Abstract
Impaired awareness of hypoglycaemia (IAH) is a complication of diabetes treatment, whereby individuals are no longer able to feel an oncoming hypoglycaemic event. IAH may be a result of central nervous system adaptation to low recurrent hypoglycaemias, however the precise pathways involved remain unknown. This study employed proteomics analysis to explore potential pathophysiological pathways in IAH, using a nested case-control design within the Dutch Type 1 Diabetes Biomarker study. The Olink® Cardiovascular II panel was used for targeted proteomics, comparing 67 individuals with IAH to 108 age- and sex-matched individuals with normal awareness of hypoglycaemia (NAH). Univariate analysis revealed that agouti-related protein (AGRP) levels were significantly lower in individuals with IAH compared to NAH (6.12 NPX vs. 6.44 NPX, FDR-adjusted P = 0.012). In multivariate models adjusted for sex and diabetes duration, AGRP remained significant before p-value adjustment (P < 0.001) but not after adjusting for false discovery rate (FDR) (P = 0.057). AGRP, known for its orexigenic effects and expression in the arcuate nucleus of the hypothalamus, is involved in glucose sensing and hypothalamic-pituitary-adrenal (HPA) axis stimulation, suggesting its potential role in the pathophysiology of IAH. This study highlights the need for further research to clarify AGRP’s role and its possible implications for managing IAH in diabetes.
Similar content being viewed by others
Introduction
The occurrence of hypoglycaemia can be a limiting factor to achieve optimal glucose regulation by insulin in individuals with type 1 diabetes. On average, individuals with type 1 diabetes have around two non-severe hypoglycaemic events per week, and at least one severe hypoglycaemic event per year1,2. Modern insulin treatment by (hybrid) closed-loop systems can mitigate these risks3. But the greatest risk factor for hypoglycaemia is an impaired ability to perceive a decrease in blood glucose and to respond to this in an adequate and timely manner2. This phenomenon is called impaired awareness of hypoglycaemia (IAH). Individuals with IAH have up to a 6-fold increased risk of having a severe hypoglycaemic event2. In addition to the cognitive disruption and detrimental psychosocial consequences of hypoglycaemia4, there are also significant deleterious effects on cardiovascular health and increased (cardiovascular) mortality5. Understanding and appropriately managing IAH can significantly reduce the risk of a hypoglycaemic event as well as improve overall quality of care.
Despite the exact pathophysiology of IAH remaining unknown, it is well understood that the counterregulatory response (CRR) is affected in individuals with IAH6. Individuals with type 1 diabetes and advanced type 2 diabetes rely on the sympathetic nervous system to mount an epinephrine response due to the relative hyperinsulinemia during hypoglycaemia, and the inability to release a sufficient glucagon response7,8. There is a general consensus that adaptive changes, or habituation, occurs in the central nervous system in response to recurrent hypoglycaemia, leading to IAH8,9. These adaptations have been observed in brain glucose transport, cerebral blood flow, glucose- and alternate fuel- metabolism in the brain6. Moreover, both animal and human models have extensively mapped the cerebral glucose sensing in response to hyper- and hypoglycaemia. Glucose thresholds for glucose-inhibited and glucose-excited neurons have been hypothesized to adapt in response to recurrent hypoglycaemia6,10. In response to a drop in blood glucose, these glucose-sensing pathways signal the hypothalamus-pituitary-adrenal axis that initiates the sympathoadrenal response10. The function of glucose-sensing brain regions and networks is therefore paramount for an adequate response to blood glucose decrease. However, we still are far from fully understanding the aetiology of IAH.
IAH is likely a consequence of a combination of genetic, environmental, and adaptive triggers. Though hypotheses driven studies have elucidated potential causes of IAH, a non-hypothesis driven approach may identify novel pathways, otherwise missed by traditional hypotheses driven approaches11. As high-throughput methods have become increasingly easier to conduct and the cost of -omics technologies more affordable, such methods have become more popular in investigating disease mechanisms. By investigating disease states at the genetic, protein, and metabolite levels, a more complete picture of disease mechanisms can be understood. In this study, we conducted a targeted proteomics analysis in plasma from individuals with type 1 diabetes with and without IAH matched for sex and age. Investigating the differentially expressed proteins between those with and without IAH can help to identify novel pathways that may be associated with the development and/or the occurrence of IAH.
