Abstract
To investigate the association between the Pan-Immune-Inflammatory Value (PIV) and the risk of latent tuberculosis infection (LTBI) using a nationally representative sample from the United States. This study utilized data from 4877 adult participants in the National Health and Nutrition Examination Survey (NHANES) 2011–2012 cycle. PIV was calculated using neutrophil, monocyte, platelet, and lymphocyte counts. LTBI was assessed using the QuantiFERON-TB Gold In-Tube (QFT-GIT) test. Multivariable logistic regression was used to evaluate the association between PIV and LTBI risk. Subgroup analyses and Restricted Cubic Spline (RCS) analysis were performed. Cox regression analysis was used to examine the association of PIV with all-cause mortality among LTBI patients. Individuals with LTBI had significantly lower PIV levels compared to those without LTBI. Logistic regression analysis showed a significant negative correlation between PIV and LTBI risk, even after adjusting for covariates. The RCS analysis confirmed a dose-response relationship. The association remained significant across various demographic subgroups. Among LTBI patients, those in the highest PIV quartile had a significantly increased risk of all-cause mortality after full adjustment. PIV is negatively associated with the risk of LTBI. Additional extensive prospective investigations are required to validate these results.
Similar content being viewed by others
Introduction
Latent tuberculosis infection (LTBI) poses a significant global public health concern, with approximately one-quarter of the world’s population estimated to be infected with mycobacterium tuberculosis1,2,3. As a non-infectious, asymptomatic immunological state, LTBI acts as a persistent reservoir, perpetuating the transmission and emergence of new tuberculosis cases2,4.
As we all known, host immune responses and inflammatory processes are critically involved in both the occurrence and progression of LTBI5. Effective cellular immunity can suppress the proliferation and spread of Mycobacterium tuberculosis, while excessive inflammatory activity may result in tissue injury and facilitate the shift from latent to active disease6. Thus, there is a pressing need for comprehensive biomarkers that accurately reflect the host’s immune-inflammatory status to enhance risk assessment and diagnostic stratification for LTBI7. The pan-immune-inflammation value (PIV) is a recently introduced biomarker that combines indicators of systemic inflammation and immune function, calculated from routine blood cell counts-including neutrophils, monocytes, platelets, and lymphocytes8,9,10. Prior studies have demonstrated that PIV exhibits superior predictive value for outcomes in cancer and for assessing the risk of inflammation-driven conditions, outperforming conventional single or composite markers9,11,12,13,14,15. While composite inflammatory indices such as the systemic immune-inflammation index (SII) and neutrophil-to-lymphocyte ratio (NLR) have been found to be negatively associated with latent tuberculosis infection (LTBI)7,16, systematic research specifically investigating the relationship between PIV and LTBI risk remains scarce.
Given this gap, the present study utilizes data from the nationally representative National Health and Nutrition Examination Survey (NHANES) in the United States to analyze the association between PIV levels and the risk of LTBI.
Methods
Data source
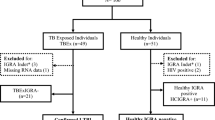
NHANES, administered by the Centers for Disease Control and Prevention (CDC), is a nationwide study that utilizes personal interviews, physical measurements, and laboratory tests to capture detailed demographic, health, and nutrition data from a representative sample of US residents, providing data critical for research and public health strategies17. This study utilized data from participants in the NHANES 2011–2012 cycle. We excluded subjects under 20 years of age, those who were pregnant, reported having active tuberculosis, or lacked data on QuantiFERON-TB Gold In-Tube (QFT-GIT), PIV, and mortality (Fig. 1). After applying these criteria, 4877 participants remained, including 497 individuals identified with LTBI and 4380 without LTBI. Ethical approval for research involving human subjects was obtained from the Ethics Review Board of the National Center for Health Statistics (https://www.cdc.gov/nchs/nhanes/about/erb.html). All methods were performed in accordance with the relevant guidelines and regulations, and the study protocol complies with the principles of the Declaration of Helsinki.
