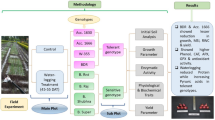
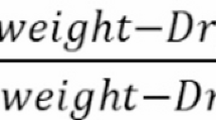Abstract
Drought is a major constraint that limits onion productivity. This current study explored the physio-biochemical, morphological, and yield attributes of 14 onion genotypes during the bulb development stage under drought conditions. A greenhouse experiment was conducted using a Completely Randomized Design (CRD) with four replications and two treatments. Drought stress was applied for a duration of 30 days. Drought reduced all morpho-physiological parameters of onion genotypes compared to the control. Drought significantly increased (p < 0.001) hydrogen peroxide (H2O2) levels in onion plants. The genotypes Goudami and Prema showed higher levels of catalase and ascorbate peroxidase. Additionally, Prema and Goudami exhibited the highest proline content, while Red_Creole had the lowest. Yields were significantly reduced (p < 0.001), resulting in a 49.58% loss compared to the control. The yield of onion genotypes was strongly positively associated with several factors, including stress tolerance index, bulb diameter, proline, drought tolerance efficiency, ascorbate peroxidase, catalase, relative water content, total chlorophyll, and stomata number. Prema, Goudami, and Red_Jewel recorded higher stress tolerance index, antioxidant activity, proline, chlorophyll, drought tolerance efficiency, stomatal aperture, and better yield; therefore, they were categorized as tolerant. The local variety, Rouge_Tama ARES, IDOL, AVON1317, AVON1074, Synthetique, Violet_Galmi, Dayo, and SAFARI were considered intermediate, displaying lower chlorophyll, stress tolerance index, potential activity of PS II, and proline. In contrast, Red_Creole was identified as a sensitive genotype, showing low morphological traits, drought tolerance efficiency, and a high percentage of yield reduction. Drought-tolerant onion genotypes identified could be essential for farmers and future improvement programs in drought-prone regions. This study emphasizes the significance of these tolerant onions for effectively responding to drought while maintaining bulb quality and productivity.
Similar content being viewed by others
Introduction
Globally, climate change is a 30-year shift in regional climatology, with the most adverse effects observed in Africa, Asia, and Central and South America1,2. The consequences of changes in climate are extreme temperature, freezing stress, water stress, like drought, floods, and soil salinity3. Water deficit is the most vital restraining factor for agricultural production. In recent decades, crop productivity, particularly for horticultural crops, has been significantly affected by severe water deficit stress4. It is well established that during drought conditions, plants struggle to uptake sufficient moisture, which in turn impacts their physiological and biochemical processes5. Furthermore, water deficits can severely affect the productivity of major crops, leading to losses of up to 50% or even complete crop failure6,7.
Onions are significant for human health owing to their nutritional content, which includes carbohydrates, proteins, lipids, minerals, vitamins, quercetin, and polyphenols8,9. Onions have promising antimicrobial activity and are used in the treatment and/or prevention of sickness, such as hypercholesterolemia, diabetes, hypertension, and syndrome coronavirus 210,11. Onion genotypes are produced in the dry seasons in most African countries, and therefore, are susceptible to drought stress. Onion has a small, shallow root system with maximum roots in about 0.18 m; however, irrigation water that moves below 0.76 m is not accessible to the onion crop12. Drought adversely impacts various morphological traits, including plant height, leaf number, leaf length, width, and area. This, in turn, can lead to a reduction in bulb yield of up to 65%13,14. A supplementary study showed that dry spells caused by climate variability have reduced global bulb production by approximately 30%15,16. The onion yield is the key characteristic used for screening genotypes’ resilience to drought, supplemented by secondary indicators such as plant water status, biochemical, and physiological characters15. The level of damage to bulb yield varies depending on the cultivar and phenological stage during which drought stress occurs13. Additionally, water stress impacts the physiological and biochemical parameters of onion plants. Numerous studies have documented reductions in chlorophyll a, chlorophyll b, carotenoid, and total chlorophyll, relative water content, membrane, and stability index during drought stress in onion plants17,18. Biochemical analyses revealed that water deficit decreases the synthesis of phenols, flavonoids, tannins, and pyruvic acid as secondary metabolites19,20, while it increases proline accumulation in onion plants20,21. To adapt to drought stress, plants must alter their morphological, physiological, and biochemical parameters. For instance, tolerant genotypes under drought stress exhibit higher values of parameters such as chlorophyll a, chlorophyll b, carotenoid, total chlorophyll, index of membrane stability, drought tolerance efficiency (> 90%), RWC, integrity of membrane, water use efficiency, activities of antioxidant enzymes, and proline content21,22,23, while sensitive genotypes demonstrate low levels of these parameters17,19. The plants close their stomata to control water loss as their response to water-deficit stress conditions24. They also produce various ROS, like superoxide anion (O2−) and hydrogen peroxide (H2O2), which can affect chlorophyll content, chlorophyll fluorescence, and photosynthetic activity24,25,26. To mitigate oxidative damage, plants produce many antioxidants, including catalase (CAT), ascorbate peroxidase (APX), and SOD (superoxide dismutase)24,27. During drought conditions, plants can keep their turgidity due to the high proline content produced to protect leaf membrane cells from damage28,29. Nevertheless, there is a lack and insufficient knowledge regarding the biochemical and physiological responses of onions to water stress14,19.
Thus, this current investigation objectives were to (1) examine the drought stress effects on morphological, physiological, and yield parameters of different onion genotypes, and (2) explore the role of biochemical traits in drought stress tolerance.
This study hypothesized that (i) induced drought conditions reduce stomata density, chlorophyll content and chlorophyll a fluorescence, resulting in stunted plant growth, reduced biomass, and yield losses; and (ii) onion plants produce increased amounts of osmolytes and antioxidant enzymes to maintain their osmotic potential and protect leaf membrane cells from damage in response to drought stress, thereby preserving physiological parameters.
Materials and methods
Plant material
This study evaluated a total of 14 onion genotypes, which include 3 commercial genotypes obtained from the East-West Seed Company in Benin: Prema, Dayo, and Red_Jewel F1. Additionally, 5 line genotypes from the WorldVeg in Mali were included: AVON1317, AVON 1074, Goudami, Violet_Galmi, and Synthetique 1. Furthermore, 5 commercial genotypes—Safari, ARES, Red_Creole, Idol, and Rouge_Tama—were provided by BENIN-SEMENCES. Finally, a local cultivar, open-pollinated from farmers in Benin Republic, was also evaluated.
Experimental design, conditions, and water stress treatments
A greenhouse experiment was conducted under controlled conditions at the University of Cologne, Germany, from February to July 2024. The experimental settings maintained a temperature of 22 °C during the day and 16 °C at night during the growth stage, and 27 °C during the day and 19 °C at night during the bulbing stage. The photoperiod consisted of 16 h of light and 8 h of darkness, with a light intensity of 450 µmol m−2 s−1 and 60% of relative humidity. To maximize seed viability and germination, and to eliminate any pathogenic organisms that could affect viability, the onion seeds were stored at − 80 °C for one day30. After the freezing period, the seeds were germinated in plastic pots filled with artificial soil placed in a plant growth chamber. Once the seeds germinated, the seedlings were transplanted into individual plastic pots (13 cm height, 13 cm diameter). The substrate used in this study had a pH of 5.9 and contained 30% organic matter, 50 mg/L of nitrogen (CaCl₂), 50 mg/L of phosphorus (CaL), 300 mg/L of potassium oxide (CaL), and 80 mg/L of magnesium (CaCl₂). Sixty-nine days after transplantation, drought stress was imposed using a modified method from15. The onion plants were categorized into two classes: a control group (C) containing 56 plants, which did not experience drought stress, and a drought stress group (D) containing 56 plants. The treatments were arranged in a randomized complete design (RCD) with four replications for each grouThe replication unit was a pot. The plants were watered twice a day to maintain optimal soil moisture levels.
The irrigation management strategy was designed to ensure an adequate water supply for plant growth before the plants were subjected to drought stress. To assess drought tolerance, the water supply was suspended for thirty consecutive days during the development of bulbs19. Thirty days of drought stress was selected for the study because leaf yellowing and senescence were observed only in the stressed plants after this period. Considering the life cycle of the genotypes used, it was found that 69 days of onion plant growth coincided with bulb initiation for most of these genotypes. Consequently, these characteristics were correlated with bulb development during the critical life stages of the onion. After the drought conditions were lifted, the stressed plants were thoroughly watered. They were then allowed to grow until they showed signs of neck bending, indicating they were ready for harvest. Throughout this period, regular watering was maintained to ensure the soil reached its field capacity. Data on agronomic, morphological, physiological, and biochemical characters were collected on the 30th day of drought stress conditions. Leaf samples from the third or fourth levels were taken across the four replications for biochemical analysis. The collected samples were immediately placed in liquid nitrogen. These frozen samples were then stored at − 80 °C until they were needed for analysis.