Results
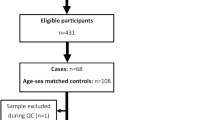
From the 611 individuals participating in Dutch type 1 diabetes Biomarkers cohort, 431 were eligible for inclusion (Fig. 1). Selection of cases (IAH) and controls, individuals with normal awareness of hypoglycaemia (NAH), led to the inclusion of 176 individuals.
In total, 162 individuals and 83 proteins passed quality control (Limit of detection (LOD) > 75%) and were included in the analysis. Characteristics of the included individuals are included in Table 1. The median age and diabetes duration were 43 and 22 years, respectively. Individuals with IAH were significantly older than those with NAH and had a longer diabetes duration. Included individuals were 41% male. Median insulin use was 50 units per day, significantly lower in the IAH group, and mean HbA1c was 61 mmol/mol (7.7%).
Differential NPX of proteins is shown in Fig. 2. Proteins with negative effect size estimates had lower levels in individuals with IAH in comparison to NAH, whilst positive effect sizes indicate higher levels in individuals with IAH. Agouti-related protein (AGRP) was significantly lower in individuals with IAH in comparison to those without IAH after false discovery rate (FDR) correction of P-values (6.12 NPX vs. 6.44 NPX, FDR adjusted P = 0.012, β = 0.98). When adjusted for sex and diabetes duration, AGRP reached near significance after adjustment for FDR (P = 0.057, β = 0.79). A further 9 proteins were found to be significant in the univariate analysis before P-value adjustment, annotated in Fig. 2.
Differentially expressed proteins in the multivariate model adjusted for sex and diabetes duration were largely similar as the univariate analysis and are shown in Fig. 3. Proteins that lost significant after multivariate adjustment are shown in supplementary Fig. 1. Sensitivity analyses with C-peptide, HbA1c, and medication use resulted in similar top differentially expressed proteins.
Figure 4 shows the ROC analysis for AGRP. The area under the curve was 0.66.
Volcano plot of differentially expressed proteins in Olink CVD II panel based on Student’s t-test. The x-axis shows effect sizes; the y-axis shows –log10(raw P-values). In red, proteins significant after FDR correction. Annotated proteins in blue are those with significant raw P-values before FDR correction.
Violin plots of the most significant differentially expressed proteins identified by multivariate regression analysis adjusting for sex and diabetes duration. P-values are adjusted for multiple testing using the false discovery rate (FDR). IAH, impaired awareness of hypoglycemia (red); NAH, normal awareness of hypoglycemia (blue).
Discussion
In this hypothesis-generating proteomics study, 8 proteins of interest were identified. AGRP, Interleukin- receptor alpha (IL-4RA), Pentraxin-related protein (PTX3), Serpin family A member 12 (SERPINA12) showed lower levels of expression in individuals with IAH. Whilst, NF-kappa-B essential modulator (NEMO), 2,4-dienoyl-CoA reductase 1 (DECR1), Interleukin-1 receptor-like 2 (IL1RL2), and Interleukin-16 (IL16) were expression as higher in those with IAH. In particular, AGRP expression was lower in individuals with IAH and significant before adjusting for sex and diabetes duration and FDR, and it reached near significance after accounting for these adjustments.
Agouti-related protein, or AGRP, is a well described peptide involved in energy balance. AGRP is expressed in the hypothalamus and adrenal glands, and its overexpression in the hypothalamus is related to hyperphagia and obesity, through its antagonistic effects on melanocortin-3 receptor and melanocortin-4 receptor12. In contrary, deficiency in AGRP has been associated with increased metabolic rate, resistance to fat accumulation, and a decrease in body weight12. AGRP expression has been hypothesized to be an important mechanism to respond to hypoglycaemia both through its effects on satiety as well as on the counter regulatory response13,14. In fasted states, the hypothalamic-pituitary-adrenal (HPA) axis is likely activated by AGRP neurons in the arcuate nucleus (ARC) through projections to the paraventricular hypothalamus (PVH) and lateral hypothalamus (LH)10. These projections can stimulate the sympathetic and parasympathetic nervous system in response to changes in glycaemic state. In particular, the catecholamine release after sympathetic activation increases glucagon release, which is essential in individuals with type 1 diabetes and advanced type 2 diabetes. Additionally, the release of corticotropin-releasing factor (CRF) in the PVH can be stimulated leading to an increased release of ACTH and an increase in cortisol15. AGRP is also highly expressed in the adrenal medulla, and has been hypothesized to exert local paracrine/autocrine effects on steroidogenesis16. Neurons in the arcuate and paraventricular nucleus are particularly of interest as they are heavily involved in glucose sensing, which may be influenced in individuals with IAH10. In rodent models, tyrosine hydroxylase expressing neurons of the nucleus of solitary tract (NTS) projecting to the ARC have been shown to regulate glucoprivic feeding through adrenergic modulation of AGRP and pro-opiomelanocortin (POMC)13. Recurrent hypoglycaemias have been hypothesized to alter the threshold of glucose sensing neurons, which may result in alteration in AGRP release in the arcuate nucleus17,18. Previous studies have shown that antecedent hypoglycaemia has a dampening effect on sympathetic activation, with lower circulating levels of cortisol and epinephrine being measured in plasma in response to hypoglycaemia19.