Definition of PIV and LTBI
The PIV, utilized as the measure of exposure, was determined using the following equation: PIV = [neutrophil count (10^9/L) × monocyte count (10^9/L) × platelet count (10^9/L)] divided by lymphocyte count (10^9/L)10. LTBI was assessed using the QFT-GIT test, which was performed according to the manufacturer’s instructions. Peripheral blood samples were incubated with Mycobacterium tuberculosis-specific antigens at 37 °C for a duration of 16 to 24 h, after which interferon-gamma (IFN-γ) concentrations were determined by enzyme-linked immunosorbent assay (ELISA). Interpretation of results followed the CDC recommendations for interferon-gamma release assays (IGRAs). Participants with a positive QFT-GIT were identified as having LTBI, and those with negative results were considered LTBI negative18. If QFT-GIT results were indeterminate, a positive purified protein derivative (PPD) test-defined as an induration of 10 mm or greater-was used to classify the individual as LTBI positive, irrespective of risk factors19,20.
All-cause mortality
Given that LTBI is a non-infectious, asymptomatic immunological state that does not itself cause death, the mortality analysis was prespecified as exploratory and descriptive, intended to assess whether baseline systemic inflammation (indexed by PIV) correlates with overall health status among individuals with LTBI. We analyzed all-cause mortality outcomes for LTBI patients using the NHANES Public-Use Linked Mortality File, which provides follow-up data on participants through probabilistic linkage to the National Death Index until December 31, 2019. The follow-up period began at the baseline examination at the mobile examination center and continued until death or the end of the observation period.
Covariates
Data on several covariates were collected and included in the analysis. These potential covariates comprised age (years), gender (female/male), and race (Mexican American/Non-Hispanic Black/Non-Hispanic White/Other Hispanic/Other Races), marital status (married or not), poverty income ratio (PIR), education level (above high school or not), smoking history (yes/no), body mass index (BMI, kg/m2), Hemoglobin A1c (HbA1c, %), diabetes (yes/no), hypertension (yes/no), cardiovascular disease (yes/no), serum uric acid (SUA, µmol/L), and the estimated glomerular filtration rate (eGFR, mL/min/1.73m2). Calculation of eGFR was based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula, integrating variables such as age, gender, race, and serum creatinine21. Diabetes and hypertension were based on self-reported medical histories. Cardiovascular diseases (CVDs) status was determined based on self-reported history of heart attack, stroke, heart failure, coronary artery disease, or angina.
Statistical analysis
Statistical analyses followed CDC recommendations and accounted for NHANES’s sampling weights. For continuous variables, median and interquartile range was reported, while categorical variables were summarized as counts and weighted proportions. Group differences were analyzed using the Kruskal-Wallis test and the chi-square test. The association between PIV and LTBI risk was evaluated using logistic regression models (Model 1: without covariate adjustment; Model 2: adjusted for age, gender, and race; Model 3: adjusted for age, gender, race, marital status, PIR, education, smoking, BMI, HbA1c, diabetes, hypertension, CVDs, SUA, and eGFR). Potential nonlinear patterns were explored with Restricted Cubic Spline (RCS)22. Subgroup analyses assessed potential effect modifiers, stratifying by age, gender, BMI, diabetes, hypertension, cardiovascular disease, and eGFR. Multivariate Cox regression examined the association of PIV with all-cause mortality among LTBI patients. Sensitivity analyses were conducted by excluding participants with major noncommunicable diseases (diabetes, hypertension, CVDs) and CKD (eGFR < 60 mL/min/1.73 m²) to assess the robustness of the results. PIV levels were also compared between individuals with LTBI and those who self-reported active TB in NHANES. All analyses were conducted with R version 4.2.1, and a two-sided P value < 0.05 denoted statistical significance.
