting the need for precise antifungal selection. The resistance of C. auris to fluconazole (0.00 ± 0.00 mm) and tioconazole (11.12 ± 0.47 mm), coupled with C. krusei’s resistance to both agents (7.98 ± 0.26 mm and 8.89 ± 0.27 mm, respectively), underscored the challenge of treating these pathogens. Nystatin’s robust activity (e.g., 24.96 ± 0.55 mm for C. glabrata and 28.53 ± 0.38 mm for C. krusei) offered a temporary solution, but its toxicity limits long-term use. The poor performance of tioconazole against C. auris (11.12 ± 0.47 mm) and C. krusei (8.89 ± 0.27 mm) emphasizes the urgency for alternative therapies, such as AgNPs, to address multidrug-resistant strains. The antifungal activity of terbinafine against C. parapsilosis strain was significantly higher than that of fluconazole and itraconazole antifungals (p < 0.05). However, the antifungal activity of terbinafine against C. parapsilosis was non significantly different compared to clotrimazole and nystatin (p > 0.05). On the other hand, Fluconazole exhibited significantly greater activity against C. albicans (36.09 ± 0.18 mm; p < 0.05) than other azoles (itraconazole: 29.99 ± 0.41 mm; ticonazole: 29.11 ± 0.47 mm), while clotrimazole showed superior efficacy against C. krusei (32.38 ± 0.29 mm; p < 0.05) compared to all tested antifungals, including nystatin (28.53 ± 0.38 mm) and other azoles (fluconazole: 7.98 ± 0.26 mm; itraconazole: 16.64 ± 0.64 mm). For C. auris strain, the antifungal activity of nystatin was significantly higher than that of natamycin and ticonazole antifungal agents (p < 0.05) while no significant difference was detected for nystatin compared to itraconazole antifungal agent (p > 0.05). Similarly, nystatin showed significantly stronger antifungal activity compared to the other antifungal agents against C. glabrata strain (p < 0.05). On the other hand, clotrimazole demonstrated significantly greater antifungal activity compared to the other antifungal agents (p < 0.05).
Screening of anticandidal activity of At-AgNPs against different fungal strains
The antimicrobial efficiency of At-AgNPs was evaluated utilizing the disk diffusion technique against candidal pathogens and compared with the aqueous root extract of A. tinctoria. Notably, the biogenically synthesized At-AgNPs exhibited significant anticandidal activity against all tested strains, with IZD ranging from 9.69 ± 0.93 mm to 15.46 ± 0.68 mm in diameter. Among the strains, C. parapsilosis displayed the highest susceptibility to At-AgNPs, with IZD of 15.46 ± 0.68 mm (Table 2), whereas C. tropicalis exhibited the lowest susceptibility, demonstrating IZD of 9.69 ± 0.93 mm. Conversely, the root extract of A. tinctoria exhibited no anticandidal action against the tested strains. Interestingly, the At-AgNPs demonstrated antifungal activity against C. glabrata strain which was significantly higher than that of terbinafine antifungal agent (p < 0.05) as the strain showed terbinafine resistance. This finding highlights the potential of At-AgNPs) as a novel therapeutic alternative to conventional antifungals, particularly in addressing drug-resistant fungal pathogens.
The MIC of At-AgNPs was evaluated using a broth microdilution assay against C. parapsilosis strain, which displayed the greatest susceptibility to At-AgNPs. In this context, MIC of At-AgNPs was 1.0 mg/l however minimum fungicidal concentration was found to be 2.0 mg/l.
Synergistic antifungal efficacy of At-AgNPs with common antifungal drugs
The combination of At-AgNPs (1.0 mg/disk) with clotrimazole (CLO, 10 µg/disk) exhibited variable synergistic effects across Candida species. Against C. albicans, the inhibition zone diameter (IZD) significantly increased from 33.84 ± 0.51 mm (CLO alone) to 37.28 ± 0.59 mm (CLO + At-AgNPs), with a relative IFA value of 0.213, indicating strong synergism (Fig. 8). However, for C. tropicalis and C. parapsilosis, the combination showed antagonism (IFA: − 0.102 and − 0.106, respectively), with reduced IZDs compared to CLO alone. Minimal synergy was observed against C. glabrata, C. auris, and C. krusei (IFA: 0.007–0.047).
In contrast, the combination of At-AgNPs (1.0 mg/disk) with fluconazole (FLU, 25 µg/disk) demonstrated limited synergy. While C. albicans and C. parapsilosis showed slight enhancement in IZDs (IFA: 0.073 and 0.066, respectively), C. glabrata and C. tropicalis exhibited antagonism (IFA: − 0.130 and − 0.034). Notably, FLU-resistant strains (C. auris and C. krusei) displayed no activity when treated with FLU alone (IZD: 0.00 mm), but the addition of At-AgNPs restored activity (IZDs: 11.99 ± 0.92 mm and 11.48 ± 0.32 mm, respectively), though IFA values indicated no formal synergy (IFA: 0.00).
Synergistic effects were more pronounced with nystatin (NST, 25 µg/disk) combined with At-AgNPs. Against C. albicans, the IZD increased from 17.89 ± 0.31 mm (NST alone) to 19.64 ± 0.28 mm (NST + At-AgNPs), with an IFA of 0.205. Similar but weaker synergy was observed for C. glabrata, C. auris, C. parapsilosis, and C. krusei (IFA: 0.022–0.076). No synergy occurred for C. tropicalis (IFA: − 0.010).
