Abstract
Glycine plays a central role in human metabolism, and an adequate supply is required for synthesizing glutathione (GSH), eliminating excess metabolites as acylglycine via the glycine conjugation detoxification pathway, and maintaining 1-carbon cycle activity. However, glycine is deficient in individuals with severe obesity, which may compromise these pathways and metabolic health. This exploratory study examines whether dietary glycine supplementation could correct glycine deficiency and impairments in glycine-dependent metabolic pathways. 19 participants with severe obesity (BMI 38.3 ± 5.3 kg/m2) were treated with dietary glycine (100 mg/kg/day) for two weeks. We found that treatment significantly increased the plasma concentration of glycine and enhanced the urinary excretion of isobutyrylglycine, tigylglycine, isovalerylglycine, and hexanoylglycine. There were no changes in body weight but significant reductions in plasma triglyceride and aminotransferases. The glutamate-serine-glycine index, an indirect marker of metabolic dysfunction-associated liver disease (MASLD), also improved. Treatment did not affect GSH but raised the plasma concentrations of serine, homocysteine, cysteine, and folate, which are 1-carbon cycle metabolites. We conclude that dietary glycine supplementation reversed obesity-associated glycine deficiency and enhanced the glycine conjugation detoxification reaction, 1-carbon cycle flux, and potentially the severity of MASLD. Glycine supplementation should be further investigated as a novel treatment for MASLD.
Clinical trials registry number NCT04658134 (https://tinyurl.com/7rthbwjb).
Similar content being viewed by others
Introduction
Glycine is the simplest amino acid in the human body but is central to human metabolism and is required in large amounts to maintain normal health. Conventionally regarded as a nutritionally non-essential amino acid, the body’s glycine supply is provided by the diet and endogenous synthesis1,2. However, glycine is deficient in individuals with obesity due to significantly slower de novo synthesis rates3. Low circulating glycine concentration correlates closely with various cardiometabolic disorders, and this may be related to the impact of glycine deficiency on the activity of glycine-dependent metabolic pathways1,2,4.
Low plasma glycine concentration correlates with insulin resistance, and healthy individuals with glycine deficiency are at higher risk of developing type 2 diabetes mellitus5,6. This association could be related to the direct effect of glycine on insulin secretion or indirectly via its role in the human antioxidant and detoxification systems1,2,4. Glycine is a precursor for glutathione (GSH) biosynthesis, and GSH is the predominant antioxidant molecule within cells7. An inadequate supply of glycine in individuals with obesity may limit the body’s ability to synthesize adequate amounts of GSH, exacerbating obesity-induced oxidative stress and thereby contributing to tissue and organ damage8,9. In addition, glycine functions as a phase II detoxicant by forming conjugates with potentially toxic endogenous and xenobiotic metabolites for excretion10. Recently, we reported that glycine deficiency led to impairment in the glycine conjugation pathway and resulted in a significant decrease in the elimination of endogenous and exogenous metabolites in individuals with severe obesity11. Since these metabolic reactions occur mainly in the liver, impairment in this pathway could lead to the accumulation of excess metabolites, including byproducts of amino acid and lipid metabolism, in the liver and thereby contribute to the pathogenesis of Metabolic Dysfunction-associated Steatotic Liver Disease (MASLD)12,13. Similarly, glycine is a primary donor of single-carbon units to maintain 1-carbon cycle14, a key metabolic process that occurs mainly in the liver and regulates various methylation, redox, and biosynthetic reactions15. Glycine deficiency could significantly disrupt 1-carbon metabolism and influence hepatic lipid metabolism by modulating methylation reactions, phosphatidylcholine production, energy homeostasis, and immune function16.
In individuals with obesity, glycine behaves as a conditionally essential amino acid3. Hence, glycine deficiency is potentially correctable using dietary supplements. Several studies have reported the ability of short-term dietary glycine supplementation to correct glycine deficiency in individuals with uncontrolled diabetes17, HIV infection18, and old age19. In addition, dietary glycine supplementation increased GSH availability and led to metabolic benefits such as reduced body weight, adiposity, insulin resistance, oxidative stress markers, and mitochondrial function17,18,20. However, the etiology and clinical impact of glycine deficiency in obesity may differ from other diseases, and dietary glycine supplementation has not been used to treat individuals with severe obesity. Hence, it remains unclear whether obesity-associated glycine deficiency can be corrected using dietary glycine supplements and whether this correction would result in similar metabolic benefits. Furthermore, defects in the glycine conjugation pathway in individuals with obesity are a relatively recent discovery11,21, and it is unknown whether glycine supplementation in humans can improve the formation and excretion of acylglycines.
This single-arm clinical trial is an exploratory study to determine the effectiveness of a two-week dietary glycine supplementation in correcting its deficiency in individuals with severe obesity and to evaluate its impact on the metabolic pathways that are dependent on glycine availability, including the glycine conjugation detoxification pathway, GSH synthesis, and the 1-carbon cycle.
Results
Baseline characteristics of study participants
21 participants were screened, and 19 were enrolled. All participants returned for their final study visit (Fig. 1). However, the mixed meal tolerance test (MMTT) was not performed on two participants due to challenging venous access. Post-treatment urine samples were also not collected from two participants due to menstrual bleeding. The average age of the participants was 42 ± 11 years, and there were more men (73.6%) than women. All participants had severe obesity with an average weight of 108 ± 16 kg and a BMI of 38.3 ± 5.3 kg/m2. 42.1% of the participants had hypertension, and 36.8% had hyperlipidemia. All subjects had normal renal function.