Morphological parameters
Leaf length, heights of plants, leaf number, leaf width, and pseudo-stem diameter of genotypes of onions subjected to well-watered and drought-stressed conditions were collected as morphological parameters after 30 days of drought imposition.
Physiological parameters
On the 30th day after the imposition of the drought conditions, physiological parameters were measured from control and drought-stressed plants at the third or fourth leaf between 8 and 11 a.m. to minimize the impact of midday environmental stress15.
Relative water content
RWC as relative water content was evaluated on the 3rd or 4th leaf. For each replicate, onion leaf segments about 5 cm long were sampled, and the fresh weights (FW) were measured. Then, for 24 h, the samples were immersed in distilled water and re-weighed to determine the turgid weight (TW). The samples of turgid leaves were then dried in an oven at 80 °C for 24 h to measure the dry weights (DW). The RWC was calculated by the means of Barrs and Weatherley31 procedures:
Stomatal density and stomatal activity
The density of stomata of the onion leaves was estimated using optical microscopy. The clear nail varnish was applied on abaxial leaf surfaces. After 5 min, the dry varnish was imprinted with epidermal cells using scotch tape and stuck on the glass slide. Microscopic epidermal images were captured from the middle area of leaves using camera-mounted light microscopes (Leica, DM750-ICC50 HD and 13613242/0.17 PLAN 40X/0.65) at 40X magnification (2,048 × 1,536 pixels or 0,529 × 0,264 mm) per leaf. Stomatal cells and stomata opened were counted.
Chlorophyll fluorescence
Chlorophyll fluorescence measurements were performed through the FluorPen instrument (FP-110; Germany). Before measurement, onion leaves (3rd or 4th fresh leaf) were selected from each pot and were dark-adapted for approximately 30 min using leaf clips, then maximum quantum yield (Fv/Fm) and potential activity of PS II (Fv/Fo) were measured.
Photosynthetic pigments
The chlorophyll contents were estimated with the help of Aron32 methods. Fresh leaf samples of 0.05 g were collected and homogenized with 1.2 ml of 80% acetone. It was then centrifuged at 10,000 rpm for 5 min at 4 °C. The supernatant was transferred, and the procedure was repeated till the residue became colorless. The supernatant was taken for the determination of photosynthetic pigments using a spectrophotometer. The blank sample was prepared with acetone. The absorbance values of the solution were read at 663 nm, 645 nm, and 470 nm, respectively, for chlorophyll a, chlorophyll b, and carotenoid. The pigment concentrations were calculated using the following formula.
where A470, A645, and A663 are absorbance values, v is the volume of extract (ml), and w is the weight of the sample used (g).
Biochemical measurements
Proline
Proline content was measured according to the methods outlined by Bates et al.33 with minor modifications. 0.05 g of fresh leaf sample was homogenized in 1.2 ml of 3% aqueous sulfosalicylic acid. After 3 h, the mixture was centrifuged at 1500 × g for 10 min at 4 °C. A total of 0.2 ml of the extract was combined with 0.2 ml of glacial acetic acid and 0.2 ml of acidic ninhydrin, then boiled in a water bath at 100 °C for one hour. The solution was cooled down by placing it on ice. 0.5 ml of toluene was added and mixed vigorously using the vortex for 15–20 s, and the toluene containing the chromophore was separated using a separatory funnel, and the absorbance was measured at 520 nm in a spectrophotometer against an appropriate toluene blank. The proline content was determined from a standard curve prepared with L-proline and expressed in (µmol/g FW). The unknown proline content is calculated from the samples using the standard graph. The proline concentration is then calculated employing the following formula:
where e is the µg/ml proline, V1 is the toluene volume, V2 is the volume of sulfosalicylic acid, and m is the weight (g) of the fresh sample used.
Total phenolic content (TPC)
TPC of the samples of leaves was measured with the help of the reagents of Folin-Ciocalteu (FC), following the method described by Julkunen-Tiitto34 with some minor modifications. To prepare the extract, 0.05 g of the fresh leaf sample was homogenized in 1.2 ml of 80% acetone, and the homogenate was then centrifuged at 10,000 rpm for 10 min. Following centrifugation, 0.25 ml of the extract was mixed with 0.25 ml of Folin-Ciocalteu reagent. After incubating the mixture in the dark at room temperature for 5 min, 0.6 ml of 20% Na2CO3 solution was added, and the mixture was incubated for an additional 45 min. The absorbance of the resulting solution was measured at 750 nm using a UV–VIS spectrophotometer. A standard curve was created using gallic acid at various concentrations (0–200 µg/ml). The phenolic content was expressed as milligrams of gallic acid equivalent per gram of fresh weight (mg GAE/g FW). Total Phenolic Content (TPC) was determined using:
where c is the sample concentration from the calibration curve (mg/ml), V is the volume (ml) of the solvent used for the extraction, and m is the weight (g) of the fresh sample used.
Hydrogen peroxide
0.05 g of fresh tissue was homogenized in 1.2 ml of 0.1% trichloroacetic acid. The homogenate was centrifuged at 12,000 g for 10 min, and 0.5 ml of supernatant was mixed with 0.5 ml of potassium phosphate buffer (10 mM, pH 7.0) and 1 M potassium iodide (1 ml). The absorbance was determined at 390 nm35 and the content was calculated by means of the standard curve with a known concentration of H2O2 and expressed as µMol g-1FW.
Activities of antioxidant enzymes
Enzymes were extracted according to Chen and Zhang36 methods. Approximately 0.05 g of leaf sample was homogenized in 1.2 ml of 100 mM phosphate buffer (pH 7.0). The homogenate was centrifuged at 15,000. g for 15 min at 4 °C, and the supernatant was used as a crude enzyme source for catalase (CAT), superoxide dismutase (SOD), and ascorbate peroxidase (APX).
Catalase (CAT) activity
The catalase activity was determined by a mixture in a sterilized Eppendorf tube of 0.5 mL of 0.2 M phosphate buffer (pH 7.0), 0.3 mL of (v/v) H2O2 (0.3%), and 0.1 ml of the enzyme. The final volume was made up of 1.2 ml by adding distilled water. The reaction was started by adding the enzyme extract, and the change in optical density was measured at 240 nm for 0 min and 3 min in a spectrophotometer37. The catalase positive control activity is defined as the amount of catalase necessary to decompose 1.0 µM of H₂O₂ per minute at pH 7.0 at 25 °C while H₂O₂ concentration falls from 10.3 mM to 9.2 mM.
Ascorbate peroxidase (APX) activity
The ascorbate peroxidase activity was determined by mixing in a sterilized Eppendorf tube of 0.6 ml 100 mM phosphate buffer (pH 7), 100 µl ascorbate (5 mM), 250 µl H2O2 (0.3%), and 250 µl enzyme extract was taken and the reaction was started after the addition of H2O2. The decrease in absorbance was recorded for 1 min at 290 nm, and the amount of ascorbate oxidized was calculated from the extinction coefficient of 2.8 mM− 1 cm− 138. Activity was calculated using the formula:
where ΔA290 is the decrease in absorbance per minute, v is reaction volume (ml), D is dilution factor, ε is molar extinction coefficient, l is cuvette path length (usually 1 cm), and w is fresh weight of tissue (g).
Superoxide dismutase (SOD) activity
The superoxide dismutase activity was determined by a mixture in a sterilized Eppendorf tube of 0.1 ml of 1.5 M Na2CO3, 0.2 ml of 200 mM methionine, 0.1 ml of 3 mM EDTA, 0.1 ml of 2.25 mM p-nitroblue tetrazolium chloride (NBT), 0.250 ml of 100 mM potassium phosphate buffer (pH 7.5), 0.2 ml of distilled water was added with 0.05 ml of enzyme samples. The tube without the enzyme served as a control. The reaction started by adding 0.1 ml of 60 µM riboflavin and placing the tubes below a light source of two 15 W fluorescent lamps for 15 min. The reaction was stopped by switching off the light and covering the tubes with a black cloth. Absorbance was recorded at 560 nm. An illuminated blank without an enzyme gave the maximum reduction of NBT, and therefore, the maximum absorbance at 560 nm. The activity of SOD was expressed as units/min/g FW39. To determine SOD activity, we first determined the % inhibition using the following formula:
where Acontrol is the absorbance without enzyme, and Asample is with enzyme.