The combination of terbinafine (TER, 30 µg/disk) with At-AgNPs showed strain-dependent effects. The most significant synergy was observed against C. krusei, where the IZD increased from 18.18 ± 0.46 mm (TER alone) to 19.98 ± 0.43 mm (TER + At-AgNPs; IFA: 0.207). Moderate synergy occurred against C. tropicalis, C. auris, and C. parapsilosis (IFA: 0.059–0.113). However, C. albicans and C. glabrata showed no improvement (IFA: − 0.010 and 0.00, respectively), with TER alone being ineffective against C. glabrata (IZD: 0.00 mm).
Interestingly, the antifungal activity of clotrimazole combination with AgNPs against C. albicans strain was significantly higher compared to that of clotrimazole alone (p < 0.05). Moreover, the antifungal activity of terbinafine combination with AgNPs was significantly higher than that of terbinafine alone against C. krusei strain (p < 0.05). Additionally, the antifungal efficiency of nystatin combination with AgNPs was significantly higher than that of nystatin alone against C. albicans strain (p < 0.05).
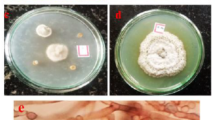
Evaluation of morphological deformations using SEM analysis
The most potent synergy was observed between At-AgNPs and clotrimazole against C. albicans. SEM evaluation of treated cells showed progressive morphological damage across treatments. Untreated controls displayed normal, smooth cellular morphology (Fig. 9). AgNP monotherapy caused substantial surface deformation, including membrane pitting and cellular shrinkage (Fig. 10). Clotrimazole alone induced moderate surface wrinkling and partial collapse (Fig. 11). The combination treatment produced the most severe alterations, with complete membrane disintegration, extensive cellular collapse, and apparent loss of cellular contents (Fig. 12). These findings demonstrate that At-AgNPs significantly potentiate clotrimazole’s membrane-disrupting effects, likely through combined physical and oxidative damage mechanisms that compromise structural integrity. This synergistic interaction could enable dose reduction while enhancing antifungal efficacy against resistant strains. The SEM examination revealed that the percentage of deformation of candidal cells was 55.5% for the examined clotrimazole treated cells compared to total cells whereas the percentage of deformed cells for the examined clotrimazole and AgNPs treated cells was 82.35%.
Discussion
The green synthesis of AgNPs was confirmed by a distinct color change, serving as a visual indicator of At-AgNPs formation. In this context, the brown coloration results from the surface plasmon resonance (SPR) effect, a distinctive optical feature exhibited by AgNPs. When AgNPs are formed, their free electrons oscillate collectively upon interaction with light, leading to the absorption of specific wavelengths and the reflection of others, resulting in the observed brown color17. The aqueous extract of Alkanna tinctoria roots comprises several phytochemicals, including alkaloids, flavonoids, phenolic compounds, and terpenoids, which function as reducing agents18. These compounds provide electrons to Ag⁺ ions in the AgNO₃ solution, thus reducing them to metallic silver (Ag⁰). Moreover, the phytochemicals serve as capping agents, stabilizing the biosynthesized AgNPs and inhibiting their aggregation. The biosynthesized AgNPs exhibited a characteristic SPR peak at 406.27 nm, consistent with typical SPR behavior of silver nanoparticles. These findings align with previous reports using Hagenia abyssinica leaf extract, which demonstrated a similar SPR peak at 406 nm19. Moreover, TEM analysis revealed spherical AgNPs with a mean particle diameter of 19.91 nm and a size distribution ranging from 5 to 30 nm. Previous research revealed that TEM analysis showed the spherical shape of AgNPs synthesized from Barleria prattensis, with a mean size of 28.57 nm ± 7.8. This organic capping layer improves the stabilization of the Bp-AgNPs by effectively inhibiting particle agglomeration20. Furthermore, the AgNPs synthesized from three Sideritis species exhibited a spherical and monodispersed morphology, with mean size ranging between 22 and 26 nm21. Collectively, our findings demonstrate a significantly smaller particle size compared to those reported in prior studies, underscoring the superior efficiency of the green synthesis method employed in the current investigation. Moreover, green synthesis using Alkanna tinctoria root extract provides a safer alternative, employing natural phytochemicals from the extract as reducing and stabilizing agents, unlike chemical synthesis methods, which require toxic reducing and stabilizing agents that can leave hazardous residues on the nanoparticles and limit their practical use22. Moreover, the current study achieved smaller nanoparticle sizes (19.91 nm) compared to those reported by Ashraf et al. (2015), who employed a chemical synthesis method using sodium citrate solution as a reducing agent to produce AgNPs ranging from 42 to 58 nm23. Moreover, other study reported the chemical method of AgNPs synthesis using sodium borohydride resulting in particle sizes ranged from 15 to 85 nm in diameter according to TEM results24. Collectively, these comparisons demonstrate that our green synthesis approach not only provides an environmentally safe alternative but also yields significantly smaller nanoparticles, highlighting the superior efficiency of this method.