In our study, we observed that AGRP protein levels were lower in individuals with IAH in comparison to NAH. Previous studies have demonstrated that circulating plasma AGRP correlate strongly with AGRP levels in the brain20. Moreover, it has been shown to cross the blood brain barrier21. We therefore hypothesize that there may be a reduction in the activation of the HPA axis through the ARC/PVH pathways, which is reflected in the circulating AGRP. This may be a result of changes in glucose-sensing thresholds through habituation of AGRP/NPY neurons in the ARC or PVH, however, may also be a consequence of a less robust catecholaminergic response. Whether our findings are causal, or a consequence of IAH remains to be investigated, however, it demonstrates the potential importance of AGRP in IAH.
In addition to AGRP, other proteins were found to be expressed differently. DECR1, or dienoyl-CoA reductase, was more highly expressed in those with IAH and is an important enzyme in beta oxidation of polyunsaturated fatty acids. In individuals with defective mitochondrial fatty acid oxidation, a hypoketotic hypoglycaemia can occur after fasting or physical exercise, as a result of insufficient acetyl-CoA moieties for ketone body production22. Specifically, in DECR (-/-) mice, fasting induced hypoglycaemia, however, this was independent of ketogenesis; suggesting a role in impairment of gluconeogenesis23. An upregulation of DECR1 could therefore be an adaptive advantage in individuals with recurring hypoglycaemias to facilitate easier transition to ketogenesis and potentially gluconeogenesis.
Some proteins related to inflammation, such as IL-4RA, IL-16, and NEMO, were also found to be differentially expressed between those with and without IAH. Previous studies have demonstrated long-term effects of hypoglycaemia on the inflammatory response, with increases in circulating lymphocytes and monocytes of up to 1 week after hypoglycaemia in individuals with type 1 and type 2 diabetes24,25. This increase has been linked to the activation of the inflammatory response and correlated strongly with the adrenaline response after a hypoglycaemia. In individuals with type 2 diabetes, IL-6, FGF-21, and FGF-23 expression was increased after antecedent induced hypoglycaemia24. In our study, we found some inflammatory proteins to be differentially expressed, however FGF-21 did not show differential expression in our study. This is comparable to existing literature, where peripheral blood mononuclear cells (PMBCs) from individuals with IAH undergoing hypoglycaemia showed less cytokine response after stimulation in comparison to those without IAH25.
SERPINA12 is an adipokine that regulates insulin action by inhibiting serine-type endopeptidase activity. Circulating SERPINA12 has been reported to be higher in individuals with obesity, insulin resistance, and type 2 diabetes26,27. This is hypothesized to be a compensatory mechanism, whereby SERPINA12 expression is increased to ameliorate insulin sensitivity27. In our study we found lower levels of SERPINA12 in individuals with IAH. This may be due to more insulin sensitivity in the IAH group, resulting in less compensatory need for SERPINA12 expression. In our study, individuals with IAH had similar HbA1c to those with NAH, however daily insulin dose was lower. This could potentially suggest that these individuals are more insulin sensitive. However, other factors such as lipid lowering medication and antihypertensive medications can influence these results. Further studies may be of interest to investigate whether this is also a compensatory mechanism in IAH.