Treatment effect of glycine supplementation on plasma amino acids
Participants tolerated the glycine supplements well, as none developed adverse effects or discontinued their treatment. The compliance was high, as 93 (84–97) % of the supplements prescribed were consumed. None of the study participants took new medications during the study period. They also maintained their usual dietary habits, as there were no changes in their dietary energy and macronutrient intake (Supplementary Table 1). As shown in Table 1, glycine supplementation caused significant increases in the plasma concentrations of glycine by 49.2 ± 29.4 µmol/L (95% CI 35.0–63.4 µmol/L), serine by 16.1 ± 9.8 µmol/L (95% CI 11.4–20.8 µmol/L), and cysteine by 17.1 ± 24.3 µmol/L (95% CI 5.3–28.9). There was also a small but significant decrease in tryptophan by 2.0 ± 3.0 µmol/L (95% CI 3.5–0.57 µmol/L). The plasma concentrations of other amino acids did not change.
Treatment effect of glycine supplementation on clinical and metabolic parameters
As shown in Table 2, glycine supplementation did not cause any significant change in body weight and parameters of body composition. There was also no change in blood pressure and plasma total-, LDL- and HDL-cholesterol. However, plasma triglyceride concentration was significantly lower. Interestingly, glycine treatment significantly reduced the plasma concentrations of alanine transaminase and aspartate transaminase but had no significant effects on albumin, alkaline phosphatase, or gamma-glutamyl transferase plasma concentrations. Glycine supplementation did not significantly change HbA1C, fasting glucose, or fasting insulin. Similarly, treatment did not affect the area under the curves (AUCs) of glucose and insulin following MMTT. Measurements of insulin resistance (HOMA-IR and the Matsuda index) were also unaffected by glycine supplementation.
Treatment effect of glycine supplementation on the glycine conjugation pathway
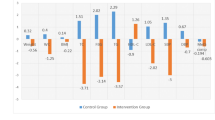
The ability of the glycine conjugation pathway to eliminate potentially toxic excess acylCoAs from the liver was quantified by measuring the plasma and urine concentrations of various acylglycines. Acetylglycine reflects the elimination of acetylCoA, while isobutyrylglycine, tigylglycine, and isovalerylglycine reflect the elimination of intermediates derived from branched-chain amino acid (BCAA) oxidation. Similarly, acylCoAs from medium-chain fatty acid oxidation are eliminated as hexanoylglycine and octanoylglycine, and long-chain fatty acid oxidation intermediates as palmitoylglycine. As shown in Fig. 2 (and Supplementary Table 2), urine concentrations of isobutyrylglycine, tigylglycine, isovalerylglycine, and hexanoylglycine increased significantly after glycine supplementation. However, urine acetylglycine, and octanoylglycine were not significantly different from baseline. The post-treatment change in acylglycine concentrations in plasma showed a similar trend to the corresponding urine values. However, only post-treatment plasma acetylglycine showed a statistically significant increase from 2.42 ± 0.97 to 3.06 ± 1.50 µmol/L (p = 0.002) (Supplementary Table 3).
Treatment effect of glycine supplementation on total energy expenditure, substrate oxidation rates, and plasma acylcarnitines
Glycine supplementation did not significantly change the resting energy expenditure or respiratory quotient. Accordingly, no treatment effects on fat, carbohydrate, or protein oxidation rates were observed (Supplementary Table 4). Acylcarnitine profiling provides an overview of cellular metabolic fuel selection. However, plasma concentrations of various acylcarnitines (Supplementary Table 5) and the urinary nitrogen excretion rate were unchanged post-treatment (Supplementary Table 4).
Treatment effect of glycine supplementation on red blood cell total glutathione, plasma malondialdehyde, folate, and homocysteine concentrations
As shown in Table 3, glycine supplementation did not significantly change red blood cell (RBC) total glutathione concentration or plasma malondialdehyde (MDA) concentration. Nonetheless, the GSG index decreased significantly, and there were also significant increases in plasma folate by 2.4 ± 4.5 µmol/L (95% CI 0.21–4.7 µmol/L) and homocysteine by 0.6 ± 1.2 µmol/L (95% CI 0.1–1.2 µmol/L).
Discussion
The primary aim of this study was to determine whether oral glycine supplementation would correct its deficiency in individuals with severe obesity and to evaluate its impact on the metabolic pathways that are dependent on glycine availability, including the glycine conjugation detoxification pathway, GSH synthesis, and the 1-carbon cycle. We found that glycine supplementation significantly increased the plasma concentration of glycine and enhanced the formation and urinary excretion of several acylglycines. Glycine supplementation lowered plasma triglyceride, hepatic aminotransferases, and the GSG index, a biomarker of MASLD, without changes in body weight, insulin resistance, energy expenditure, or whole-body substrate oxidation rates. Plasma concentration of several metabolites involved in the 1-carbon cycle increased post-treatment, but the treatment did not affect RBC GSH or oxidative stress marker concentrations. These findings indicate that oral glycine supplementation corrected glycine deficiency in individuals with severe obesity, which in turn increased flux through the glycine conjugation pathway and the one-carbon cycle. Most importantly, these changes were associated with marked improvements in several biomarkers of liver health.