On the other hand, EDX analysis confirmed the elemental composition of the nanoparticles, with silver (Ag) dominating at 79.24% of the total mass. Additional signals revealed carbon (6.03%) and oxygen (8.44%), likely originating from the plant-derived capping agents. The presence of carbon (C) at 6.03% and oxygen (O) at 8.44% suggests the involvement of organic compounds derived from the root extract, likely acted as both stabilizing and reducing agents during the nanoparticle synthesis25. Furthermore, the presence of chlorine (Cl⁻) ions at 6.29% is consistent with prior research, which has established that Cl⁻ ions are abundant in plants and play a vital role in maintaining cellular homeostasis and facilitating photosynthetic processes. This may account for the prominent Cl⁻ peak observed in the EDX spectrum of the biogenic AgNPs26. The total mass percentage of 100.00% confirmed the precision and reliability of the EDX measurements. Collectively, these findings underscore the efficacy of the root extract in the synthesis and stabilization of AgNPs, while also highlighting the contribution of organic capping agents and plant-derived ions to the nanoparticles’ structural integrity and dispersion. Moreover, FTIR analysis demonstrated the presence of different capping agents over the surface of AgNPs as phenolic, alcoholic, aldehydes, and amines. Interestingly, the XRD pattern’s distinct peaks at 2θ = 38.515° (111), 44.540° (200), 64.566° (220), 77.508° (311), and 81.762° (222) confirm the face-centered cubic (fcc) crystalline structure of At-AgNPs, matching standard metallic silver (JCPDS 04-0782). The existence of these peaks verifies the successful biosynthesis of crystalline AgNPs, with the measured 2θ values and related lattice planes closely matching the standard diffraction pattern for metallic silver, hence affirming the crystalline nature of At-AgNPs27. The low PDI value of At-AgNPs (0.202) confirms a monodisperse, homogeneous sample28. However, the observed size discrepancy between DLS (120.6 nm) and TEM arises because DLS measures nanoparticles with their hydrate layer and phytochemical capping agents, while TEM provides exact physical dimensions29. The zeta potential (− 23.34 mV) indicates moderate colloidal stability, attributed to negatively charged functional groups from A. tinctoria root extract adsorbed on the nanoparticle surface30.
The tested Candida strains exhibited distinct resistance patterns when evaluated for antifungal susceptibility using the disk diffusion method. C. albicans and C. glabrata demonstrated resistance to terbinafine, while C. auris and C. krusei were resistant to fluconazole. Additionally, C. glabrata showed resistance to itraconazole, C. auris to natamycin and tioconazole, and C. krusei to tioconazole. Among all the antifungal agents tested, nystatin was the most effective against the different Candida species, showing the highest inhibitory activity. The resistance patterns observed in this study align with known mechanisms of antifungal resistance. Terbinafine resistance in C. albicans and C. glabrata likely results from mutations in the ERG1 gene or overexpression of efflux pumps such as CDR1 and CDR2, which disrupt ergosterol biosynthesis by targeting squalene epoxidase31. Similarly, fluconazole resistance in C. auris and C. krusei can be attributed to ERG11 mutations, increased efflux pump activity (MDR1, CDR1), or, in the case of C. krusei, inherent structural differences in the ERG11-encoded enzyme32. The widespread resistance to azoles (fluconazole, itraconazole) and polyenes (natamycin) highlights the growing challenge in treating candidal infections, particularly with the rising prevalence of multidrug-resistant strains like C. auris. While nystatin demonstrated strong efficacy, its clinical use is limited due to toxicity concerns, emphasizing the need for alternative antifungal agents. In this context, silver nanoparticles (AgNPs) synthesized using Alkanna tinctoria root extract were investigated as a potential solution. Their unique mechanism of action, distinct from conventional antifungals, may help overcome existing resistance mechanisms, offering a promising therapeutic alternative that warrants further exploration. Remarkably, the results demonstrated that while the Alkanna tinctoria root extract itself showed no antifungal activity (0.00 ± 0.00 mm inhibition zones across all Candida species), the AgNPs synthesized from the extract exhibited significant inhibitory effects. The AgNPs (1.0 mg) produced measurable inhibition zones ranging from 9.69 ± 0.93 mm (C. tropicalis) to 15.46 ± 0.68 mm (C. parapsilosis), with particularly strong activity against C. parapsilosis. The positive control (terbinafine 30 µg) showed variable efficacy, with high activity against C. tropicalis (22.55 ± 0.56 mm) and C. parapsilosis (38.42 ± 1.16 mm) but no effect on C. glabrata (0.00 ± 0.00 mm), consistent with earlier resistance patterns.
Our findings represented significant advancements over previous reports of green-synthesized AgNPs, particularly in nanoparticle characteristics and antifungal efficacy. While Arsène et al. successfully produced cubic AgNPs with a hydrodynamic diameter of 80.31 ± 10.03 nm and an SPR peak at 429.83 nm using Aloe vera extract, the biosynthesized A. tinctoria-derived AgNPs demonstrated superior physicochemical properties. The smaller particle size (19.91 nm by TEM versus 80.31 nm by DLS in previous work) enabled better membrane penetration and cellular uptake33. Furthermore, At-AgNPs exhibited broader antifungal activity, including against resistant strains like C. glabrata (10.25 ± 0.40 mm) and C. auris (12.36 ± 0.29 mm), whereas prior studies only reported dose-dependent efficacy against C. albicans isolates (inhibition zones: 0–22 mm). Most notably, we demonstrated the enhanced synergistic effects with conventional antifungals. In this regard, the biogenic At-AgNPs improved clotrimazole efficacy by approximately 10% (37.28 mm versus 33.84 mm alone), while previous research focused solely on the antifungal activity of AgNPs alone. Moreover, a previous report have demonstrated the antifungal activity of AgNPs synthesized using date palm leaf extract, reporting inhibition zone diameters (IZD) of 18 mm and 21 mm against Aspergillus niger and C. albicans, respectively34. However, this study didn’t examine the synergistic potential of AgNPs with conventional antifungals. Our study addresses this gap by evaluating the ability of AgNPs to enhance the efficacy of standard antifungal agents.