One unit of SOD activity is defined as the amount of enzyme required to cause 50% inhibition of NBT reduction. Activity is expressed as units per g fresh weight.
Bulb yield parameters measurements and drought tolerance indices
At maturity, the bulbs were gathered, and the yield parameters were measured: bulb diameter (mm), bulb length (mm) using digital calipers, and the weight of the bulbs (g) using a precision weighing balance. In addition, the drought tolerance indices were determined (Table 1).
Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) to compare the means of the genotypes in the control and drought conditions separately with the Least Significant Difference (LSD). Additionally, a two-way ANOVA with genotypes and treatments as fixed factors for all parameters was used to determine the main and interaction effects. Treatments (Control and Drought) means were compared using the student’s t-test to determine whether they were significantly different at the 0.05 probability level. The analysis of correlation was done by calculating a two-tailed Pearson correlation coefficient with significance levels of 0.05. The principal component analysis (PCA), and biplot PCA identified the most variables that discriminate the most the varieties. We performed cluster analysis to project the variables and the genotypes on the axes and to regroup the genotypes on the basis of their performance and relationship under water stress. All analyses were implemented using R software, R 4.4.044. Graphical representation was performed using R software, R 4.4.0, and Microsoft Excel 2020.
Results
Influence of drought conditions on morphological parameters of onion
The morphological parameters of onion genotypes subjected to a deficit of water were significantly lower (p < 0.001) compared to the control (Table 2). Under drought conditions, SAFARI and ARES had the highest plant height, while Violet_Galmi, Local, and Goudami had the highest number of leaves. The genotypes SAFARI, Red_Jewel, and Prema recorded the highest leaf length, 37.82 cm, 33.70 cm, and 33.50 cm, respectively, under drought conditions. The highest mean values for leaf diameter were found in Local (2.56 cm), SAFARI (2.54 cm), and Red_Jewel (2.51 cm). The Genotypes Goudami (7.35 mm), Local (7.20 mm), and SAFARI (7.07 mm) outperformed the other genotypes with pseudo-stem diameter.
Physiological markers for drought tolerance in onion
Relative water content
The relative water content of onion genotypes significantly decreased (p < 0.001) in all onion genotypes exposed to 30 days of drought during the development of bulbs compared to their performance under well-watered conditions. Under water deficit, Goudami (71.80%), AVON1074 (70.22%), Red_Jewel (68.04%), and Prema (66.44%) had the highest values for the relative water content (RWC), while, minimum values were observed in Red_Creole (44.39%) and Rouge_Tama (44.07%) (Fig. 1). The biggest decrease was observed in SAFARI (41.58%) and Synthetique (37.72%). AVON1074 (12.51%) and Dayo (18.93%) were the two genotypes that lost the least water.
Stomatal density and stomatal activity
The drought stress caused a significant decrease (p < 0.001) in the stomatal number and stomata opened in all onion genotypes. Under normal water, the highest stomatal number was observed in AVON1074 (15.75), Goudami (15.50), and Violet_Galmi (14.25). Under drought, the highest stomatal numbers were observed in Goudami (10.50), Prema (10.25), and Local (10.25), respectively (Fig. 2A).
In response to drought stress, we also counted the number of open stomata (Fig. 2B). Compared to the control group, a significant decrease in the number of open stomata was observed in onions subjected to drought conditions. Among the genotypes, Prema, Goudami, and AVON1074 exhibited the highest numbers of open stomata under water deficit conditions. The genotypes Violet_Galmi (n = 7) and AVON1074 (n = 7) reduced the number of stomata the most. AVON1317 (n = 1) and Synthetique (n = 1) reduced the number of stomata the least. For open stomata, the genotypes Violet_Galmi (n = 12.25), Local (n = 11.25), and AVON1074 (n = 10.25) showed the highest reduction in open stomata. In contrast, the genotypes Synthetique (n = 5.5), Prema (n = 6.5), and IDOL (n = 6.5) showed the least reduction.
Chlorophyll a fluorescence
The chlorophyll fluorescence was significantly reduced in the majority of the genotypes under drought conditions as compared to the control. The genotypes AVON1074, AVON1317, SAFARI, Goudami, IDOL, Prema, Rouge_Tama, Synthetique, and Violet_Galmi showed a significant decrease (p < 0.05) in the potential activity of PS II (Fv/Fo) and maximum quantum yield (Fv/Fm) under drought stress compared to the control condition, whereas the genotypes ARES, Dayo, Local, Red_Creole, and Red_Jewel maintained Fv/Fo statistically similar to the control (p > 0.05) (Fig. 3A and B). Under drought stress, Red_Jewel, Prema, and IDOL had the maximum values of the potential activity of PS II and the highest maximum quantum yield. The biggest decrease of Fv/Fo and Fv/Fm was observed in AVON1317 (2.08 and 0.063, respectively), whereas Red_Jewel (0.64 and 0.019, respectively) showed the least reduction.
Boxplots showing differences between two conditions (control and drought conditions) for fourteen genotypes. (A) The potential activity of PS II, and (B) the maximum quantum yield. The asterisks ‘*’ designate data that are significantly different between the conditions (*p ≤ 0.05, (**p ≤ 0.01, Student’s t-test).
Photosynthetic pigments
Significant differences in photosynthetic pigments were found among the fourteen onion genotypes under drought stress. Similar significant differences in photosynthetic pigments, except for carotenoid content, were found in onion genotypes under normal water conditions. Under normal water, Prema, Red_Jewel, and Goudami had the highest chlorophyll a value, Prema, Red_Jewel, Goudami, and Local had the highest chlorophyll b values, and Prema, Red_Jewel, Goudami, and Local had the highest total chlorophyll values (Supplementary Fig. S1A-C and Fig. 4). In comparison to the control condition, chlorophyll a, carotenoid content, chlorophyll b, and total chlorophyll significantly reduced under water deficit conditions. Under drought conditions, Red_Jewel, Prema, and Goudami had the highest values for chlorophyll a (Supplementary Fig. S1A). The highest values for chlorophyll b were determined in Prema and ARES under drought conditions (Supplementary Fig. S1B). There was no significant difference in the carotenoid content in onion genotypes under normal water conditions. However, under deficit water conditions, a significant difference was observed in carotenoid content among onion genotypes, with the highest value recorded in Prema (Supplementary Fig. S1C). Prema and Red_Jewel recorded the highest values of total chlorophyll under drought stress (Fig. 4). The biggest decrease of total chlorophyll was observed in Local (0.0300 mg/gFW), Rouge_Tama (0.0250 mg/gFW), and Red_Creole (0.0225 mg/gFW). In contrast, the genotype Prema (0.0075 mg/gFW) showed the least reduction.
Biochemical markers for drought resilience in onion
Proline content
In comparison to the control group, proline content significantly increased in onion plants subjected to drought stress. Among the genotypes, Prema, Goudami, and Red_Jewel exhibited the highest proline concentrations at both control and drought treatments, while the lowest was observed in Red_Creole (Fig. 5A). The biggest increase of proline was observed in Prema (25.90 µmol/g FW) and Goudami (24.45 µmol/g FW). In contrast, the genotypes Local (7.28 µmol/g FW) and Red_Creole (8.37 µmol/g FW) showed the least increase.
Phenol content
Both genotype and water stress had a significant influence on the phenol content. As with other parameters, Goudami and Red_Jewel presented the maximum values for phenol content at drought treatment (Fig. 5B). The highest increase in phenol was observed in IDOL, while the largest decrease was recorded in Red_Jewel.
(A) Proline content and (B) phenol content in various onion genotypes under both normal and drought conditions. The data are presented as means ± standard error from four biological replicates. Different letters indicate values that are significantly different at p ≤ 0.05, according to the LSD test.
Hydrogen peroxide accumulation
Drought treatment significantly increased the H2O2 content in onion plants. The highest increase in H2O2 was observed in Local (5.45 µmol/min/g WF) and Red Creole (4.25 µmol/min/g WF), whereas the least increase was recorded in Red Jewel (2 µmol/min/g WF), when compared to the control plants (Fig. 6).