Glycine is conventionally regarded as a nutritionally non-essential amino acid. However, glycine deficiency develops when the human body cannot synthesize adequate glycine to meet its metabolic demand. In the late stages of pregnancy, slower glycine de novo synthesis limits the ability of adolescent mothers to maintain glycine flux and compromises fetal growth22. Similarly, we found that a diminished de novo glycine synthesis rate led to glycine deficiency in individuals with severe obesity3. These findings suggest simple measures, such as dietary supplementation, could be used to correct glycine deficiency. Glycine is the smallest amino acid and is rapidly absorbed. A single dose of dietary glycine raises plasma glycine concentration after 10 min23, and earlier clinical trials showed that two weeks of dietary glycine supplementation at a similar dose to what was used in this study significantly raised glycine levels in individuals with glycine deficiency from HIV infection18, uncontrolled diabetes17, and old age19,24. However, glycine supplementation has not been attempted in individuals with severe obesity, and the amount or duration of glycine required to correct glycine deficiency in individuals with severe obesity is unknown. Participants in this study consumed an average of 83 g of protein, translating to approximately 2.5 g of glycine per day. We demonstrated that treatment with glycine supplements at 100 mg/kg/day (4 times the amount of the participants’ dietary glycine intake) for two weeks raised plasma glycine concentration by 35%. This finding confirms glycine de novo synthesis is impaired in the obese state and should be considered a conditionally essential amino acid. In addition, because the study subjects were glycine deficient, the additional 1.9 g of nitrogen intake from glycine supplementation was unlikely to have resulted in a state of positive nitrogen balance and increased urine nitrogen excretion.
Glycine participates in multiple metabolic reactions and is involved in the human body’s detoxification system. AcylCoAs, produced during cellular substrate metabolism, become metabolically toxic at high levels and must be cleared to maintain normal cellular function. The glycine conjugation pathway is a phase II detoxification reaction catalyzed by the mitochondrial enzyme glycine-N-acyltransferase. This reaction involves transferring the acyl group from acyl-CoA esters to the N-terminus of glycine to form N-acylglycines, which are cleared from the human body via urinary excretion. In obesity, accelerated substrate turnover rates result in a huge influx of substrates that require oxidation and clearance in the mitochondria. However, when the influx of substrates exceeds mitochondrial oxidative capacity, these intermediates accumulate in the liver as acylCoAs. This pathway serves as an alternative elimination route, but the short supply of glycine in the obese state may impair glycine conjugation and compromise this detoxification system. Several human and rodent studies found that obesity-associated glycine deficiency was associated with decreased urine excretion of acylglycines derived from acetylCoA and BCAA oxidation intermediates21,24,25. Using stable-isotope tracers, we recently confirmed that obesity-associated glycine deficiency compromised the synthesis and clearance of endogenous and xenobiotic acylCoAs as acylglycines3.
The metabolic consequence of impaired detoxification via this glycine-dependent pathway is unclear. However, since this reaction occurs primarily in the liver, glycine deficiency could impede the elimination of excess acylCoAs from the liver of individuals with obesity and contribute to MASLD pathogenesis. This also means that interventions that enhance this elimination pathway could be used to treat MASLD. In this study, we demonstrated that the correction of glycine deficiency with dietary supplements increased the urinary excretion of acylglycine derived from the intermediates of the BCAAs (isobutyrylglycine, tigylglycine, and isovalerylglycine) and fatty acid oxidation (hexanoylglycine). The higher urinary excretion of isobutyrylglycine, tigylglycine, and isovalerylglycine also suggests that glycine supplementation increases hepatic BCAA catabolism. However, the plasma BCAA concentrations were not affected. In addition to the liver, the plasma concentration of BCAAs is also determined by the release and uptake of BCAAs from other organs, such as skeletal muscle and adipose tissue26. Thus, the lack of post-treatment alteration in plasma BCAA concentration could be attributed to the effect of glycine supplementation on BCAA metabolism in different organs. These tissue-specific effects were observed in mice receiving glycine supplementation27, but it needs to be further investigated in humans.
Our findings were consistent with an earlier study that showed greater excretion of acylglycines and reductions in hepatic short- and intermediate-chain acylCoAs without altering blood BCAA levels in Zucker Fatty Rats receiving dietary glycine supplements27. We also detected statistically significant post-treatment reductions in plasma triglyceride, hepatic transaminases, and the GSG index. We did not perform any imaging or histological examination of liver tissue. However, these parameters are markers of MASLD28,29, and the GSG index can predict the presence of biopsy-proven hepatic fibrosis30. Thus, we hypothesize that glycine supplementation reduces hepatic steatosis and inflammation by enhancing the elimination of excess acylCoAs in the liver as acylglycines.