The anticandidal mechanism of At-AgNPs employs a multimodal strategy that disrupts the structural and functional integrity of candidal cells, resulting in their lysis. In this context, AgNPs exhibit fungicidal effects mainly by interacting with the fungal cell membrane, disrupting its integrity, increasing permeability, and inducing leaking of internal contents, finally leading to cell lysis35. Additionally, AgNPs stimulate the production of reactive oxygen species (ROS) in cells, resulting in oxidative stress that damages lipids, proteins, and DNA, hence compromising cellular homeostasis and metabolic functions36,37. The At- AgNPs block critical enzymatic processes by attaching to sulphur- and phosphorus-containing groups in proteins and DNA, hence affecting functions such as cellular respiration, energy generation, and DNA replication38. Furthermore, AgNPs may infiltrate the cell wall and nucleus, resulting in DNA damage, strand breakage, and the inhibition of replication and transcription, so obstructing cell division39. Mitochondrial dysfunction represents a significant pathway, since AgNPs interfere with the electron transport chain, diminishing ATP synthesis and intensifying ROS generation40.
The emergence of antifungal-resistant strains underscores the urgent need for innovative combinatorial strategies. Leveraging the inherent antifungal efficacy of At-AgNPs, we systematically assessed their synergistic interactions with conventional antifungals against the tested Candida strains. This study’s findings illustrate the capability of At-AgNPs to augment the antifungal efficacy of conventional antifungal drugs, such as clotrimazole, fluconazole, nystatin, and terbinafine, against different candidal pathogens.
The most significant synergistic impact was seen with the combination of At-AgNPs and clotrimazole against C. albicans, shown by the substantial increase in the inhibition zone diameter (37.28 mm for the combination compared to 33.84 mm for clotrimazole alone). This improvement may be ascribed to the combined mechanistic effects of AgNPs and clotrimazole. Silver nanoparticles are recognized for their capability to damage fungal cell membranes via ROS generation and the compromise of candidal membrane integrity, while clotrimazole inhibits the manufacture of ergosterol, a vital constituent of candidal cell membranes41. The synergistic action probably increases membrane permeability and cellular damage, resulting in heightened antifungal activity. The synergistic impact seen with the combination of nystatin and terbinafine may be elucidated by the complimentary mechanisms of both drugs. Nystatin interacts with ergosterol, forming holes in the fungal membrane, whilst AgNPs further compromise the membrane integrity42. Terbinafine, an inhibitor of squalene epoxidase, interrupts ergosterol production43. When used in conjunction with AgNPs, it may enhance antifungal efficacy by simultaneously targeting membrane integrity and ergosterol synthesis44.
The variability in synergistic patterns among different Candida strains, such as C. albicans, C. krusei, and C. auris, can be attributed to differences in their intrinsic resistance mechanisms and membrane compositions. For instance, C. krusei and C. auris are known for their inherent fluconazole resistance due to alterations in drug target enzymes (e.g., lanosterol 14α-demethylase) and efflux pump overexpression. However, the combination of antifungals with AgNPs demonstrated improved antifungal activity against resistant strains, likely mediated by enhanced membrane permeability and drug internalization45. This work highlights the synergistic impact of combining AgNPs with conventional antifungals to address resistant fungal infections, especially when monotherapy is inadequate.
While this study demonstrates the promising antifungal synergy of At-AgNPs, several limitations should be noted. The lack of cytotoxicity evaluation against mammalian cells limits assessment of therapeutic selectivity, and physiological relevance requires verification through in vivo studies examining pharmacokinetics and safety profiles. Subsequent research efforts should focus on three key areas: establishing biosafety parameters through cytotoxicity profiling, validating efficacy in animal models, and investigating biofilm disruption potential given its clinical significance in Candida infections. Deeper mechanistic exploration of molecular targets, including membrane proteins and oxidative stress pathways, would further optimize these combinatorial approaches for clinical translation.
This study highlights the successful green synthesis of small (19.91 nm), monodisperse (PDI: 0.202) AgNPs utilizing A. tinctoria aqueous extract, which exhibit superior antifungal activity particularly against resistant Candida strains as C. glabrata. The synergistic activity of AgNPs with conventional antifungals (clotrimazole, nystatin, terbinafine) demonstrated a breakthrough in combating resistance. For instance, combining AgNPs with clotrimazole increased inhibition zones by ~ 10% against C. albicans, leveraging dual mechanisms: AgNPs disrupt membranes via ROS, while antifungals target ergosterol synthesis. These findings offer a promising solution to the growing crisis of antifungal resistance, providing a basis for developing novel combination therapies for fungal infections, particularly in immunocompromised patients. Future clinical studies should validate these results in vivo to accelerate translation into treatment protocols.