Results
Baseline characteristics
A total of 4877 adults were stratified by LTBI status for demographic and clinical characteristic comparisons (Table 1). Compared to the non-LTBI group, participants in the LTBI group were older and had a higher proportion of males (P < 0.001). The LTBI group also exhibited a lower PIR, a lower percentage of higher education attainment, and a higher prevalence of smoking history (P < 0.05). For clinical indicators, the LTBI group had higher levels of HbA1c and a higher prevalence of diabetes and hypertension (P < 0.01). There were no statistically significant differences between groups regarding BMI, CVDs, and SUA levels (P > 0.05). Moreover, the LTBI group exhibited lower eGFR as well as significantly lower PIV (P < 0.01). Table 2 shows that participants in higher PIV quartiles were older and had lower PIR and education levels (P < 0.01). Higher PIV was associated with increased smoking, higher BMI and HbA1c, and greater rates of diabetes, hypertension, and CVDs (P < 0.001). Significant differences in SUA and eGFR were observed across quartiles (P < 0.001). The prevalence of LTBI was lowest in the highest PIV group (P = 0.001).
Logistic regression analysis of PIV and LTBI risk
Logistic regression revealed a significant positive link between PIV (as a continuous variable) and LTBI risk (see Table 3). In unadjusted Model 1, increased log (PIV) values were associated with decreased LTBI risk (OR = 0.565, 95%CI 0.415–0.768, P < 0.001). This relationship persisted after adjusting for covariates in Models 2 (adjusted for age, gender, and race) and 3 (adjusted for age, gender, race, marital status, PIR, education, smoking, BMI, HbA1c, diabetes, hypertension, CVDs, SUA, and eGFR), with adjusted ORs of 0.598 (95%CI 0.431–0.830, P = 0.002) and 0.597 (95%CI 0.415–0.858, P = 0.005), respectively. When categorizing PIV into quartiles (Q1-Q4) and fully adjusting for confounders, the risk of LTBI changed across quartiles (Q1: reference; Q2: OR = 1.167 (95%CI 0.879–1.550); Q3: OR = 0.979 (95%CI 0.727–1.318); Q4: OR = 0.610 (95% CI 0.439–0.848) (P for trend = 0.003).
RCS and subgroup analyses
The results from RCS analysis, adjusted for confounding factors including age, gender, race, marital status, PIR, education, smoking, BMI, HbA1c, diabetes, hypertension, CVDs, SUA, and eGFR, reinforced the presence of a dose-response association between PIV and LTBI, as depicted in Fig. 2 (P for nonlinear = 0.062). To assess the robustness of this relationship, subgroup analyses were conducted across different demographic groups, while controlling for the above covariates. As shown in Fig. 3, the positive association between PIV and LTBI risk remained significant irrespective of demographic factors, including age, gender, BMI, diabetes, hypertension, cardiovascular disease, and eGFR, with no significant interaction detected (P for interaction > 0.05).
Cox regression analysis of PIV and all-cause mortality risk in LTBI patients
Among 497 patients diagnosed with LTBI, 64 all-cause deaths were recorded over a median follow-up period of 93 months. Baseline Cox regression analysis indicated that elevated baseline log (PIV) was not associated with an increased all-cause mortality risk (P = 0.178). Nevertheless, after comprehensive adjustment (age, gender, race, marital status, PIR, education, smoking, BMI, HbA1c, diabetes, hypertension, CVDs, SUA, and eGFR), individuals in the highest quartile faced a 2.748-fold greater risk of all-cause mortality compared to those in the lowest quartile (OR = 2.748, 95%CI 1.131–6.677, P = 0.026).(Table 4).
Sensitivity analyses and PIV in active-TB patients
Sensitivity analyses were performed to evaluate the robustness of the findings, excluding participants with major non-communicable diseases (diabetes, hypertension, CVDs) and those with CKD (eGFR < 60 mL/min/1.73 m2). The association between PIV and LTBI remained directionally consistent and of similar magnitude compared with the primary analysis (Supplementary Table 1). In the unadjusted Model 1 and the adjusted Models 2 (adjusted for age, gender, and race) and 3 (adjusted for age, gender, race, marital status, PIR, education, smoking, BMI, HbA1c, SUA, and eGFR), the ORs were 0.559 (95%CI 0.353–0.887, P = 0.013), 0.648 (95%CI 0.400–1.050, P = 0.078), and 0.586 (95%CI 0.344–0.997, P = 0.049), respectively. Additionally, we compared PIV levels between participants with LTBI and those with self-reported active TB (Supplementary Table 2). PIV levels tended to be higher in individuals with active TB than in those with LTBI (203.44 (146.15-333.49) vs. 197.27 (128.06–294.00), P = 0.191); however, this difference did not reach statistical significance, likely due to the relatively small sample size.