Antioxidant enzyme activity
Significant differences in catalase and ascorbate peroxidase were found among the onion genotypes under control conditions; Goudami and Prema presented the highest values for catalase activity, and the highest APX was recorded in Red_Jewel and Prema (Supplementary Fig. S2A-B). The drought stress caused a significant increase (p < 0.001) in antioxidant enzyme activity in all onion genotypes under stress (Supplementary Fig. S2A-C). The highest increase in catalase was observed in Synthetique (0.140 Unit/min/gFW) and SAFARI (0.118 Unit/min/gFW), whereas the least increase was recorded in Rouge_Tama (0.018 Unit/min/gFW) and Violet_Galmi (0.018 Unit/min/gFW). Rouge_Tama (92.37 Unit/min/gFW) and Red_Creole (89.25 Unit/min/gFW) recorded the highest increase for superoxide dismutase, while the lowest was Goudami (43.20 Unit/min/gFW) and Dayo (45.75 Unit/min/gFW).
The heatmap shows that the variation in fold changes in the activities of antioxidant enzymes was influenced by genotypes and stress conditions, with the influence of genotype predominating over that of drought. The highest increase was recorded by Goudami (0.38 µmol/min/g FW) and Prema (0.37 µmol/min/g FW) for APX, while the smallest increase was recorded by Local (0.11 µmol/min/g FW) and Synthetique (0.12 µmol/min/g FW) under drought stress. Also, Goudami and Prema exhibited strong induction of the CAT under drought. Under water stress, Goudami, Prema, and Red_Jewel exhibited the highest values for catalase activity, while the lowest value was observed in Rouge_Tama; the highest APX activities were observed in Goudami, Prema, and Red_Jewel; and the highest superoxide dismutase was observed in Red_Creole and Rouge_Tama, while the lowest value was noted in Goudami, Red _Jewel, and Prema (Fig. 7).
A heatmap showing fold-changes of activities of antioxidant enzymes in onion genotypes under control (C) and drought (D) conditions. Each row represents one genotype, and each column represents one parameter (Catalase (CAT), Superoxide Dismutase (SOD), Ascorbate Peroxidase (APX)) under each condition (C, D). The color intensity indicated the relative value (z-score) of each parameter, with yellow indicating low values, white representing intermediate values, and gray representing the highest values.
Agronomic markers and indices of drought tolerance associated with drought resistance in onion genotypes
The drought stress effects on bulb parameters are presented in Table 3. There was a significant (p < 0.001) difference in onion genotypes subjected to drought stress. The average bulb yield was significantly decreased by 49.58% compared to the control treatment (Table 4). The highest yield of bulbs was recorded in Prema (39.95 g), followed by Goudami (36.20 g) and Red_Jewel (33.65 g), while the lowest bulb yield was observed in Red_Creole (7.99 g) and Rouge_Tama (12.42 g). Additionally, water stress reduced bulb diameters and size. Prema (30.22 mm), Red_Jewel F1 (30.17 mm), and Goudami (27.52 mm) had the largest bulb diameter, while the lowest bulb diameters were observed in Red_Creole (15.05 mm). Red_Jewel (59.80 mm) and Goudami (48.85 mm) had the biggest bulb size, while the lowest bulb size was observed in Red_Creole (31.25 mm).
Based on drought indices, genotypes were divided into four groups according to percent bulb yield reduction and drought tolerance efficiency (Table 4). Three onion genotypes, namely Prema (29.22%), Goudami (34.60%), and Red_Jewel (36.83%)—demonstrated the lowest yield reductions and the highest levels of drought tolerance efficiency, categorizing them as highly drought-tolerant. In addition, three other varieties, Dayo (43.09%), SAFARI (43.74%), and IDOL (44.39%), exhibited yield reductions higher than 40% and demonstrated a drought tolerance efficiency of 50%, classifying them as tolerant varieties. Additionally, six genotypes—Local, ARES, Synthetique, AVON1074, Violet_Galmi, and AVON1317—showed yield reductions of 50% and drought tolerance efficiencies between 40% and 50%, identifying them as moderately tolerant genotypes. Lastly, two genotypes, Rouge_Tama (64.46%) and Red_Creole (68.33%), exhibited a drought tolerance efficiency of only 30%, classifying them as sensitive genotypes.
ANOVA of physiological, morphological, biochemical, and agronomic traits
ANOVA showed that all morpho-physiological traits were significantly different under drought stress (p < 0.001). The cultivar had also significant effect on all morpho-physiological parameters, except for the potential activity of PSII (Fv/Fo) and maximum quantum yield (Fv/Fm) (Table 5). PH, LL, Fv/Fm, and Fo/Fv were not significantly affected by the interaction between treatments and genotypes during 30 days of water stress, but the other traits were significantly reduced.
Analysis of variance for biochemical and yield characteristics of onion genotypes showed that treatments, genotypes, and their interaction affected all biochemical and yield parameters, except for phenol, which was not affected by treatment (Table 6).
Relationships of traits subjected to drought conditions
Analysis of correlation was conducted on the physiological, morphological, biochemical, and yield-contributing traits of genotypes of onions subjected to drought conditions (Fig. 8). The Pearson correlation matrix is illustrated in Fig. 8. Plant height and leaf length (0.96***), pseudostem diameter and leaf diameter (0.81***) were significantly and positively correlated. Bulb diameter had a strong and positive relationship with chlorophyll a (0.81***), proline (0.8***), catalase (0.88***), and bulb yield (0.91***), but had a negative correlation with superoxide dismutase (− 0.86***). The yield of bulbs was positively linked to chlorophyll a (0.81***), total chlorophyll (0.81***), and proline (0.92***). The potential activity of PS II had a strong and positive association with maximum quantum yield (0.99***). The total chlorophyll was positively linked to chlorophyll a (0.86***), and chlorophyll b (0.81***), whereas proline with opening stomata (0.80***), and relative water content (0.81***). Catalase activity was positively correlated with relative water content (0.83***), and proline (0.84***). Antioxidant ascorbate peroxidase showed correlation with relative water content (0.84***), chlorophyll a (0.82***), and proline (0.91***). The drought tolerance efficiency exhibited a strong and positive relationship with ascorbate peroxidase (0.80***), bulb diameter (0.82***), and bulb yield (0.93***). The yield reduction percentage had a negative association with ascorbate peroxidase (− 0.80***), bulb diameter (− 0.82***), and bulb yield (− 0.93***). Stress tolerance index had a significant and positive relationship with chlorophyll a (0.83***), total chlorophyll (0.86***), proline (0.94***), catalase (0.83***), ascorbate peroxidase (0.91***), bulb diameter (0.90***), and bulb yield (0.99***). The stress tolerance index exhibited a positive relationship with drought tolerance efficiency (0.86***), but showed a negative correlation with yield reduction percentage (− 0.86***). The drought susceptibility index had a negative association with ascorbate peroxidase (− 0.80***), bulb diameter (− 0.82***), and bulb yield (− 0.93***).
Pearson correlation coefficients among the morphological, agronomic, biochemical, and physiological variables subjected to drought conditions. Plant Height (PH). Number of Leaves (NL). Leaf Length (LL). Leaf Diameter (LD). Pseudostem Diameter (PD). Number Stomata (NS). Opening Stomatal (OS). Maximum quantum yield (Fv/Fm). Potential activity of PS II (Fv/Fo). RWC (Relative Water Content). Chlorophyll a (Ch a). Chlorophyll b (Ch b). Total Chlorophyll (T Chl). Carotenoid (Car). Hydrogen Peroxide (H2O2). Proline (Pro). Phenol (Phe). Catalase (CAT). Ascorbate Peroxidase (APX). Superoxide Dismutase (SOD). Bulb Diameter (BD). Bulb Length (BL). Bulb Weight (BW). Drought Tolerance Efficiency (DTE). Yield Reduction (YLR). Drought Susceptibility Index (DSI).
Principal component analysis and ascending hierarchical classification
At the bulb development stage under drought conditions, the first two components accounted for 68.42% of the overall variability (Fig. 9A). The bulb yield, stress tolerance index, bulb diameter, proline, drought tolerance efficiency, ascorbate peroxidase, catalase, chlorophyll a, relative water content, total chlorophyll, opening stomatal, bulb length, phenol, number of stomata, chlorophyll b contributed positively to the PC1, however, the yield reduction percentage, drought susceptibility index, superoxide dismutase, hydrogen peroxide negatively contributed to the same component. The pseudo-stem diameter, leaf diameter, leaf length, plant height, and number of leaves were positively correlated with PC2, but the carotenoid content had a negative association with this principal component.