Conclusions
The rising prevalence of multidrug-resistant Candida infections underscores the urgent need for innovative therapeutic strategies. Our study demonstrates that green-synthesized At-AgNPs synergistically enhance conventional antifungals, particularly against resistant strains like C. auris and C. krusei, offering a promising avenue to combat antifungal resistance. The observed synergy with clotrimazole and nystatin highlights potential clinical applications, such as topical formulations for mucocutaneous candidiasis or medical device coatings to prevent biofilm-associated infections. To translate these findings, future research should prioritize in vivo validation of efficacy and safety in systemic and localized infection models, coupled with mechanistic studies to elucidate how At-AgNPs potentiate antifungal activity (e.g., via membrane disruption, efflux pump inhibition, or oxidative stress amplification). Additionally, rigorous biofilm eradication assays and cytotoxicity profiling in human cell lines are essential to assess clinical feasibility. Scalable, eco-friendly synthesis methods must also be optimized to ensure reproducible production. If successful, this approach could revolutionize infection management by reducing drug doses, minimizing toxicity, and curbing the economic burden of prolonged therapies particularly for disability patients.
These findings have important clinical implications for disability prevention, particularly in vulnerable populations where invasive candidiasis can lead to sepsis-related neurological damage, chronic organ dysfunction, or prolonged hospitalization. The observed synergy with clotrimazole and nystatin suggests practical applications in topical formulations for patients with limited mobility (preventing cutaneous candidiasis) or medical device coatings to reduce biofilm-associated infections in catheter-dependent individuals. By potentially lowering required drug doses, this approach may decrease medication-related complications that can exacerbate disability burdens. Future research should focus on safety optimization and scalable production to realize these benefits, ultimately aiming to reduce infection-related disabilities and improve quality of life for high-risk patients.
Methods
Chemicals and plant material
The Silver Nitrate (AgNO3) with ≥ 99.0% purity was purchased from Sigma Aldrich. The plant materials (A. tinctoria roots) were collected from local markets in Riyadh, Saudi Arabia. The plant material was identified by the Herbarium of Botany and Microbiology Department, College of Science, King Saud University and deposited with voucher number of KSU-202557.
Green synthesis and characterization of AgNPs
The roots were thoroughly rinsed with distilled water and left to dry completely. Once dried, the roots of A. tinctoria were finely powdered using a mechanical grinder. Next, 10 g of the powder was added to 250 mL flasks containing 100 mL of distilled water. The flask, covered with foil, was placed on a rotary shaker at 200 rpm for 24 h at room temperature. The root extract in water was passed through Whatman filter paper (No. 1) to achieve a clear filtrate. This solution was subsequently stored at 4 °C for later use. For AgNP biosynthesis, a solution containing 1 m M of silver nitrate (AgNO3) was prepared, and 10 mL of A. tinctoria aqueous extract was added to 90 mL of AgNO3 solution in a 250 mL flask. The flask was heated to 70 °C over a magnetic stirrer for 60 min. Following that, the solution’s colour shifted to brownish, indicating the synthesis of AgNPs. The At-AgNPs was centrifuged at 10,000 rpm for 60 min. After centrifugation, the supernatant was collected and discarded. To remove any impurities, the pellets were washed three times with distilled water and alcohol. Finally, the precipitates were allowed to dry at room temperature for further analysis. The At-AgNPs were physicochemically characterized using different techniques as UV, TEM, EDX, XRD, FTIR and zeta potential analysis.
Characterization of AgNPs
The biogenic AgNPs were initially analyzed using UV-Vis spectroscopy to assess their optical properties. The sample was dispersed in distilled water, and absorption spectra were recorded between 200 and 800 nm using a UV-Vis-NIR spectrophotometer (Shimadzu, Japan), with distilled water serving as the reference. For morphological examination, the nanoparticles were purified by repeated washing with deionized water, deposited onto carbon-coated copper grids, and imaged using a high-resolution transmission electron microscope (JEOL, Japan) operating at 100 kV. Elemental composition was verified through energy-dispersive X-ray spectroscopy (EDX) coupled with scanning electron microscopy (JEOL, Japan). Surface functional groups were identified via Fourier-transform infrared (FTIR) spectroscopy (Shimadzu, USA) in the 400–4000 cm⁻¹ range, with samples prepared as KBr pellets. Crystalline structure was analyzed by X-ray diffraction (XRD) using a Shimadzu diffractometer with Cu-Kα radiation, where diffraction patterns were compared with standard reference data (JCPDS). Additionally, colloidal stability and hydrodynamic size were evaluated using dynamic light scattering (Malvern Instruments, UK) to measure zeta potential and particle size distribution.