Glycine supplements’ ability to reduce MASLD severity in individuals with obesity needs additional validation. Nonetheless, the therapeutic potential of glycine as a novel treatment for MASLD is supported by recent preclinical studies that demonstrated the ability of glycine-based supplements to reduce hepatic fat, inflammation, and fibrosis in rodents and non-human primates with MASLD31,32. However, these improvements were attributed to glycine’s ability to enhance GSH synthesis, redox balance, and fatty acid oxidation. GSH is the most abundant intracellular antioxidant synthesized using glycine, cysteine, and glutamine, and a lack of GSH could contribute to oxidative stress and the development of obesity-associated complications. Glycine may be rate-limiting for GSH synthesis9, and we recently reported impaired RBC GSH synthesis in individuals with obesity-associated glycine deficiency8. Thus, glycine supplementation could increase GSH availability and improve redox balance. In individuals with elevated oxidative stress due to aging, HIV infection, and uncontrolled diabetes, glycine and cysteine supplementation raised GSH concentration and lowered oxidative stress17,18,20. By contrast, in our study, RBC GSH and plasma MDA concentrations did not improve following glycine supplementation. Glycine utilization for GSH synthesis may depend on individuals' redox status. In the largest randomized controlled trial using glycine (and cystine) supplementation, treatment of 114 healthy older adults also did not improve RBC GSH or reduce oxidative stress. However, GSH levels did increase in a subset of participants with low GSH and high MDA levels at baseline19. Our study participants were relatively young and free from any serious chronic diseases. Therefore, oxidative stress levels and GSH demand in these participants may not be enough to divert glycine supply for GSH synthesis.
With glycine supplementation, we observed a significant increase in plasma serine by 17%. Glycine and serine are interconvertible via the serine hydroxymethyltransferase (SHMT), and the increase in serine reflects the rapid conversion of glycine to serine33,34. In addition, total cysteine, folate, and homocysteine concentrations were significantly higher post-treatment. Glycine and serine are principal donors of single-carbon units to the one-carbon metabolism cycle, a key metabolic process in the human body that involves folate-mediated movement of single-carbon units for methylation, redox, and biosynthetic reactions15. Homocysteine, as a component of the one-carbon cycle, can be converted to methionine via the trans-methylation pathway or cysteine via the trans-sulfuration pathway15,35. Thus, we interpret the higher post-treatment values of serine, folate, homocysteine, and cysteine as the consequence of the greater entry of one-carbon units, hence, flux along the one-carbon cycle. As the liver is also the major site for one-carbon metabolism, which is dysregulated in MASLD16, glycine treatment may benefit liver health through this mechanism. This notion is supported by a recent pre-clinical study that showed that the downregulation of hepatic SHMT2 in mice led to changes in hepatic glycine and serine content, which reduced hepatic methylation potential and modulated hepatic fat synthesis, fibrosis, and inflammation36.
The slight elevation of homocysteine observed in our study is unexpected since serine and folate lower homocysteine levels in individuals with hyperhomocysteinemia37,38. Higher plasma homocysteine levels have been associated with a greater incidence of coronary heart disease39. The increase in plasma homocysteine concentration in this study was marginal at 0.6 µmol/L, and the causal relationship between hyperhomocysteinemia and adverse health outcomes is still uncertain40,41. Nonetheless, to truly understand the safety of consuming dietary glycine supplements at the current dose, future long-term studies will be needed to confirm the effects of dietary glycine supplements on homocysteine levels and adverse clinical outcomes. Further, dedicated experiments are also required to examine its metabolic impact on various methylation reactions.
We did not detect significant body weight or composition changes in the study participants following glycine supplementation. This is unsurprising as they maintained the same dietary energy and macronutrient intake throughout the study. Furthermore, indirect calorimetry measurements and plasma acylcarnitine profiling indicate that glycine supplementation did not shift energy expenditure or metabolic fuel selection. Glycine correlates inversely with insulin resistance and diabetes risk. However, the ability of glycine to modulate glucose regulation remains unresolved. While some human studies have reported the potential effects of a single dose of oral glycine to reduce glucose excursion or increase insulin secretion23,42, other studies using oral or intravenous glycine failed to demonstrate any response43,44. Similarly, individuals with metabolic syndrome treated with high doses of oral glycine for 3 months did not demonstrate any significant change in plasma insulin concentration45. Our study was consistent with these findings as we did not detect any statistically significant post-treatment changes in glucose and insulin values at fasting or following glucose challenge.
This study demonstrates that glycine supplementation in individuals with severe obesity enhances the glycine conjugation detoxification pathway and one carbon cycle. These two metabolic reactions occur primarily in the liver, and the associated improvement in MASLD markers suggests glycine supplements could be developed as a low-risk, cost-effective treatment for NAFD. However, there are several limitations. We showed that dietary glycine supplementation increased plasma glycine concentration by 35%, achieving a value similar to healthy normal-weight individuals (~ 200 µmol/L) from another study3. However, our study did not include a healthy control group to confirm the correction of glycine deficiency. Further, as an exploratory study with a limited sample size and short study duration, this study is designed to demonstrate the ability of dietary glycine to raise post-treatment plasma glycine concentration and not improvements in clinical parameters or outcomes. Our study also did not perform liver imaging or histological examination of liver tissue, but MASLD is almost always present in patients with severe obesity46. Previous glycine supplementation trials were also limited by their study design, sample size, and duration, and have yet demonstrated a definite improvement in clinical outcomes47,48. Therefore, future randomized controlled trials with an adequately powered sample size and longer study duration will be necessary to confirm the clinical efficacy and safety of dietary glycine supplementation. Specifically, liver imaging or biopsy will be required to verify the effectiveness of dietary glycine supplementation in treating MASLD. Additional studies will also be required to mechanistically link enhanced urine acylglycine excretion and one carbon cycle activity with MASLD pathogenesis. Further, the downstream metabolic sequelae from enhanced acylglycine excretion and 1-carbon cycle activity in other organs and tissues should be explored in future efforts.