Limitations of our study include the restriction of the proteomics analysis to the CVD II O link panel and not an MS approach. This decision was made in order to balance the exploratory nature of the study with interpretability, and to sustain enough power in the analyses. Moreover, another limitation is that the analysis was limited to the cardiovascular panel and the measurement in plasma, which may not be ideal to investigate adaptations in the brain. However, as mentioned previously, circulating plasma AGRP has been shown to correlate well with brain AGRP secretion and is known to cross the blood brain barrier21. Therefore, despite no direct measurements in the CSF, which is an invasive procedure, circulating AGRP likely accurately represents cerebral fluctuations.
Further limitations include the use of an arbitrary unit of protein expression makes the comparability to other populations challenging. Differences measured in expression levels were also small and due to the cross-sectional nature of this study, the associations found will need to be further investigated in either experimental or longitudinal studies to elucidate any causal associations. As well as include measurements in healthy controls to enable comparison to normal levels.
Strengths of this study include the novelty of this proteomics approach in investigating IAH and the large sample size. Moreover, the differential expression of AGRP was robust with great significance in univariate analyses and a consistent signal after adjusting for potential confounders. Sensitivity analysis including potential confounders such as HbA1c, fasting glucose, and C-peptide also did not alter the results of the study.
In conclusion, we found significantly lower levels of AGRP in individuals with IAH. IL-4RA, NEMO, PTX3, SERPINA12, DECR1, IL1RL2, and IL16 were also differentially expressed between those with and without IAH. Findings in this study will need to be confirmed and validated in future studies but provides interesting new pathways to investigate in relation to IAH.
Methods
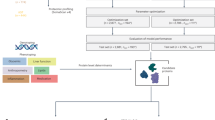
In this nested case-control study, plasma proteins from individuals with and without IAH were measured from the Dutch type 1 diabetes Biomarkers study. This study has been described in detail elsewhere28. In summary, this was a prospective cohort and data/biobank of individuals ≥ 16 years of age with type 1 diabetes. Data from baseline measurements were included if individuals had completed the validated Dutch translation of the Clarke questionnaire and were Western European29. Individuals were excluded if they had a diabetes duration of < 10 years and had cardiovascular disease (CVD), as IAH is more prevalent in individuals with longer diabetes duration. In addition, CVD is known to influence metabolic profiles30.
Definition of IAH
Individuals were considered to have IAH if they scored ≥ 3 on the Dutch Clarke questionnaire (maximum score = 5), this scoring has been validated in previous studies29. Nearest neighbour matching was used to match all individuals with IAH to sex and age matched controls (NAH) by 1:1.6 using the MatchIt package in R.
Blood samples
Blood samples for serum C-peptide measurements were collected in the fasting state, when possible, between 8:00 and 10:00. Routine laboratory data including HbA1c, lipid profile, serum creatinine and urinary albumin excretion were either collected on the same day, or obtained from medical records within the last year. C-peptide was measured by an immunoradiometric assay (IM3639, Beckman Coulter, Brea, California, USA). The limit of quantitation was 3.8 pmol/L, and interassay coefficient of variation (c.v.) was 9.1% at 6.5 pmol/L.
Protein level
Blood samples were taken in 10 ml EDTA BD Vacutainer® after an overnight fasting period whenever feasible. Following collection, the samples were centrifuged at 1300 g for 10 min, and the resulting supernatants were divided into 2 ml tubes. These aliquoted samples were then stored at -80 °C until needed for analysis. Plasma samples selected for inclusion were transported on dry ice to the Helmholtz Institute and subsequently maintained at -80 °C until they were thawed for analysis.
Protein level was measured using the Olink®Target 96 Cardiovascular panel II. This panel includes 92 proteins known to be involved in cardiovascular disease, as well as inflammatory markers and some exploratory proteins (Supplementary Table 1). Proteins are measured through proximity extension assay technology (Olink Proteomics AB, Uppsala, Sweden), using 1 µL of plasma per sample, according to the manufacturer’s instructions. Data are expressed as Normalized Protein Expression (NPX) units on a log2-scale. NPX values are an indication of relative expression of each protein.
Quality control was conducted using internal controls to assess the performance of the assays and the quality of individual samples. Intra and inter assay coefficient of variances (CV) were also calculated, and proteins with < 15% intra-assay CV and < 25% inter-assay CV were selected for further analysis. Moreover, only proteins measured in at least 75% of the samples were included in the analysis.