Discussion
This study is the first to systematically investigate the relationship between the PIV and LTBI, and it uncovers a significant negative correlation between these two factors. Furthermore, after adjusting for confounding factors, we found that highest PIV quartile levels were positively associated with an increased risk of all-cause mortality among LTBI patients. These findings provide valuable new perspectives for tuberculosis infection prevention and control strategies, as well as for the assessment of host immune status.
PIV, which integrates peripheral blood indices such as neutrophils, platelets, monocytes, and lymphocytes, serves as a comprehensive biomarker of immune and inflammatory status10. It has been validated as a prognostic factor in multiple conditions, including cancer and cardiovascular disorders9,23,24. Nevertheless, its significance in non-infectious, asymptomatic immunological diseases like LTBI has not been previously established. In this cross-sectional analysis, individuals with LTBI had lower PIV than IGRA-negative controls; However, this association should be interpreted cautiously and does not imply causation. Physiologically, early Mycobacterium tuberculosis infection rarely prompts systemic inflammation, and LTBI hinges on finely balanced adaptive immunity25. Reduced neutrophil, monocyte, and platelet counts may reflect subdued inflammation, while maintained lymphocyte levels support adaptive responses25,26. Distinct from active tuberculosis, LTBI individuals generally sustain lower systemic inflammation27. This negative correlation with PIV may reflect the “immunological standstill” characteristic of LTBI, where inflammatory activation remains limited, favoring pathogen persistence and immune equilibrium27,28. Importantly, PIV captures systemic inflammatory burden rather than pathogen- or pathway-specific immunity. Given the dynamic and heterogeneous nature of LTBI-encompassing possible sterilizing immunity (“resisters”), transient subclinical infection, and shifting host-pathogen equilibria-single, static PIV measurements may not capture relevant immunologic nuances. Accordingly, our findings are hypothesis-generating and suggest that accessible inflammatory composites might complement, rather than replace, TB-specific immune markers.
We also observed higher PIV levels are associated with an increased mortality risk in individuals with LTBI. Elevated PIV indicates heightened systemic inflammation, which could result from reactivation of latent tuberculosis, coexisting chronic diseases, or other underlying conditions. Prior studies have also shown that elevated PIV is linked to higher all-cause mortality, particularly in chronic disease and cancer29,30. In the LTBI subgroup, higher PIV likely serves as a nonspecific indicator of overall health status and inflammatory burden rather than a marker of LTBI-related lethality. These exploratory findings warrant cautious interpretation and replication in cohorts with richer comorbidity and cause-of-death data.
Several limitations should be acknowledged in this study. Firstly, the cross-sectional nature of the NHANES dataset does not permit inference of causality between PIV levels and risk of LTBI. Secondly, despite adjustments for relevant covariates, unmeasured confounders such as human immunodeficiency virus (HIV) serostatus, chronic obstructive pulmonary disease (COPD), and co-infections may have influenced our results. Additionally, peripheral blood cell counts may not fully capture tissue-specific immune perturbations relevant to TB pathogenesis, particularly within the lung microenvironment where TB primarily manifests. Thirdly, PIV and related laboratory markers were assessed only once, preventing analysis of their temporal fluctuations or impacts on LTBI and mortality risk over time. Finally, because our sample comprised exclusively of US residents, generalizability to other settings with differing demographics or burdens of tuberculosis may be limited.
Conclusion
This study reveals that lower PIV is associated with a higher risk of LTBI. Within the LTBI population, elevated PIV levels were associated with increased all-cause mortality in exploratory analyses, likely reflecting overall systemic inflammation and comorbidity burden.
Data availability
This study draws on data from the NHANES database, available to the public at https://wwwn.cdc.gov/nchs/nhanes. For access to the summary data underpinning these results, please contact the corresponding author.