In conclusion, we found that dietary glycine supplements corrected obesity-associated glycine deficiency, increased urine acylglycine excretion, and plasma metabolites associated with one-carbon metabolism. The post-treatment improvement in hepatic markers suggests that glycine supplements should be investigated as a potential novel treatment for MASLD.
Methods
Study participants
Study participants (age 21–65 years) with severe obesity (BMI > 32.5 kg/m2) were recruited from the Singapore General Hospital’s weight management clinic from January 2021 until November 2021. They were excluded if they had Diabetes Mellitus, uncontrolled thyroid disorders, excessive alcohol intake (> 1 unit per day for women and > 2 units per day for men), systemic steroid usage, weight-loss medication usage, significant cardiac, renal, or liver dysfunction. This study received approval from the SingHealth Centralized Institutional Review Board (CIRB Ref: 202008-00040), and all participants provided written informed consent. This study complied with the International Standard of Good Clinical Practice (ICH E6-GCP) procedures and the principles of the Declaration of Helsinki (1964).
Study procedures
At baseline, study participants reported in the morning following a 10-h fast. Blood samples were collected for metabolic profiling, followed by anthropometric measurement, body composition analysis, and MMTT. Study participants also submitted urine samples collected from the night before the study visit (~ 10 h). Participants were then treated with oral glycine supplementation for two weeks and reassessed.
Participants maintained their usual diet throughout the study and recorded their food intake for 3-days before their study visit, which a clinical research coordinator further verified. Total energy and macronutrient intakes were analyzed using the nutrient analysis software on the local food database (Dietplan7, Frestfield Software, UK). Height was measured on a stadiometer. Waist circumference was measured midway between the inferior margin of the last rib and the iliac crest. Hip circumference was the widest circumference over the great trochanters. Body composition, fat-free mass, fat mass, and fat mass percentage were measured using tetrapolar bioelectric impedance analysis (Tanita Body Composition scale, model TBF-300, Tanita Corporation, Tokyo, Japan). All parameters were measured three times and averaged.
The participant’s CO2 exhalation and O2 consumption were measured using an indirect calorimeter (Quark RMR, Cosmed, Rome, Italy). This procedure was performed for 30 min when participants were fasting. Parameters were obtained to calculate resting energy expenditure, respiratory quotient, and carbohydrate and fat oxidation rates.
MMTT was performed to measure insulin resistance and glucose tolerance. During MMTT, a liquid meal (Ensure©Plus, Abbott Nutrition) was given at 6 kcal/kg (max 360 kcal). The liquid meal consisted of 30% energy from fat, 15% from protein, and 55% from carbohydrates. Blood samples were collected before and at 30, 60, 90, and 120 min after MMTT for plasma glucose and insulin measurements.
Glycine supplement
The glycine supplement (NOW Foods, Illinois, USA) was purchased commercially as bottled capsules. Each glycine capsule contained 1 g of pure glycine and was manufactured according to Good Manufacturing Product standards. Oral glycine supplements at 100 mg/kg/day were prescribed to study participants for 14 days. Glycine tablets were consumed in four divided doses, with the last dose taken at 10 pm. Subjects were asked to record the intake of their supplements, including the doses they missed. The number of glycine capsules dispensed and returned was carefully recorded, and compliance was calculated as the percentage of glycine consumed over the glycine supplements prescribed.
Laboratory measurements
Plasma glucose, insulin, and urine nitrogen
Clinical biochemistry measurements were performed using standard methods on fasting samples. Plasma insulin concentration was measured using an immunoassay method (Abbott Architect i200; Abbott Diagnostics), and plasma glucose concentration by the glucose oxidase method (YSI Glucose Analyzer; YSI). Urine nitrogen was measured using the Kjeldahl Nitrogen Analyzer (Hanon Automatic Kjeldahl Nitrogen Analyzer, Hanon Advanced Technology Group Co., Ltd, Jinan, China).
Plasma amino acids
Plasma amino acid concentrations were measured by ultra-high performance liquid chromatography (ACQUITY H-Class System, Waters Corporation, MA, USA) using pre-column derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (Waters AccQ × Tag™ assay kit, MA, USA) and norvaline (Sigma Aldrich, MO, USA) as internal standard as previously described11.
Plasma and urine acylglycines
The plasma and urine concentrations of acetylglycine, isobutyrylglycine, tigylglycine, isovalerylglycine, hexanoylglycine, and octanoylglycine were measured. Palmitoylglycine concentration could not be reliably measured as the internal standard of this metabolite could not be prepared due to its poor solubility. Samples were butylated according to the method of Hobert et al.49 & Fisher et al.50 and analyzed by LC tandem MS (TSQ Altis, Thermo Scientific) as previously described11. Urine acylglycine concentrations were further corrected for renal function and expressed as mmol/mol creatinine (crt).
Plasma acylcarnitines
The plasma concentrations of acetylcarnitine, propionylcarnitine, butyrylcarnitine, and isovalerylcarnitine, octanoylcarnitine, myristoylcarnitine, and palmitoylcarnitine were quantified by in vitro isotope dilution method using internal standards from labeled carnitine standards set B (NSK-B, Cambridge Isotope Laboratories Inc.) and analyzed by LC tandem MS (TSQ Altis; Thermo Scientific)11.