The biplot of the principal component analysis revealed that Prema, Goudami, and Red_Jewel were characterized by high BD, BL, BW, NS, OS, chlorophyll a, b, total chlorophyll, Fv/Fm, Fv/Fo, RWC, proline, phenol, catalase, ascorbate peroxidase, DTE, and STI. The genotypes Local and SAFARI had the highest values of LL, PH, LD, NL, and PD. Red_Creole and Rouge_Tama were characterized by high superoxide dismutase, yield reduction (YLR), and drought susceptibility index (DSI) (Fig. 9B). Local had the highest hydrogen peroxide content.
Cluster analysis grouped the 14 genotypes into three clusters based on Ward’s clustering method using the package of square Euclidean distance among characters (Fig. 10).
The hierarchical tree of genotypes subjected to drought conditions at the development of bulbs showed that cluster III including Prema, Goudami, and Red_Jewel is characterized by the highest values for the stress tolerance index, ascorbate peroxidase, bulb yield, proline, chlorophyll a, total chlorophyll, drought tolerance efficiency, bulb diameter, catalase, and opened of stomata, but low values for the variables YLR and DSI (Fig. 10 and Supplementary Table). Local, Rouge_Tama ARES, IDOL, AVON1317, AVON1074, Synthetique, Violet_Galmi, Dayo, and SAFARI of cluster II had the lowest values for ascorbate peroxidase, total chlorophyll, chlorophyll a, carotenoid, stress tolerance index, potential activity of PS II, and proline. The Red_Creole of cluster I presented the lowest values for PH, LL, PD, BD, LD, CAT, and NL, but the highest values for SOD.
Discussion
In the current investigations, water-deficit stress at the stage of development of bulbs considerably reduced all morphological traits of onion genotypes compared to the controls. Similar results were found in onion field and greenhouse experiments, where 20–45 days of drought negatively affected morphological parameters such as leaf number, length, width, and area, with a high rate of leaf senescence14,17,19. Our earlier research on onion genotypes reinforces our current findings: drought stress significantly impacts morphological characteristics. It’s evident that the challenges posed by water scarcity take a substantial toll on these important traits16. Reduced plant growth under drought may be caused by reduced cell division, cell differentiation, and expansion, thus leading to a reduction in growth, notably in the number of leaves, plant height, leaf width, and length45,46,47, which constitutes the morphological adaptation mechanism to drought.
Drought stress also negatively affected physiological characteristics. Relative water content is an important physiological trait to estimate plant water status under drought stress. Relative water contents (RWC) of onion genotypes considerably decreased (p < 0.001) in all onion genotypes exposed to 30 days of drought during the bulb development compared to their respective control. This finding underscores the significance of evaluating relative water content in understanding how onion plants respond to drought stress, which aligns with trends observed in previous studies. Water deficit negatively impacted the leaf water status of plants19,48. The reduction in leaf water content could result in decreased metabolic reactions and photosynthetic activity. However, different genotypes responded differently to water stress: Goudami, AVON1074, Red_Jewel, and Prema maintained higher leaf water content, which is an indication of the ability to absorb water from the soil and conserve water in the leaf during periods of drought stress. Under water deficit conditions, high relative water content (RWC) in onion plants allows high values for leaf area, total chlorophyll content, antioxidant enzyme activity, membrane stability index, and bulb yield15.
When plants are exposed to drought, stomatal closure is a common and the first adaptation response of plants to the onset of drought conditions. During periods of drought stress, onion plants demonstrate a response by decreasing the number of stomata and closing them more tightly. Remarkably, this is the first documented instance of such behavior in onion genotypes. While this adaptive strategy aids in water conservation, it also presents challenges for the overall health of the plants. These findings highlight the importance of stomatal characteristics in understanding how onion plants react to drought conditions. These results correlate with the previous studies that reported that drought stress caused a reduction in stomatal frequency and stomatal closure, which regulate transpiration/CO2 uptake by leaves and decrease photosynthetic performance49,50. Recent studies have also shown that rice leaves close stomata under water stress conditions to maintain cellular water24, and a reduction in stomatal density in water-stressed tomato plants has been reported51.
Here, in onion genotypes, Fv/Fm and Fv/Fo declined under water-deficit stress (Fig. 3A-B). Similar findings have been reported on the onion52 and tomato47,53. According to Raja et al.27, drought stress decreased the maximum quantum yield (Fv/Fm) and the potential activity of PS II (Fv/Fo) in tomato leaves, supporting the role of alternative electron sink (either from PSII or PSI) and cyclic electron flow in protecting PSII and PSI from light, and also in generating ATP to counteract drought stress. The photochemical quantum yield of photosystem II (Fv/Fm) is a very important variable that is measured when all centers are open54. It is used to identify PSII damage and possible photoinhibition in leaves of plants subjected to drought conditions55,56. The decrease in Fv/Fm was an explanation for the reduction in Rubisco activity and the partial inactivation of PSII during water stress54. The recent development of Chlorophyll a fluorescence (ChlF) may be a potentially valuable new approach to determining the photochemical efficiency of leaves, specifically, providing detailed information on the status and function of Photosystem II (PSII) reaction centers, light-harvesting antenna complexes, and both the donor and acceptor sides of PSII57,58. Recently, some investigators used chlorophyll fluorescence parameters to evaluate the fluorescence response to drought in Acer genotypes59, in cucumbers58 and found that Fv/Fm was significantly decreased under drought stress. In our study, Fv/Fm was able to help unravel the tolerance status of the genotypes to water deficit conditions.
In this current study, chlorophyll a, b, total chlorophyll, and carotenoid content decreased in genotypes of onions subjected to drought conditions. These observations highlight the significance of chlorophyll analysis in enhancing our understanding of how onions respond to water deficit, consistent with previous studies. A decrease in chlorophyll content under water deficit was also reported in previous studies on onion14,17,21, tomato27, Camarosa60, and rice61. During the water deficit decrease in chlorophyll content might be due to the degradation of chlorophyll biosynthetic enzymes. The total chlorophyll content, as well as the chlorophyll a and b content, has a direct effect on the ability of the plant to absorb light for photosynthesis.
In contrast to the decrease in physiological variables, drought stress induces the accumulation of compatible solutes (proline). Proline content was significantly higher (p < 0.001) in Prema, Goudami, and Red_Jewel under drought stress for 30 days at bulb development. Maximum proline was recorded in cultivar Prema subjected to drought stress conditions in India62. This increase in proline helps the plant to acclimate to drought stress. Several recent studies have shown that proline accumulation plays a crucial role in stressed plants, including osmotic pressure regulation and maintenance of protein and cell membrane stability63,64,65, and as also reported in previous studies by Hanci and Cebeci21 and Gökçe et al.17,66.
The results of the current investigations revealed that total phenolic content was significantly higher in genotypes Goudami and Red_Jewel under water stress. The increase in phenol in certain genotypes corroborates the findings of19,62. The plant accumulates higher total phenolics in response to water deficit stress as one of the tolerance mechanisms.
Drought stress causes oxidative stress in plants, resulting in the production of various reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), hydroxyl radicals (OH−), and superoxide radical (O2−). Onion plants subjected to water deficit conditions resulted in elevated hydrogen peroxide (H2O2) levels; the highest H2O2 contents were observed in Local, Red_Creole, and Rouge_Tama. The accumulation of higher H2O2 contents in stressed onion also agrees with the earlier study of Chaudhry et al. (2023) on onion genotypes. Overproduction of hydrogen peroxide in plants under drought stress inactivates enzymes, damages key cellular components, induces lipid peroxidation, and fatty acid de-esterification67.
In the current research investigations, a significant increase was observed in catalase, ascorbate peroxidase, and superoxide dismutase activities under drought. Antioxidant enzymes were regulated differently in onion genotypes, which is crucial for withstanding drought conditions. Goudami, Prema, and Red_Jewel presented the maximum values as a result of drought stress (Fig. 7). Catalase (CAT) and ascorbate peroxidase (APX) are enzymes that detoxify (H2O2) in plants. CAT catalyzes the dismutation of H2O2 to H2O and O2, and APX reduces H2O2 into H2O37,68. The levels of superoxide dismutase increased in the 14 genotypes under drought stress. This superoxide dismutase is well known for its role in plant growth and development, carrying out the dismutation of superoxide radicals (O2−) to molecular oxygen and hydrogen peroxide (H2O2), and in providing tolerance to abiotic stress conditions by controlling oxidative stress69,70. The increasing activity of catalase, APX, and SOD under drought conditions has also been reported in previous studies24,27.