Antifungal sensitivity testing
Six strains of Candida species, including C. albicans ATCC 60,193, C. auris ATCC 5130, C. tropicalis ATCC 66,029, C. glabrata ATCC 20,013, C. krusei ATCC 14,243, and C. parapsilosis ATCC 22019, were used to evaluate their susceptibility to various conventional antifungal agents. These strains were evaluated for their sensitivity to widely used antifungal agents through the standard disk diffusion technique. The antifungal drugs tested–fluconazole, terbinafine, itraconazole, clotrimazole, nystatin, tioconazole, and natamycin were dissolved in methanol using ultrasonic sonication. Sterile filter paper discs (6 mm in diameter) were impregnated with the respective antifungal solutions at concentrations of 25, 30, 10, 10, 25, 30, and 50 µg/disc, respectively. To prepare the candidal suspension, 24-hour-old fungal colonies were collected with a sterile loop and suspended in a 0.85% sterile NaCl solution. The turbidity of the fungal suspension was adjusted to match the 0.5 McFarland standard, corresponding to approximately 10⁶ CFU/mL. Mueller Hinton agar (MHA) medium, supplemented with 0.5 µg/mL methylene blue and 2% glucose, was prepared and poured into Petri dishes. Each plate was inoculated with 0.2 mL of the standardized fungal inoculum. Antifungal discs were placed onto the agar surface, and the plates were incubated at 37 °C for 48 h. Methanol-only discs served as negative controls whereas terbinafine disks (30 µg) were used as positive controls. Following incubation, the inhibition zone diameters (IZD) were evaluated using Vernier calipers to assess the sensitivity of the Candida strains to the antifungal agents. The results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines, categorizing the strains as sensitive, intermediate, or resistant.
Screening of anticandidal activity of AgNPs
The antifungal effectiveness of A. tinctoria root extract (1.0 mg) and its biogenic At-AgNPs against various Candida strains was assessed using disk diffusion assay. To prepare the fungal suspension, 24-hour-old cultures of Candida were collected with a sterile loop and suspended in 0.85% sterile NaCl solution. The turbidity of the suspension was adjusted to 0.5 McFarland standard, corresponding to a cell density of approximately 106 CFU/mL. MHA medium, supplemented with 2% glucose and 0.5 µg/mL methylene blue, was prepared and poured into Petri dishes. Each plate was inoculated with 0.5 mL of the adjusted fungal suspension, which was evenly spread using sterile swabs. The At-AgNPs were suspended in methanol and sonicated to achieve uniform dispersion. Sterile discs were loaded with 1.0 mg of the At-AgNPs solution and also 1.0 mg of the root extract. Terbinafine was used as a positive control, with discs containing 30 µg of the drug. Methanol-only discs served as negative controls. The treated plates were refrigerated at 4 °C for 2 h to facilitate diffusion of the At-AgNPs, followed by overnight incubation at 37 °C. The IZD were evaluated using Vernier calipers.
The MIC of At-AgNPs was determined using the broth microdilution method, following the protocol described in CLSI document M27-Ed4. This was tested against C. parapsilosis, which exhibited the highest susceptibility to At-AgNPs. To detect the MFC, samples from MIC wells with no visible microbial growth were streaked onto MHA plates. For 48 h, these plates were kept in an incubator set at 35 ± 2 °C, and the least concentration that completely inhibited candidal growth was identified as the MFC.
Synergistic patterns of At-AgNPs with standard antifungal agents
The synergistic potential of At-AgNPs (1.0 mg) was assessed in combination with widely used antifungal agents: clotrimazole, fluconazole, nystatin, and terbinafine, representing distinct antifungal classes such as azoles, polyenes, and allylamines. Sterile discs were prepared with standard concentrations of the antifungal agents: 25 µg fluconazole, 10 µg clotrimazole, 25 µg nystatin, and 30 µg terbinafine. Another group of discs was prepared with the same antifungal concentrations combined with MIC of At-AgNPs (1.0 mg). Additionally, discs containing only methanol served as negative controls, while discs loaded solely with At-AgNPs (1.0 mg) were used to evaluate their individual antifungal efficacy.
The MHA plates were inoculated as previously described, and the prepared discs were placed onto the seeded agar surface. The inoculated plates were refrigerated at 4 °C for 2 h to facilitate At-AgNPs diffusion, followed by incubation at 25 °C for 24 h. After incubation, the plates were examined for IZD, and their IZD were measured using Vernier calipers. Synergistic effects were quantified using the formula: (B − A)/A × 100, where A denotes the IZD of the antifungal agent alone, and B denotes the IZD when combined with At-AgNPs. The fold increase in the inhibition area (IFA) was calculated using the following formula: IFA = (B² − A²)/A².
SEM analysis for detection of morphological deformations
To examine the structural changes induced by At-AgNPs on C. albicans, SEM analysis was conducted. Agar sections were carefully removed from the inhibition zones of At-AgNPs, Clotrimazole, and their combined treatment. These sections were then fixed for 1 h at 25 °C in a 3% (v/v) glutaraldehyde solution, prepared in 0.1 M sodium phosphate buffer at pH 7.2. After fixation, the agar pieces were rinsed four times with the same buffer solution. Subsequently, the samples underwent post-fixation in 1% (w/v) osmium tetroxide (OsO4) for an hour, followed by another four buffer rinses. Dehydration was achieved using a graded ethanol series (30–100%), with each concentration applied for 15 min. Once dried, the samples were affixed to stubs with double-sided carbon tape and sputter-coated with a thin layer of gold using a Polaron SC 502 coater. The prepared specimens were subsequently examined using a JEOL JSM-6380 LA scanning electron microscope for detailed analysis.