Red blood cell glutathione and plasma malondialdehyde
Total intracellular GSH concentration was measured in RBC based on in vitro isotope dilution using [Gly-13C2, 15N]-GSH (Cambridge Isotope Laboratories) as an internal standard8. Acetonitrile/water (1:1) buffer was added to RBC, and then the samples were lysed by freezing and thawing. After centrifugation, the supernatant was transferred and dried. Dithiothreitol (60 mmol/L in 0.1 mol sodium tetraborate/L) was added to convert oxidized glutathione (GSSG) to GSH and was then alkylated by adding iodoacetamide (0.5 mol/L) in 0.1 mol ammonium bicarbonate/L. Alkylated GSH was converted into its DANS [5-(dimethylamino)-1-napthalene sulfonamide] derivative and analyzed using a Kinetex C18 2.6μ 100 × 2.1 mm column (Phenomenex, Torrance, CA) on a triple quadrupole mass spectrometer (TSQ Altis; Thermo Scientific, San Jose, CA). The ions were then analyzed using SRM mode. The transitions observed were m/z 598 to 380 & 601 to 380. Malondialdehyde (MDA), a naturally occurring product of lipid peroxidation and an indicator of oxidative stress, was measured with ELISA using TBARS Assay (Cayman Chemical Company, Ann Arbor, MI, USA).
Calculations
Insulin resistance
Insulin resistance was calculated based on the HOMA-IR51. Insulin sensitivity was calculated using post-MMTT glucose and insulin values as the Matsuda index52. The AUCs for glucose and insulin following MMTT were also quantified using the trapezoidal rule.
Resting energy expenditure, substrate oxidation rate
Resting energy expenditure (REE) was calculated using Weir’s equation53 and glucose and fat oxidation according to the equation by Frayn54 as listed below
VCO2 and VO2 are gaseous exchanges (L/min) obtained from the calorimeter, and N is the urinary nitrogen excretion (g/min).
Glutamate-serine-glycine index
The GSG index is a novel indicator of MASLD severity29,30. This index was calculated using the plasma concentrations of glutamate/(serine + glycine).
Statistical analysis
The primary objective of this study was to evaluate whether oral glycine supplements correct glycine deficiency. We previously reported that plasma glycine concentration in individuals with morbid obesity was approximately 26% (or 40 ± 13 µmol/L) lower than controls with healthy weight3. We predict that two weeks of treatment with dietary glycine supplements at 100 mg/kg, which is equivalent to four folds in the daily glycine intake, will raise plasma glycine concentration by at least 25% or 40 µmol/L (assuming a standard deviation of 49 µmol/L). A sample size of 14 will be required to detect this change with a power of 80% and a Type 1 error of 0.05. We recruited a larger sample size (n = 19) for a 25% loss follow-up.
Measured outcomes are the post-treatment changes in plasma amino acid concentrations and metabolic measurements. Data were first examined for normality, and for data that followed a normal distribution, significant changes after glycine supplementation were tested using paired t-test. The Wilcoxon’s Signed Rank test was used for data that did not follow a normal distribution. Data are presented as mean ± standard deviation (SD) or median (inter-quartile range). A 2-tailed P value < 0.05 was considered statistically significant. Statistical testing was performed using STATA version 17 (StataCorp, Texas, USA) and Prism version 10 (GraphPad Software Inc.
Data availability
Data described in the manuscript, code book, and analytic code will be available by submitting a request to the corresponding author.
Abbreviations
- GSH:
-
Glutathione
- BCAA:
-
Branched-chain amino acid
- SHMT:
-
Serine hydroxymethyltransferase
- MASLD:
-
Metabolic Dysfunction-associated Steatotic Liver Disease
- MMTT:
-
Mixed-meal Tolerance Testing
- HOMA-IR:
-
Homeostatic Model Assessment for Insulin Resistance
- AUC:
-
Area under the curve
- GSG:
-
Glutamate-serine-glycine
- RBC:
-
Red blood cell
References
Alves, A., Bassot, A., Bulteau, A. L., Pirola, L. & Morio, B. Glycine metabolism and its alterations in obesity and metabolic diseases. Nutrients https://doi.org/10.3390/nu11061356 (2019).
Adeva-Andany, M. et al. Insulin resistance and glycine metabolism in humans. Amino Acids 50, 11–27. https://doi.org/10.1007/s00726-017-2508-0 (2018).
Hong Chang Tan, J. W. H., E Shyong Tai, Shaji Chacko, Vieon Wu, Chun Fan Lee, Jean-Paul Kovalik, Farook Jahoor. De novo glycine synthesis is reduced in adults with morbid obesity and increases following bariatric surgery. Front. Endocrinol. (2022).
Yan-Do, R. & MacDonald, P. E. Impaired, “Glycine”-mia in Type 2 diabetes and potential mechanisms contributing to glucose homeostasis. Endocrinology 158, 1064–1073. https://doi.org/10.1210/en.2017-00148 (2017).
Floegel, A. et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 62, 639–648. https://doi.org/10.2337/db12-0495 (2013).
Gall, W. E. et al. Alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE 5, e10883. https://doi.org/10.1371/journal.pone.0010883 (2010).
Lu, S. C. Regulation of glutathione synthesis. Mol. Aspects Med. 30, 42–59. https://doi.org/10.1016/j.mam.2008.05.005 (2009).