Water-deficit stress significantly decreased the yields of onion bulbs and its yield components15,70,71,72,73. We found that the drought stress during bulb development significantly (p < 0.001) reduced the onion bulb trait. Bulb productivity under drought stress was considerably decreased to 49.58% compared to the well-watered plants. In comparison to our findings, a lower (14%) and a higher (65%) yield reduction due to water deficit stress was reported by Almaroai and Eissa13,74, respectively. These differences in the magnitude of yield reduction across studies could be due to differences in the time of stress imposition, duration of stress, and growth stage at which plants were exposed to drought. The reduction in bulb characteristics could be due to the drought-induced reduction in chlorophyll content, stomatal density, stomatal closure, photosynthesis in the leaves, and the processes of food translocation to developing bulbs.
Remarkable variations in genotypes were identified across all agronomic, biochemical, and physiological characters studied under drought conditions, highlighting the significant genetic diversity present. This indicated that the genotypes tested exhibited genetic variability for these traits. Therefore, notable differences in genotypic variation could be utilized to compare onion genotypes and classify them into groups on the basis of variability observed in agronomic performances, biochemical, and physiological traits, and drought tolerance indices under water deficit conditions. This method could also be employed to screen the onion cultivars for other abiotic stresses, helping to identify genotypes with the most divergent responses to specific environmental challenges. The identified resilient genotypes may possess drought-tolerance genes, which can serve as valuable resources in breeding programs aimed at developing drought-tolerant onions. Furthermore, these exceptionally resistant genotypes can be mated through crossing with high-yielding onion varieties to introduce drought-tolerance genes without compromising their intrinsic yield potentials under water deficit or drought conditions.
The correlation analysis revealed that the stress tolerance index had a positive association with drought tolerance efficiency, while it was negatively correlated with the percentage of yield reduction. Additionally, drought tolerance efficiency demonstrated a strong positive relationship with bulb yield. There was also a solid positive relationship between the stress tolerance index and several factors: relative water content, chlorophyll content, proline levels, catalase activity, ascorbate peroxidase levels, and bulb yield. Therefore, the genotypes with a high-stress tolerance index and low yield reduction percentage were tolerant with high onion bulb yield subjected to drought conditions. The drought susceptibility index had a positive relationship with yield reduction percentage but had a negative association with the yield of onion bulbs. Thus, the genotypes that showed higher yield reduction percentages were sensitive. These results thus demonstrated the importance of these parameters and drought indices in the selection of drought-tolerant genotypes. The present results corroborate the previous studies on onion genotypes subjected to drought stress15,17,72. The levels of superoxide dismutase (SOD) and proline are inversely related, with high proline levels compensating for SOD’s function. The strength of this relationship varies according to the duration and intensity of stress, and the stage of plant development. Similarly, severe drought stress significantly reduced SOD activity in all provenances and increased proline concentration75.
In our results regarding PCA analysis, stress tolerance index, bulb diameter, proline, drought tolerance efficiency, ascorbate peroxidase, catalase, chlorophyll a, relative water content, total chlorophyll, number of stomata, and chlorophyll b significantly influence bulb yield. In PC1, STI, and DTE demonstrated the highest variability, indicating that these parameters may be a promising characteristic for screening many onion varieties under drought stress. Consistent results have been found in various onion genotypes, strengthening the validity of our findings related to these specific genotypes15.
A biplot formed between Principal Component 1 (PC1) and Principal Component 2 (PC2) revealed a distinct pattern in the grouping of onion genotypes along the line of the vector. Genotypes that demonstrated exceptional performance for specific traits were positioned closer to this vector line. Those genotypes and parameters that are located at a far distance from the origin exhibit better breeding potential compared to others. In the current study, Prema, Goudami, and Red_Jewel were found at a significant distance from the origin, making them suitable candidates for use in onion breeding programs as tolerant varieties. Additionally, Prema was located near the line of the vector and was linked to drought-tolerant characteristics, such as drought tolerance index (DTE), index of stress tolerance (STI), the number of stomata opened, total chlorophyll content, proline levels, and activities of antioxidant enzymes. Thus, Prema could be employed in the development of a drought-tolerant variety of onions. Therefore, the specialty of this work was the use of the biplot methods as a visualization tool for exploring morpho-physiological and biochemical data, and showing simultaneous display of genotypes and variables, as well as representing the relationships between genotypes and those between variables in graphs. Gedam et al.15 and Gökçe et al.17 utilized similar methods to group onion genotypes based on their performance under drought conditions. Their studies identified drought-tolerant genotypes that exhibited promising traits associated with drought tolerance, making them suitable candidates for onion breeding programs.
We grouped onion genotypes into three clusters utilizing their characters and the indices of drought tolerance (Fig. 10). Prema, Goudami, and Red_Jewel were grouped in the third (III) cluster and are tolerant to drought stress. These genotypes recorded a higher stress tolerance index, antioxidant enzyme activity, proline, chlorophyll content, drought tolerance efficiency, opening of stomata, and lower YLR and DSI. Local, Rouge_Tama ARES, IDOL, AVON1317, AVON1074, Synthetique, Violet_Galmi, Dayo, and SAFARI were grouped in cluster II were considered as moderate drought-tolerant varieties with a low chlorophyll content, stress tolerance index, potential activity of PS II, and proline level. The Red_Creole of cluster I was categorized as sensitive with a low morphological trait, DTE, and high yield reduction percentage. Under drought conditions, tolerant onion genotypes are characterized by high proline accumulation18 and high activities of antioxidant enzymes, total chlorophyll, RWC, DTE, and STI15. In contrast, sensitive onion genotypes subjected to drought conditions accumulate low levels of proline, lower water content, and yield reduction76. Onion genotypes, in our previous investigation, were classified into three clusters on the basis of their morphophysiological, agronomic characteristics, and stress indexes under drought stress76. Our findings provide breeders with new insights into the screening methods, including physiological and biochemical markers, that can be used to identify drought-tolerant onion genotypes for their breeding programs. To encourage farmers to adopt these tolerant genotypes, further validation is needed through studies that assess genotype-by-environment interactions in field conditions under water stress.
Conclusion
The present study demonstrates that drought stress has a negative impact on morphological, agronomic, and physiological traits of onion to different extents in different genotypes. The results indicate that under drought conditions, tolerant genotypes (Prema, Goudami, and Red_Jewel) accumulate proline and antioxidants to mitigate the adverse effects, while the susceptible genotype (Red_Creole) experiences increased concentrations of hydrogen peroxide. According to the biplot analysis, the tolerant genotypes Prema, Goudami, and Red_Jewel exhibited high values for bulb diameter, bulb length, bulb weight, number of stomata, opening stomata, total chlorophyll, chlorophyll fluorescence, relative water content, proline, phenolic compounds, catalase, ascorbate peroxidase, drought tolerance index, stress tolerance index, and mean productivity. Thus, overall, Prema, Goudami, and Red_Jewel showed good performance under water deficit conditions, demonstrating superior agronomic, physiological, biochemical parameters, and tolerance indices. In contrast, the Red_Creole genotype exhibited a significant yield reduction and a higher drought susceptibility index. Therefore, Prema, Goudami, and Red_Jewel are the varieties that can be recommended to onion growers and could be valuable for future improvement programs in drought-prone areas. Our study tackles an important research gap by examining the impact of drought stress on onions, focusing on a combination of phenotypic, physiological, biochemical, and agronomic traits. Future research on onion stress tolerance should evaluate these genotypes in environments prone to drought and employ genetic techniques to identify the genes associated with drought tolerance.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Wingler, A. The function of Trehalose biosynthesis in plants. Phytochemistry 60, 1. https://doi.org/10.1016/S0031-9422(02)00137-1 (2002).
Solecki, W., Roberts, D. & Seto, K. C. Strategies to improve the impact of the IPCC special report on climate change and cities. Nat. Clim. Change. 14, 685–691. https://doi.org/10.1038/s41558-024-02060-9 (2024).
Report, S. Climate Change 2023 Synthesis Report (IPCC, 2023).
Kunchge, N., Kumar, K. & Firke Vegetable crops (chili pepper and onion): approaches to improve crop productivity and abiotic stress tolerance. Improv. Crop Resist. Abiotic Stress. 1, 951–978 (2012).
Chaudhry, U. K., Junaid, M. D. & Gökçe, A. F. Influence of environmental adversities on physiological changes in plants. Dev. Clim. Crop. 1, 85–110 (2021).