Statistical analysis
The data were statistically analyzed using GraphPad Prism v8.0 (GraphPad Software, Inc., USA) by applying one-way ANOVA followed by Tukey’s post hoc test at a significance level of p < 0.05. All experiments, including inhibition zone diameter measurements, were performed in triplicate, and results are expressed as mean ± standard error (SEM) of three independent replicates.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Garvey, M. & Rowan, N. J. Pathogenic drug resistant fungi: a review of mitigation strategies. Int. J. Mol. Sci. 24, 1584 (2023).
Sanyaolu, A. et al. Candida auris: an overview of the emerging drug-resistant fungal infection. Infect. Chemother. 54, 236 (2022).
Talapko, J. et al. Candida albicans—the virulence factors and clinical manifestations of infection. J. Fungi. 7, 79 (2021).
Kaur, R., Dhakad, M. S., Goyal, R. & Kumar, R. Emergence of non-albicans Candida species and antifungal resistance in intensive care unit patients. Asian Pac. J. Trop. Biomed. 6, 455–460 (2016).
Battles, H. & Gilmour, R. Beyond mortality: survivors of epidemic infections and the bioarchaeology of impairment and disability. Bioarchaeology Int. 6 (40-), 23 (2022).
Alanazi, K. H. et al. An overview of healthcare-associated Candida auris outbreaks in ministry of health hospitals–Saudi Arabia 2020–2022; retrospective multicentric study. PloS One. 20, e0313589 (2025).
Alshahrani, F. S. et al. in Healthcare. 3150 (MDPI).
Evren, K., Öncel, B. & Kömeç, S. Antifungal susceptibility pattern of Candida auris strains. J. Global Antimicrob. Resist. 39, 62 (2024).
Khairy, A. et al. Antifungal susceptibility pattern of Candida auris strains: analysis of clinical strains in a tertiary-care educational university hospital. new. Microbiol. 47, 152–156 (2024).
Soltys, L., Olkhovyy, O., Tatarchuk, T. & Naushad, M. Green synthesis of metal and metal oxide nanoparticles: principles of green chemistry and Raw materials. Magnetochemistry 7, 145 (2021).
Dey, N. et al. Role of nanomaterials in deactivating multiple drug resistance efflux pumps—a review. Environ. Res. 204, 111968 (2022).
Thapa, M. & Choudhury, S. R. Green synthesized nanoparticles: physicochemical properties and mode of antimicrobial activities. Compr. Anal. Chem. 94, 49–79 (2021).
Manisekaran, R. et al. Silver-nanoparticles‐based composites for antimicrobial applications: an update. ChemistrySelect 9, e202403772 (2024).
Verma, N. et al. Anticandidal efficacy of green synthesized silver nanoparticles using Trans-himalayan plant extracts against drug resistant clinical isolates of Candida auris. Indian J. Microbiol. 64, 1912–1928 (2024).
Pugazhenthi, E. et al. Cleome rutidosperma leaf extract mediated biosynthesis of silver nanoparticles and anti-candidal, anti-biofilm, anti-cancer, and molecular docking analysis. Biomass Convers. Biorefinery. 14, 28971–28983 (2024).
Mehboob, J. et al. Biosynthesis of silver lavandula angustifolia, alkanna tinctoria and its antimicrobial activities—a comparative study. Pakistan J. Weed Sci. Res. 27 (2021).
Raj, A. & Thomas, R. K. in Optical and Molecular Physics 43–69Apple Academic Press, (2021).
Huq, M. A., Ashrafudoulla, M., Rahman, M. M., Balusamy, S. R. & Akter, S. Green synthesis and potential antibacterial applications of bioactive silver nanoparticles: a review. Polymers 14, 742 (2022).
Melkamu, W. W. & Bitew, L. T. Green synthesis of silver nanoparticles using Hagenia abyssinica (Bruce) JF Gmel plant leaf extract and their antibacterial and anti-oxidant activities. Heliyon 7, , e08459(2021).
Ali, S. S. M., Aljawobaei, W., Rao, V. J., Nagar, P. S. & Robin, P. Sustainable synthesis of silver nanoparticles using Barleria prattensis extract: characterization and evaluation of their biological and catalytic activities. Biomass Convers. Biorefinery, 15, 17811–17828 (2025).
Ceylan, R., Demirbas, A., Ocsoy, I. & Aktumsek, A. Green synthesis of silver nanoparticles using aqueous extracts of three sideritis species from Turkey and evaluations bioactivity potentials. Sustainable Chem. Pharm. 21, 100426 (2021).
Yassin, M. T., Mostafa, A. A. F., Al-Askar, A. A. & Al-Otibi, F. O. Facile green synthesis of silver nanoparticles using aqueous leaf extract of Origanum Majorana with potential bioactivity against multidrug resistant bacterial strains. Crystals 12, 603 (2022).
Mostafa, A. A. et al. Evaluation of biological activities of chemically synthesized silver nanoparticles. Journal of Nanomaterials 789178 (2015). (2015).
Mohsen, E., El-Borady, O. M., Mohamed, M. B. & Fahim, I. S. Synthesis and characterization of Ciprofloxacin loaded silver nanoparticles and investigation of their antibacterial effect. J. Radiation Res. Appl. Sci. 13, 416–425 (2020).
Jaffar, S. S. et al. Green synthesis, characterization and antimicrobial efficacy of silver nanoparticles from Kappaphycus Alvarezii extract. Res. Chem. Intermed. 50, 3435–3452 (2024).