Tan, H. C. et al. The impact of bariatric surgery on glutathione synthesis in individuals with severe obesity. Antioxidants (Basel). https://doi.org/10.3390/antiox13080967 (2024).
McCarty, M. F., O’Keefe, J. H. & DiNicolantonio, J. J. Dietary glycine is rate-limiting for glutathione synthesis and may have broad potential for health protection. Ochsner. J. 18, 81–87 (2018).
Badenhorst, C. P., van der Sluis, R., Erasmus, E. & van Dijk, A. A. Glycine conjugation: Importance in metabolism, the role of glycine N-acyltransferase, and factors that influence interindividual variation. Expert Opin. Drug Metab. Toxicol. 9, 1139–1153. https://doi.org/10.1517/17425255.2013.796929 (2013).
Tan, H. C. et al. The impact of obesity-associated glycine deficiency on the elimination of endogenous and exogenous metabolites via the glycine conjugation pathway. Front. Endocrinol. Lausanne. 15, 1343738. https://doi.org/10.3389/fendo.2024.1343738 (2024).
Kawano, Y. & Cohen, D. E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 48, 434–441. https://doi.org/10.1007/s00535-013-0758-5 (2013).
Grenier-Larouche, T. et al. Altered branched-chain alpha-keto acid metabolism is a feature of NAFLD in individuals with severe obesity. JCI Insight. https://doi.org/10.1172/jci.insight.159204 (2022).
Lamers, Y. et al. Production of 1-carbon units from glycine is extensive in healthy men and women. J. Nutr. 139, 666–671. https://doi.org/10.3945/jn.108.103580 (2009).
Locasale, J. W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583. https://doi.org/10.1038/nrc3557 (2013).
da Silva, R. P., Eudy, B. J. & Deminice, R. One-carbon metabolism in fatty liver disease and fibrosis: One-carbon to rule them all. J. Nutr. 150, 994–1003. https://doi.org/10.1093/jn/nxaa032 (2020).
Sekhar, R. V. et al. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 34, 162–167. https://doi.org/10.2337/dc10-1006 (2011).
Nguyen, D., Hsu, J. W., Jahoor, F. & Sekhar, R. V. Effect of increasing glutathione with cysteine and glycine supplementation on mitochondrial fuel oxidation, insulin sensitivity, and body composition in older HIV-infected patients. J. Clin. Endocrinol. Metab. 99, 169–177. https://doi.org/10.1210/jc.2013-2376 (2014).
Lizzo, G. et al. A Randomized controlled clinical trial in healthy older adults to determine efficacy of glycine and N-acetylcysteine supplementation on glutathione redox status and oxidative damage. Front. Aging 3, 852569. https://doi.org/10.3389/fragi.2022.852569 (2022).
Kumar, P. et al. Supplementing glycine and N-acetylcysteine (GlyNAC) in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, physical function, and aging hallmarks: A randomized clinical trial. J. Gerontol. A Biol. Sci. Med. Sci. 78, 75–89. https://doi.org/10.1093/gerona/glac135 (2023).
Newgard, C. B. et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9, 311–326. https://doi.org/10.1016/j.cmet.2009.02.002 (2009).
Hsu, J. W. et al. Unlike pregnant adult women, pregnant adolescent girls cannot maintain glycine flux during late pregnancy because of decreased synthesis from serine. Br. J. Nutr. 115, 759–763. https://doi.org/10.1017/S0007114515005279 (2016).
Gannon, M. C., Nuttall, J. A. & Nuttall, F. Q. The metabolic response to ingested glycine. Am. J. Clin. Nutr. 76, 1302–1307. https://doi.org/10.1093/ajcn/76.6.1302 (2002).
Glynn, E. L. et al. Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia 58, 2324–2335. https://doi.org/10.1007/s00125-015-3705-6 (2015).
White, P. J. et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab. 5, 538–551. https://doi.org/10.1016/j.molmet.2016.04.006 (2016).
She, P. et al. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am. J. Physiol. Endocrinol. Metab. 293, E1552-1563. https://doi.org/10.1152/ajpendo.00134.2007 (2007).
White, P. J. et al. Muscle-liver trafficking of BCAA-derived nitrogen underlies obesity-related glycine depletion. Cell Rep. 33, 108375. https://doi.org/10.1016/j.celrep.2020.108375 (2020).
Neuman, M. G., Cohen, L. B. & Nanau, R. M. Biomarkers in nonalcoholic fatty liver disease. Can. J. Gastroenterol. Hepatol. 28, 607–618. https://doi.org/10.1155/2014/757929 (2014).
Masoodi, M. et al. Metabolomics and lipidomics in NAFLD: Biomarkers and non-invasive diagnostic tests. Nat. Rev. Gastroenterol. Hepatol. 18, 835–856. https://doi.org/10.1038/s41575-021-00502-9 (2021).
Gaggini, M. et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology 67, 145–158. https://doi.org/10.1002/hep.29465 (2018).
Rom, O. et al. Glycine-based treatment ameliorates NAFLD by modulating fatty acid oxidation, glutathione synthesis, and the gut microbiome. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aaz2841 (2020).
Qu, P. et al. DT-109 ameliorates nonalcoholic steatohepatitis in nonhuman primates. Cell Metab. 35, 742-757.e710. https://doi.org/10.1016/j.cmet.2023.03.013 (2023).