References
Suárez, I. et al. The diagnosis and treatment of tuberculosis. Dtsch. Arztebl Int. 116 (43), 729–735. https://doi.org/10.3238/arztebl.2019.0729 (2019).
Mangione, C. M. et al. Screening for latent tuberculosis infection in adults: US preventive services task force recommendation statement. Jama 329 (17), 1487–1494. https://doi.org/10.1001/jama.2023.4899 (2023).
Houben, R. M. & Dodd, P. J. The global burden of latent tuberculosis infection: A re-estimation using mathematical modelling. PLoS Med. 13 (10), e1002152. https://doi.org/10.1371/journal.pmed.1002152 (2016).
Qi, M., Qiao, R. & He, J. Q. The association between triglyceride-glucose index and related parameters and risk of tuberculosis infection in American adults under different glucose metabolic states: A cross-sectional study. BMC Public. Health. 25 (1), 966. https://doi.org/10.1186/s12889-025-21793-6 (2025).
Cliff, J. M., Kaufmann, S. H., McShane, H., van Helden, P. & O’Garra, A. The human immune response to tuberculosis and its treatment: A view from the blood. Immunol. Rev. 264 (1), 88–102. https://doi.org/10.1111/imr.12269 (2015).
Li, X. et al. Clofazimine inhibits innate immunity against mycobacterium tuberculosis by NF-κB. mSphere 9 (8), e0025424. https://doi.org/10.1128/msphere.00254-24 (2024).
Wang, L. et al. Association of systemic inflammation response index with latent tuberculosis infection and all-cause mortality: A cohort study from NHANES 2011–2012. Front. Immunol. 161538132. https://doi.org/10.3389/fimmu.2025.1538132 (2025).
Kuang, T. et al. Pan-immune inflammation value as a prognostic biomarker for cancer patients treated with immune checkpoint inhibitors. Front. Immunol. 151326083 https://doi.org/10.3389/fimmu.2024.1326083 (2024).
Hai-Jing, Y., Shan, R. & Jie-Qiong, X. Prognostic significance of the pretreatment pan-immune-inflammation value in cancer patients: an updated meta-analysis of 30 studies. Front. Nutr. 101259929 https://doi.org/10.3389/fnut.2023.1259929 (2023).
Corti, F. et al. The Pan-Immune-Inflammation value in microsatellite instability-high metastatic colorectal cancer patients treated with immune checkpoint inhibitors. Eur. J. Cancer. 150155–150167. https://doi.org/10.1016/j.ejca.2021.03.043 (2021).
Guven, D. C. et al. The association between the Pan-Immune-inflammation value and cancer prognosis: A systematic review and meta-analysis. Cancers. 14 (11). https://doi.org/10.3390/cancers14112675 (2022).
Yang, X. C. et al. Prognostic value of pan-immune-inflammation value in colorectal cancer patients: A systematic review and meta-analysis. Front. Oncol. 121036890 https://doi.org/10.3389/fonc.2022.1036890 (2022).
Qi, X. et al. Clinical utility of the pan-immune-inflammation value in breast cancer patients. Front. Oncol. 131223786. https://doi.org/10.3389/fonc.2023.1223786 (2023).
Okyar Baş, A. et al. Pan-immune inflammation value: A novel biomarker reflecting inflammation associated with frailty. Aging Clin. Exp. Res. 35 (8), 1641–1649. https://doi.org/10.1007/s40520-023-02457-0 (2023).
Shen, Y., Chen, L. & Che, G. Could pretreatment pan–immune–inflammation value predict survival in esophageal cancer? Ann. Surg. Oncol. 31 (6), 3868–3869. https://doi.org/10.1245/s10434-024-15100-7 (2024).
Huang, Y., Ao, T., Zhen, P. & Hu, M. Neutrophil-to-lymphocyte ratio and its association with latent tuberculosis infection and all-cause mortality in the US adult population: A cohort study from NHANES 2011–2012. Front. Nutr. 111467824. https://doi.org/10.3389/fnut.2024.1467824 (2024).