Singh, V. V., Garg, H. S., Meena & Meena, M. L. Drought stress response of Indian mustard (Brassica juncea L.) genotypes. Int. J. Curr. Microbiol. Appl. Sci. 7, 2519–2526 (2018).
Amé, A. Sélectionner le maïs population sur la tolérance à la sécheresse: quels leviers de sélection mettre en oeuvre pour s’ adapter? (2020).
Konate, M. et al. Evaluation des potentialités nutritives et l’aptitude à la conservation de onze variétés d’oignon (Allium cepa L.) bulbe introduites au Burkina Faso. Int. J. Biol. Chem. Sci. 11, 2018. https://doi.org/10.4314/ijbcs.v11i5.6 (2005).
Sagar, N. A., Pareek, S. & Gonzalez-Aguilar, G. A. Quantification of flavonoids, total phenols and antioxidant properties of onion skin: a comparative study of fifteen Indian cultivars. J. Food Sci. Technol. 57, 2423–2432. https://doi.org/10.1007/s13197-020-04277-w (2020).
Teshika, J. D. et al. Traditional and modern uses of onion bulb (Allium Cepa L.): a systematic review. Crit. Rev. Food Sci. Nutr. 59, S39–S70. https://doi.org/10.1080/10408398.2018.1499074 (2019).
Salem, M. A. et al. Investigation of the phytochemical composition, antioxidant, antibacterial, anti-osteoarthritis, and wound healing activities of selected vegetable waste. Sci. Rep. 13, 1–25. https://doi.org/10.1038/s41598-023-38591-y (2023).
Drinkwater, W. O. & Janes, B. E. Effects of irrigation and soil moisture on maturity, yield and storage of two onion hybrids. Proc. Am. Soc. Hortic. Sci. 66, 267–278 (1955).
Ghodke, H. et al. Physiological and biochemical responses in onion crop to drought stress. Int. J. Curr. Microbiol. App Sci. 7, 2054–2062 (2018).
Chaudhry, U. K. & Gokce, A. F. Effects of Salinity and Drought Stresses on the Physio-Morphological Attributes of Onion Cultivars at Bulbification Stage Effects of Salinity and Drought Stresses on the Physio-Morphological Attributes of Onion Cultivars at Bulbification Stage. https://doi.org/10.17957/IJAB/15.1611 (2020).
Gedam, A. et al. Screening of onion (Allium Cepa L.) genotypes for drought tolerance using physiological and yield based indices through multivariate analysis. Front. Plant. Sci. 12, 600371. https://doi.org/10.3389/fpls.2021.600371 (2021).
Sansan, O. C. et al. Onion (Allium cepa L.) and drought: Current situation and perspectives. Scientifica (Cairo). 1, 6853932 (2024).
Gökçe, Z. N. Ö., Gökçe, A. F., Junaid, M. D. & Chaudhry, U. K. Morphological, physiological, and biochemical responses of onion (Allium Cepa L.) breeding lines to single and combined salt and drought stresses. Euphytica 218 (3). https://doi.org/10.1007/s10681-022-02980-7 (2022).
Chaudhry, U. K., Öztürk, Z. N. & Gökçe, A. F. Assessment of salt and drought stress on the biochemical and molecular functioning of onion cultivars. Mol. Biol. Rep. 51, 37. https://doi.org/10.1007/s11033-023-08923-2 (2023).
Ghodke et al. Physiological and biochemical responses in onion crop to drought stress. Int. J. Curr. Microbiol. Appl. Sci. 7 (1). https://doi.org/10.20546/ijcmas.2018.701.247 (2018).
Vidya Vani, M., Vani, V. M. & Basha, O. Evaluation of biochemical responses of onion (Allium Cepa l.) seedlings under drought stress. Int. J. Recent. Sci. Res. 10, 31924. https://doi.org/10.24327/ijrsr.2019.1004.3364 (2019).
Hanci, F. & Cebeci, E. Investigation of proline, chlorophyll and carotenoids changes under drought stress in some onion (Allium Cepa L.) cultivars. Turkish J. Agric. Nat. Sci. 2, 1499–1504 (2014).
Caldwell, T. J., Lada, R. & Hooper, D. Physiological responses of onion (Allium Cepa l.) seedlings exposed to drought. Acta Hortic. 618, 321–327. https://doi.org/10.17660/ActaHortic.2003.618.37 (2003).
Ezin, V., Tossou, T. A. W., Chabi, I. B. & Ahanchede, A. Diallel analysis of Cowpea (Vigna unguiculata (L.) Walp.) genotypes under water deficit stress. BMC Plant. Biol. 23, 539 (2023).
Mondal, K., Kar, R. K., Chakraborty, A. & Dey, N. Concurrent effect of drought and heat stress in rice (Oryza sativa L.): physio-biochemical and molecular approach. 3 Biotech. 14, 1–15 (2024).
Eom, S. H. & Hyun, T. K. Transcriptomic responses of Garlic (Allium sativum L.) to heat and drought stresses. Phyt 92, 11 (2023).
I et al. Garlic ecotypes utilise different morphological, physiological and biochemical mechanisms to Cope with drought stress. Plants 12, 1824 (2023).
Raja, V., Qadir, S. U., Alyemeni, M. N. & Ahmad, A. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in solanum lycopersicum. 3 Biotech. 10, 1–18 (2020).
Pamungkas, S. S. T. & Farid, N. Drought stress: responses and mechanism in plants. Rev. Agric. Sci. 10, 168–185 (2022).
Jurkonienė, S. et al. Proline enhances resistance and recovery of oilseed rape after a simulated prolonged drought. Plants 12, 2718 (2023).
Dorna, H., Jarosz, M., Szopińska, D., Szulc, I. & Rosińska, A. Germination, vigour and health of primed allium Cepa L. seeds after storage. Acta Sci. Pol. Hortorum Cultus. 12, 43–58 (2013).
Barrs, H. D. & Weatherley, E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J. Biol. Sci. 15, 413–428. https://doi.org/10.1071/BI9620413 (1962).
Aron, D. Copper enzymes isolated chloroplasts, polyphenoloxidase in beta vulgaris. Plant. Physiol. 24, 1–15 (1949).
Bates, L. S., Waldren, R. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant. Soil. 39, 205–207. https://doi.org/10.1007/BF00018060 (1973).
Julkunen-Tiitto, R. Phenolic constituents in the leaves of Northern willows: methods for the analysis of certain phenolics. J. Agric. Food Chem. 33, 213–217 (1985).
Velikova, V., Yordanov, I. & Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant. Sci. 151, 59–66 (2000).
Chen, T. & Zhang, B. Measurements of proline and malondialdehyde content and antioxidant enzyme activities in leaves of drought stressed cotton. Bio-protocol 6, e1913 (2016).
Anjum, N. A. et al. Catalase and ascorbate peroxidase—representative H2O2-detoxifying Heme enzymes in plants. Environ. Sci. Pollut Res. 23, 19002–19029. https://doi.org/10.1007/s11356-016-7309-6 (2016).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant. Cell. Physiol. 22, 867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232 (1981).
Senthilkumar, M. et al. Estimation of superoxide dismutase (SOD). In Plant-Microbe Interactions: Laboratory Techniques (ed Senthilkumar, M.) 117–118. https://doi.org/10.1007/978-1-0716-1080-0_29 (Springer, 2021).
Cabello, R. et al. Comparison of yield based drought tolerance indices in improved varieties, genetic stocks and landraces of potato (Solanum tuberosum L). Euphytica 193, 147–156. https://doi.org/10.1007/s10681-013-0887-1 (2013).
Fernandez, A. Effective selection criteria for assessing plant stress tolerance. In Proceeding Int. SymAdapt. Veg. Other Food CroTemWater Stress. Aug. 13–16, Shanhua, Taiwan, 1992 257–270. https://cir.nii.ac.jp/crid/1571980074940839040 (1992).
Fischer, M. R. Drought resistance in spring wheat cultivars. I grain yield responses. Aust J. Agric. Res. 29, 897–912. https://doi.org/10.1071/ar9780897 (1978).
Raman, A. et al. Drought yield index to select high yielding rice lines under different drought stress severities. Rice 5, 31. https://doi.org/10.1186/1939-8433-5-31 (2012).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2024).
Hayat, S., Hasan, S. A., Fariduddin, Q. & Ahmad, A. Growth of tomato (Lycopersicon esculentum) in response to Salicylic acid under water stress. J. Plant. Interact. 3, 297–304 (2008).
Farooq, M., Wahid, A., Kobayashi, N., Fujita, D. & Basra, S. M. A. Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev. 29, 185–212 (2009).