Statistical analysis
All statistical analyses were conducted using R Statistical Software (version 4.3.1). Descriptive statistics of demographic and anthropometric data are given as means with standard deviations, median with 25th and 75th quantile, or counts with percentages. Statistical differences were analysed with the students t-test, Kruskal-Wallis test, or Χ2 test, where appropriate.
Proteomics data analysis was conducted using the OlinkAnalyzer package in R31. Univariate analyses were conducted using Student’s t-test to compare protein expression between groups. Multivariate analyses was conducted using ANCOVA models to adjust for covariates, sex and diabetes duration. For proteins with significant raw P-values in the multivariate analysis, post-hoc ANOVA was applied. Sensitivity analyses were conducted by running models adjusted for HbA1c, fasting glucose, C-peptide and medication use. For both t-tests and ANCOVA analyses, raw P-values and P-values adjusted for false discovery rate (FDR) using the Benjamini-Hochberg procedure are reported. Additionally, receiver operating characteristic (ROC) analysis was conducted for AGRP, including calculation of the area under the curve (AUC) and visualization.
No formal sample size calculations were made as this was an exploratory study.
Data availability
The data are not publicly available due to privacy or ethical restrictions. However, the data of the study are available upon reasonable request to the Dutch type 1 Diabetes Biomarkers Study group. Contact information: bwo@umcg.nl.
References
Amiel, S. A. et al. Minimizing hypoglycemia in diabetes. Diabetes Care. 38(8), 1583–1591 (2015).
Cryer, P. E. Hypoglycemia in type 1 diabetes mellitus. Endocrinol. Metab. Clin. North. Am. 39(3), 641–654 (2010).
Diouri, O. et al. Hypoglycaemia detection and prediction techniques: A systematic review on the latest developments. Diabetes Metab. Res. Rev. 37(7), e3449 (2021).
Chatwin, H. et al. The impact of hypoglycaemia on quality of life outcomes among adults with type 1 diabetes: A systematic review. Diabetes Res. Clin. Pract. 174, 108752 (2021).
Heller, S. R. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 7(5), 385–396 (2019).
McCrimmon, R. J. Consequences of recurrent hypoglycaemia on brain function in diabetes. Diabetologia 64(5), 971–977 (2021).
Tesfaye, N. & Seaquist, E. R. Neuroendocrine responses to hypoglycemia. Ann. N Y Acad. Sci. 1212, 12–28 (2010).
Sankar, A., Khodai, T., McNeilly, A. D., McCrimmon, R. J. & Luckman, S. M. Experimental models of impaired Hypoglycaemia-Associated Counter-Regulation. Trends Endocrinol. Metab. 31(9), 691–703 (2020).
McNeilly, A. D. & McCrimmon, R. J. Impaired hypoglycaemia awareness in type 1 diabetes: lessons from the lab. Diabetologia 61(4), 743–750 (2018).
Stanley, S., Moheet, A. & Seaquist, E. R. Central mechanisms of glucose sensing and counterregulation in defense of hypoglycemia. Endocr. Rev. 40(3), 768–788 (2019).
Ning, M. & Lo, E. H. Opportunities and challenges in omics. Transl Stroke Res. 1(4), 233–237 (2010).
Ilnytska, O. & Argyropoulos, G. The role of the Agouti-Related protein in energy balance regulation. Cell. Mol. Life Sci. 65(17), 2721–2731 (2008).
Aklan, I. et al. NTS catecholamine neurons mediate hypoglycemic hunger via medial hypothalamic feeding pathways. Cell. Metab. 31(2), 313– (2020). – 26.e5.
Strembitska, A. et al. Lipid biosynthesis enzyme Agpat5 in AgRP-neurons is required for insulin-induced hypoglycemia sensing and glucagon secretion. Nat. Commun. 13(1), 5761 (2022).
Fernandes, A. C. A. et al. Arcuate AgRP, but not POMC neurons, modulate paraventricular CRF synthesis and release in response to fasting. Cell. Biosci. 12(1), 118 (2022).
Doghman, M., Soltani, Y., Rebuffet, V., Naville, D. & Bégeot, M. Role of Agouti-related protein in adrenal steroidogenesis. Mol. Cell. Endocrinol. 265-266, 108–112 (2007).