Lamers, Y., Williamson, J., Gilbert, L. R., Stacpoole, P. W. & Gregory, J. F. 3rd. Glycine turnover and decarboxylation rate quantified in healthy men and women using primed, constant infusions of [1,2-(13)C2]glycine and [(2)H3]leucine. J. Nutr. 137, 2647–2652. https://doi.org/10.1093/jn/137.12.2647 (2007).
Matthews, D. E., Conway, J. M., Young, V. R. & Bier, D. M. Glycine nitrogen metabolism in man. Metabolism 30, 886–893. https://doi.org/10.1016/0026-0495(81)90067-6 (1981).
Jahoor, F. Effects of decreased availability of sulfur amino acids in severe childhood undernutrition. Nutr. Rev. 70, 176–187. https://doi.org/10.1111/j.1753-4887.2011.00462.x (2012).
Chen, G. et al. SHMT2 reduces fatty liver but is necessary for liver inflammation and fibrosis in mice. Commun. Biol. 7, 173. https://doi.org/10.1038/s42003-024-05861-y (2024).
Verhoef, P. et al. Dietary serine and cystine attenuate the homocysteine-raising effect of dietary methionine: A randomized crossover trial in humans. Am. J. Clin. Nutr. 80, 674–679. https://doi.org/10.1093/ajcn/80.3.674 (2004).
Kaye, A. D. et al. Folic acid supplementation in patients with elevated homocysteine levels. Adv. Ther. 37, 4149–4164. https://doi.org/10.1007/s12325-020-01474-z (2020).
Humphrey, L. L., Fu, R., Rogers, K., Freeman, M. & Helfand, M. Homocysteine level and coronary heart disease incidence: A systematic review and meta-analysis. Mayo Clin. Proc. 83, 1203–1212. https://doi.org/10.4065/83.11.1203 (2008).
Herrmann, W. & Herrmann, M. The controversial role of HCY and vitamin B deficiency in cardiovascular diseases. Nutrients https://doi.org/10.3390/nu14071412 (2022).
McCaddon, A. & Miller, J. W. Homocysteine-a retrospective and prospective appraisal. Front. Nutr. 10, 1179807. https://doi.org/10.3389/fnut.2023.1179807 (2023).
Gonzalez-Ortiz, M., Medina-Santillan, R., Martinez-Abundis, E. & von Drateln, C. R. Effect of glycine on insulin secretion and action in healthy first-degree relatives of type 2 diabetes mellitus patients. Horm. Metab. Res. 33, 358–360. https://doi.org/10.1055/s-2001-15421 (2001).
Kasai, K., Kobayashi, M. & Shimoda, S. I. Stimulatory effect of glycine on human growth hormone secretion. Metabolism 27, 201–208. https://doi.org/10.1016/0026-0495(78)90165-8 (1978).
Muller, W. A., Aoki, T. T. & Cahill, G. F. Jr. Effect of alanine and glycine on glucagon secretion in postabsorptive and fasting obese man. J. Clin. Endocrinol. Metab. 40, 418–425. https://doi.org/10.1210/jcem-40-3-418 (1975).
Diaz-Flores, M. et al. Oral supplementation with glycine reduces oxidative stress in patients with metabolic syndrome, improving their systolic blood pressure. Can. J. Physiol. Pharmacol. 91, 855–860. https://doi.org/10.1139/cjpp-2012-0341 (2013).
Polyzos, S. A., Kountouras, J. & Mantzoros, C. S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 92, 82–97. https://doi.org/10.1016/j.metabol.2018.11.014 (2019).
Holecek, M. Glycine as a conditionally essential amino acid and its relationship to l-serine. Metabolism 170, 156330. https://doi.org/10.1016/j.metabol.2025.156330 (2025).
Soh, J. et al. The effect of glycine administration on the characteristics of physiological systems in human adults: A systematic review. Geroscience 46, 219–239. https://doi.org/10.1007/s11357-023-00970-8 (2024).
Hobert, J. A., Liu, A. & Pasquali, M. Acylglycine analysis by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). Curr. Protoc. Hum. Genet. 91, 172511–172512. https://doi.org/10.1002/cphg.19 (2016).
Fisher, L. et al. A novel method for quantitation of acylglycines in human dried blood spots by UPLC-tandem mass spectrometry. Clin. Biochem. 54, 131–138. https://doi.org/10.1016/j.clinbiochem.2018.01.020 (2018).
Matthews, D. R. et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Matsuda, M. & DeFronzo, R. A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999).
de V Weir, J. B. New methods for calculating metabolic rate with special reference to protein metabolism.
Frayn, K. N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. Respir. Environ. Exercise Physiol. 55, 628–634 (1983).
Funding
This study was supported by the Khoo Mentored Research Grant (Duke-NUS KMRA 2020/0013).
Author information
Authors and Affiliations
Contributions
HCT, JWH, EST, PMY and JPK designed research; HCT, JWH, SC, VW conducted research; HCT and JWH analyzed data; and HCT, JWH, and FJ wrote the paper. HCT had primary responsibility for the final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tan, H., Hsu, J.W., Tai, E. et al. Metabolic impact of dietary glycine supplementation in individuals with severe obesity. Sci Rep 15, 36433 (2025). https://doi.org/10.1038/s41598-025-20511-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-20511-x