Sousaraei, N. et al. Screening of tomato landraces for drought tolerance based on growth and chlorophyll fluorescence analyses. Hortic. Environ. Biotechnol. 62, 521–535 (2021).
Hassan, M. A. et al. Drought stress in rice: morpho-physiological and molecular responses and marker-assisted breeding. Front. Plant. Sci. 14, 1–16. https://doi.org/10.3389/fpls.2023.1215371 (2023).
Pirasteh-Anosheh, H. et al. Stomatal Responses Drought Stress 24–40. https://doi.org/10.1002/9781119054450.ch3 (2016).
Zandalinas, S. I., Mittler, R., Balfagón, D., Arbona, V. & Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 162, 2–12 (2018).
Galdon-Armero, J. et al. The ratio of trichomes to stomata is associated with water use efficiency in solanum lycopersicum (tomato). Plant. J. 96, 607–619 (2018).
Semida, W. M., Abdelkhalik, A., Rady, M. O. A., Marey, R. A. & Abd El-Mageed, T. A. Exogenously applied proline enhances growth and productivity of drought stressed onion by improving photosynthetic efficiency, water use efficiency and up-regulating osmoprotectants. Sci. Hortic. (Amsterdam). 272, 109580. https://doi.org/10.1016/j.scienta.2020.109580 (2020).
Ahmad, T., Mohd, K. & Qazi, S. Differential drought stress tolerance in five varieties of tomato (Solanum lycopersicum L.): an evaluation of photosynthesis and antioxidant system. J. Soil. Sci. Plant. Nutr. 23, 2810–2831. https://doi.org/10.1007/s42729-023-01236-0 (2023).
Zhang, Y. J. et al. Effect of water stress on photosynthesis, chlorophyll fluorescence parameters and water use efficiency of common Reed in the Hexi corridor. Russ J. Plant. Physiol. 66, 556–563 (2019).
Rahbarian, R., Khavari-Nejad, R., Ganjeali, A., Bagheri, A. & Najafi, F. Drought stress effects on photosynthesis, chlorophyll fluorescence and water relations in tolerant and susceptible Chickpea (Cicer arietinum L.) genotypes. Acta Biol. Cracov. Ser. Botânica. 53, 1 (2011).
Ezin, V., Tosse, A. G. C., Chabi, I. B. & Ahanchede, A. Adaptation of cowpea (Vigna unguiculata (L.) Walp.) to water deficit during vegetative and reproductive phases using physiological and agronomic characters. Int. J. Agron. 2021, 9665312 (2021).
Kalaji, H. M. et al. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 38, 4. https://doi.org/10.1007/s11738-016-2113-y (2016).
Song, G., Wang, Q., Zhuang, J. & Jin, J. Dynamics of leaf chlorophyll fluorescence parameters can well be tracked by coupling VIS-NIR-SWIR hyperspectral reflectance and light drivers in partial least-squares regression. Sci. Hortic. (Amsterdam). 325, 112651. https://doi.org/10.1016/j.scienta.2023.112651 (2024).
Banks, J. M. Chlorophyll fluorescence as a tool to identify drought stress in Acer genotypes. Environ. ExBot. 155, 118–127. https://doi.org/10.1016/j.envexpbot.2018.06.022 (2018).
Ödemiş, B., Kazgöz, D., Candemir & Evrendilek, F. Responses to drought stress levels of strawberry grown in greenhouse conditions. Hortic. Stud. 37, 113–122. https://doi.org/10.16882/hortis.805196 (2020).
Phunthong, C. & Pitaloka, M. K. Rice mutants, selected under severe drought stress, show reduced stomatal density and improved water use efficiency under restricted water conditions. Front. Plant. Sci. 8756, 1–12. https://doi.org/10.3389/fpls.2024.1307653 (2024).
Vani, M. V. & Riazunnisa, K. Evaluation of biochemical responses of onion (Allium cepa L.) seedlings under drought stress. Int. J. Recent Sci. Res. 10, 31924–31927. https://doi.org/10.24327/ijrsr.2019.1004.3364 (2019).
Chun, S. C. et al. Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front. Microbiol. 9, 1–13 (2018).
Laura, A., Bianucci, E., Giordano, W., Castro, S. & Becker, D. F. Plant physiology and biochemistry proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant. Physiol. Biochem. 151, 566–578. https://doi.org/10.1016/j.plaphy.2020.04.010 (2020).
Wang, Z., Yang, Y., Yadav, V., Zhao, W. & He, Y. Drought-induced proline is mainly synthesized in leaves and transported to roots in watermelon under water deficit. Hortic. Plant. J. 8, 615–626. https://doi.org/10.1016/j.hpj.2022.06.009 (2022).
Gökçe, A. F., Gökçe, Z. N. Ö., Junaid, M. D. & Chaudhry, U. K. Evaluation of biochemical and molecular response of onion breeding lines to drought and salt stresses. Sci. Hortic. (Amsterdam). 311, 802. https://doi.org/10.1016/j.scienta.2022.111802 (2023).
Ansari, W. et al. Influence of drought stress on morphological, physiological and biochemical attributes of plants: a review. Biosci. Biotechnol. Res. Asia. 16, 697–709. https://doi.org/10.13005/bbra/2785 (2019).
Rajput, V. D. et al. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology (Basel). 10, 267 (2021).
Tyagi, S. et al. Role of superoxide dismutases (SODs) in stress tolerance in plants. In Molecular Approaches in Plant Biology and Environmental Challenges (ed Singh, S.) 51–77. https://doi.org/10.1007/978-981-15-0690-1_3 (Springer, 2019).
Batool, R. et al. in Molecular Mechanisms of Superoxide Dismutase (SODs)-Mediated Defense in Controlling Oxidative Stress in Plants BT—Antioxidant Defense in Plants: Molecular Basis of Regulation 157–179 (eds Aftab, T. & Hakeem, K. R.). https://doi.org/10.1007/978-981-16-7981-0_8 (Springer, 2022).
Zheng, J. et al. Effects of Water Deficits on Growth, Yield and Water Productivity of Drip-Irrigated Onion (Allium cepa L.) in an Arid Region of Northwest China. https://doi.org/10.1007/s00271-012-0378-5 (2012).
Wakchaure, G. C. et al. Growth, bulb yield, water productivity and quality of onion (Allium Cepa L.) as affected by deficit irrigation regimes and exogenous application of plant bio-regulators. Agric. Water Manag. 199, 1–10. https://doi.org/10.1016/j.agwat.2017.11.026 (2018).
Nurga, Y., Alemayehu, Y. & Abegaz, F. Effect of deficit irrigation levels at different growth stages on yield and water productivity of onion (Allium Cepa L.) at Raya Azebo woreda, agriculture is one of the main consumers of fresh-water resources in the world. It 30, 155–176 (2020).
Almaroai, Y. A. & Eissa, M. A. Role of marine algae extracts in water stress resistance of onion under semiarid conditions. J. Soil. Sci. Plant. Nutr. 1, 1092–1101 (2020).
Duan, H. et al. The role of leaf superoxide dismutase and proline on intra-specific photosynthesis recovery of Schima Superba following drought. Sci. Rep. 14, 8824. https://doi.org/10.1038/s41598-024-59467-9 (2024).
Ghodke, A. et al. Comparative transcriptome analyses in contrasting onion (Allium Cepa L.) genotypes for drought stress. PLoS ONE. 15, e0237457. https://doi.org/10.1371/journal.pone.0237457 (2020).
Acknowledgements
The authors are grateful to Bayer Foundation [Award number: OBJS-2023-018] for supporting this research. We are greatly thankful to the Institute for Plant Sciences at the University of Cologne for providing research facilities. SK research is funded by the German Research Foundation (DFG) under Germany´s Excellence Strategy—EXC 2048/1—project 390686111.
Author information
Authors and Affiliations
Contributions
Conceptualization: OCS, VE, and SK; Methodology: OCS, IK and AA, Analysis and interpretation of results: OCS, IK, VE and SK, draft manuscript: OCS, VE and SK, writing-review and editing: VE, SK, AA and OCS, Supervision: VE, IK, AA and SK. All authors contributed to the article and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sansan, O.C., Ezin, V., Ahanchede, A. et al. Assessment of agro-physiological and biochemical markers for drought tolerance in onion (Allium cepa L.) genotypes during bulb development. Sci Rep 15, 36235 (2025). https://doi.org/10.1038/s41598-025-20181-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-20181-9