Yoon, N. A. & Diano, S. Hypothalamic glucose-sensing mechanisms. Diabetologia 64(5), 985–993 (2021).
Briski, K. P., Nedungadi, T. P. & Koshy Cherian, A. Effects of hypoglycaemia on neurotransmitter and hormone receptor gene expression in laser-dissected arcuate neuropeptide Y/agouti-related peptide neurones. J. Neuroendocrinol. 22(6), 599–607 (2010).
Nwokolo, M. A. O. et al. Restoration of hypoglycemia awareness alters brain activity in type 1 diabetes. Diabetes Care. 44(2), 533–540 (2021).
Page-Wilson, G. et al. Plasma Agouti-Related protein and cortisol levels in Cushing disease: evidence for the regulation of Agouti-Related protein by glucocorticoids in humans. J. Clin. Endocrinol. Metab. 104(3), 961–969 (2019).
Kastin, A. J., Akerstrom, V. & Hackler, L. Agouti-related protein(83–132) aggregates and crosses the blood-brain barrier slowly. Metabolism 49(11), 1444–1448 (2000).
Houten, S. M., Violante, S., Ventura, F. V. & Wanders, R. J. The biochemistry and physiology of mitochondrial fatty acid β-Oxidation and its genetic disorders. Annu. Rev. Physiol. 78, 23–44 (2016).
Miinalainen, I. J. et al. Mitochondrial 2,4-dienoyl-CoA reductase deficiency in mice results in severe hypoglycemia with stress intolerance and unimpaired ketogenesis. PLoS Genet. 5(7), e1000543 (2009).
Verhulst, C. E. M. et al. Sustained Proinflammatory effects of hypoglycemia in people with type 2 diabetes and in people without diabetes. Diabetes 71(12), 2716–2727 (2022).
Ratter, J. M. et al. Hypoglycaemia induces recruitment of non-classical monocytes and cytotoxic lymphocyte subsets in type 1 diabetes. Diabetologia 61(9), 2069–2071 (2018).
Escoté, X. et al. Role of omentin, vaspin, cardiotrophin-1, TWEAK and NOV/CCN3 in obesity and diabetes development. Int. J. Mol. Sci. 18(8) (2017).
Dimova, R. & Tankova, T. The role of Vaspin in the development of metabolic and glucose tolerance disorders and atherosclerosis. Biomed. Res. Int. 2015, 823481 (2015).
Wellens, M. J. et al. Residual C-peptide secretion and hypoglycemia awareness in people with type 1 diabetes. BMJ Open. Diabetes Res. Care 9(1) (2021).
Janssen, M. M., Snoek, F. J. & Heine, R. J. Assessing impaired hypoglycemia awareness in type 1 diabetes: agreement of self-report but not of field study data with the autonomic symptom threshold during experimental hypoglycemia. Diabetes Care. 23(4), 529–532 (2000).
McGarrah, R. W., Crown, S. B., Zhang, G. F., Shah, S. H. & Newgard, C. B. Cardiovascular metabolomics. Circ. Res. 122(9), 1238–1258 (2018).
Nevola, K. et al. OlinkAnalyze: Facilitate Analysis of Proteomic Data from Olink [software]. R package version 3.5.1 (2023).
Acknowledgements
The authors wish to thank all participants of the Dutch Type 1 Diabetes Biomarker Study, and the research staff of Diabeter, UMCG, Haaglanden Medical Center, and Ikazia Hospital which supported in the data collection.
Author information
Authors and Affiliations
Consortia
Contributions
RV authored the manuscript and contributed to the design, analysis and interpretation of data. AP and SH contributed to the acquisition of data and critically reviewed the manuscript. DM and HJA contributed to the design, acquisition and critically reviewed the manuscript. BW and MK contributed to the conception, design and acquisition of data and supervised the analysis, as well as critically reviewed the manuscript. BW and MK are guarantors of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the medical ethical review committee of the University Medical Center Groningen, Groningen, the Netherlands. Participants had given written informed consent to participant in the study, in accordance with the declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Varkevisser, R.D.M., Petrera, A., Hauck, S.M. et al. Targeted proteomics analysis in type 1 diabetes identifies lower agouti-related protein levels in individuals with impaired hypoglycaemia awareness. Sci Rep 15, 36448 (2025). https://doi.org/10.1038/s41598-025-20491-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-20491-y