Aljeldah, M. M., Yassin, M. T., Mostafa, A. A. F. & Aboul-Soud, M. A. Synergistic antibacterial potential of greenly synthesized silver nanoparticles with fosfomycin against some nosocomial bacterial pathogens. Infection Drug Resistance, 16, 125–142 (2023).
Mtavangu, S. G., Machunda, R. L. van der Bruggen, B. & Njau, K. N. In situ facile green synthesis of Ag–ZnO nanocomposites using tetradenia riperia leaf extract and its antimicrobial efficacy on water disinfection. Sci. Rep. 12, 15359 (2022).
Tuzun, B. S., Fafal, T., Ozguney, I. & Kivcak, B. Green synthesis of silver nanoparticles by using anthemis tricolor boiss., factorial design for parameter optimization, characterization and in-vitro biological activities. J. Pharm. Innov. 19, 32 (2024).
Chatterjee, N., Pal, S. & Dhar, P. Green silver nanoparticles from bacteria-antioxidant, cytotoxic and antifungal activities. Next Nanatechnol. 6, 100089 (2024).
Alharbi, N. S. & Alsubhi, N. S. Green synthesis and anticancer activity of silver nanoparticles prepared using fruit extract of Azadirachta indica. J. Radiation Res. Appl. Sci. 15, 335–345 (2022).
Wu, S., Wang, Y., Liu, N., Dong, G. & Sheng, C. Tackling fungal resistance by biofilm inhibitors. J. Med. Chem. 60, 2193–2211 (2017).
Ibe, C., Otu, A. & Pohl, C. H. Mechanisms of resistance to cell wall and plasma membrane targeting antifungal drugs in Candida species isolated in Africa. Expert Rev. Anti-infective Therapy, 1, 91-104 (2025).
Arsène, M. M. J. et al. Antifungal activity of silver nanoparticles prepared using Aloe Vera extract against Candida albicans. Veterinary World. 16, 18 (2023).
Aty, A. E. A. A. Hafr al Batin Phoenix dactylifera L. Leaves extract as efficient catalyst for green synthesis of new silver nanoparticles with broad spectrum antimicrobial activity: characterization and evaluation compared to fungi. Arabian J. Sci. Engineering, 1–16 (2025).
Liu, F., Chen, Y., Huang, Y., Jin, Q. & Ji, J. Nanomaterial-based therapeutics for enhanced antifungal therapy. J. Mater. Chem. B. 12, 9173–9198 (2024).
Barkat, M. A. et al. Current progress in synthesis, characterization and applications of silver nanoparticles: precepts and prospects. Recent Pat. Anti-infect. Drug Discov. 13, 53–69 (2018).
Rodrigues, A. S. et al. Advances in silver nanoparticles: a comprehensive review on their potential as antimicrobial agents and their mechanisms of action elucidated by proteomics. Front. Microbiol. 15, 1440065 (2024).
Girma, A. et al. Green-synthesised silver nanoparticles: antibacterial activity and alternative mechanisms of action to combat multidrug-resistant bacterial pathogens: a systematic literature review. Green Chem. Lett. Rev. 17, 2412601 (2024).
Do, H. T. T. et al. Advances in silver nanoparticles: unraveling biological activities, mechanisms of action, and toxicity. Appl. Nanosci. 15, 1 (2025).
Singh, J. et al. Comprehensive antifungal investigation of green synthesized silver nanoformulation against four agriculturally significant fungi and its cytotoxic applications. Sci. Rep. 14, 5934 (2024).
Biswas, B. & Thakur, A. in Recent Advances in Human Fungal Diseases: Progress and Prospects 43–64Springer, (2024).
Simm, C. et al. Gladiolin produced by pathogenic burkholderia synergizes with amphotericin B through membrane lipid rearrangements. mBio 15, e02611–02624 (2024).
Nowosielski, M. et al. Detailed mechanism of squalene epoxidase Inhibition by terbinafine. J. Chem. Inf. Model. 51, 455–462 (2011).
Ullah, A. et al. Synergistic antifungal activity of terbinafine in combination with Light-Activated Gelatin–Silver nanoparticles against Candida albicans strains. Pharmaceutics 17, 125 (2025).
Hussain, M. A. et al. Combination therapy of clinically approved antifungal drugs is enhanced by conjugation with silver nanoparticles. Int. Microbiol. 22, 239–246 (2019).
Acknowledgements
The authors extend their appreciation to the King Salman Center For Disability Research for funding this work through Research Group no KSRG-2024-366.
Funding
King Salman Center For Disability Research.
Author information
Authors and Affiliations
Contributions
“Conceptualization, M.T.Y., K.S.A. and F.O.A.; writing—original draft preparation, M.T.Y.; Investigation, K.S.A.; writing—review and editing, F.O.A. and M.T.Y.; visualization, K.S.A..; supervision, A.A.A. and F.O.A.; project administration, F.O.A. and A.A.A.; funding acquisition, F.O.A. All authors have read and agreed to the published version of the manuscript.”
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alnahdi, K.S., Al-Otibi, F.O., Al-Askar, A.A. et al. Anticandidal activity of greenly synthesized silver nanoparticles formulated using Alkanna tinctoria roots against multidrug resistant candidal pathogens. Sci Rep 15, 34645 (2025). https://doi.org/10.1038/s41598-025-19831-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-19831-9