Wu, W. T. et al. Data mining in clinical big data: The frequently used databases, steps, and methodological models. Mil Med. Res. 8 (1), 44. https://doi.org/10.1186/s40779-021-00338-z (2021).
Chen, G., Wang, H. & Wang, Y. Clinical application of QuantiFERON-TB gold in-tube in the diagnosis and treatment of tuberculosis. Eur. J. Clin. Microbiol. Infect. Dis. 39 (4), 607–612. https://doi.org/10.1007/s10096-019-03768-9 (2020).
Khan, K. et al. Tuberculosis infection in the united states: National trends over three decades. Am. J. Respir Crit. Care Med. 177 (4), 455–460. https://doi.org/10.1164/rccm.200706-950OC (2008).
Liu, Y. et al. Association between hematological inflammatory markers and latent TB infection: insights from NHANES 2011–2012 and transcriptomic data. Front. Cell. Infect. Microbiol. 151556048. https://doi.org/10.3389/fcimb.2025.1556048 (2025).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150 (9), 604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006 (2009).
Arnes, J. I., Hapfelmeier, A., Horsch, A. & Braaten, T. Greedy knot selection algorithm for restricted cubic spline regression. Front. Epidemiol. 31283705 https://doi.org/10.3389/fepid.2023.1283705 (2023).
Jin, C., Li, X., Luo, Y., Zhang, C. & Zuo, D. Associations between pan-immune-inflammation value and abdominal aortic calcification: A cross-sectional study. Front. Immunol. 151370516 https://doi.org/10.3389/fimmu.2024.1370516 (2024).
Gu, P. et al. The predictive value of pan-immune inflammatory index for early recurrence of atrial fibrillation after cryoablation. BMC Cardiovasc. Disord. 24 (1), 669. https://doi.org/10.1186/s12872-024-04329-5 (2024).
Chi, H., Pepper, M. & Thomas, P. G. Principles and therapeutic applications of adaptive immunity. Cell 187 (9), 2052–2078. https://doi.org/10.1016/j.cell.2024.03.037 (2024).
Bonilla, F. A. & Oettgen, H. C. Adaptive immunity. J. Allergy Clin. Immunol. 125 (2 Suppl 2), S33–40. https://doi.org/10.1016/j.jaci.2009.09.017 (2010).
Barry, C. E. et al. The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nat. Rev. Microbiol. 7 (12), 845–855. https://doi.org/10.1038/nrmicro2236 (2009).
Sia, J. K. & Rengarajan, J. Immunology of Mycobacterium tuberculosis infections. Microbiol. Spectr. 7 (4). https://doi.org/10.1128/microbiolspec.GPP3-0022-2018 (2019).
Mardan, M. et al. Comprehensive analysis of pan-immune inflammation and all-cause mortality in rheumatoid arthritis: A database-driven approach, 1999–2018. Front. Immunol. 161549955 https://doi.org/10.3389/fimmu.2025.1549955 (2025).
Lee, L. E. et al. Pan-immune-inflammation value at diagnosis independently predicts all-cause mortality in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Clin. Exp. Rheumatol. 39 (2), 88–93. https://doi.org/10.55563/clinexprheumatol/m46d0v (2021).
Author information
Authors and Affiliations
Contributions
Xiaoyan Ye conceived and designed the study, supervised the research process, and was responsible for the overall manuscript revision. Xia Kang contributed to data cleaning, statistical analysis, and interpretation of the results. Hui Zhang participated in literature review, data extraction, and drafting of the manuscript. Wenting Wang assisted in methodology development and figure preparation. All authors critically revised the manuscript, approved the final version for submission, and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethical approval for research involving human subjects was obtained from the Ethics Review Board of the National Center for Health Statistics (https://www.cdc.gov/nchs/nhanes/about/erb.html).
Informed consent
All participants gave their written informed consent prior to enrollment in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ye, X., Kang, X., Zhang, H. et al. Pan-Immune-Inflammatory value and risk of latent tuberculosis infection. Sci Rep 15, 36089 (2025). https://doi.org/10.1038/s41598-025-20080-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-20080-z