Abstract
Asphaltene precipitation in carbonate reservoirs presents a significant flow assurance challenge. This study investigates a novel ZnO/SiO₂/xanthan/eucalyptus nanocomposite (NCs) for inhibiting asphaltene deposition. A multiscale analysis was employed, incorporating adsorption isotherms, atomic force microscopy (AFM). and core-flooding under realistic reservoir conditions (90 °C, up to 3700 psi). Adsorption isotherm analysis confirmed that the Langmuir model provided the best data fit, indicating monolayer adsorption with a high capacity (Qm = 185.2 mg/g). Interfacial tension (IFT) measurements demonstrated NCs altered the IFT-pressure slope by 45.71%, indicating enhanced inhibition. AFM analysis revealed NCs significantly reduced surface roughness, decreasing average roughness (Ra) from 56.70 to 11.42 nm. Core-flood experiments confirmed NCs mitigated permeability impairment by over 50% and reduced asphaltene precipitation by up to 4.00 wt.% during natural depletion. The results demonstrate that the synthesized NCs are a highly effective inhibitor for mitigating asphaltene-related formation damage in carbonate reservoirs.
Similar content being viewed by others
Introduction
Asphaltene precipitation during oil production is a major flow assurance challenge that causes significant operational disruptions and economic losses1. Crude oil consists of four primary components: asphaltenes, saturates, aromatics, and resins. While non-paraffinic solvents such as pentane and heptane cannot dissolve asphaltenes, aromatic solvents like toluene can effectively dissolve them2. Asphaltene precipitation occurs due to thermodynamic equilibrium shifts caused by variations in temperature, pressure, and composition2. Once precipitated, asphaltenes can cause significant damage to wellbore tubing and production equipment. Alhumaidan et al.3 reported that precipitated asphaltenes can be removed through mechanical, solvent, or surfactant-based methods.
Common methods to remediate asphaltene deposition include mechanical removal, solvent washing, and surfactant injection. However, due to the high cost and operational challenges associated with these techniques, the application of adsorbents has gained interest for heavy oil upgrading and flow assurance4. Adsorbents have been employed in heavy oil upgrading due to the high costs associated with alternative techniques5. The adsorption of asphaltenes onto solid surfaces is primarily influenced by their thiophenic, pyrrolic, pyridinic, and carboxylic functional groups. Selecting suitable adsorbents can mitigate asphaltene aggregation in industrial processes6. According to Jagadisan et al.7, asphaltenes can adsorb onto solid surfaces in either monolayer or multilayer configurations. Key factors affecting adsorption include solvent polarity, resin content, and asphaltene molecular characteristics. Based on their results, the adsorbed mass of asphaltenes can reach approximately 100 mg/m2 on gold and stainless steel surfaces, compared to about 60 mg/m2 on silicon dioxide, for a 50 ppm solution, highlighting a significant material-dependent adsorption capacity7. Nanoparticles, typically ranging in size from 1 to 100 nm, have numerous applications, including enhancing contact angle, improving oil recovery, and modifying interfacial tension between water and oil8,9,10. For example, based on Shirazi et al.8, the addition of 0.05 wt% TiO₂ nanoparticles to diluted modified seawater resulted in a significant improvement in wettability alteration, with contact angles decreasing from approximately 140° to a range of 50–80°. In addition, a negligible change in IFT occurred from a range of ~ 5–6 dyne/cm, and a drastic increase in oil recovery from ~ 0.2 to 0.3% OOIP/day to 0.4–0.5% OOIP/day during spontaneous imbibition, with the best performance (0.54% OOIP/day) observed for the dSW2Ca-based nanofluid. Recent studies suggest that nanocomposites can effectively delay asphaltene precipitation and deposition11. Because of their exceptional stability, extensive surface area, and pore accessibility, nanoparticles exhibit strong potential for asphaltene adsorption in oil and gas reservoirs. Furthermore, they are capable of reducing particle agglomeration in subsurface reservoirs as well as surface processing equipment12. Nanoparticles have the ability to adsorb asphaltenes present in crude oil, thereby inhibiting their deposition and aggregation. By dissolving asphaltene and inhibiting precipitation on rock surfaces, nanoparticles can delay or prevent asphaltene aggregation.
Various analytical techniques, including atomic force microscopy (AFM)13,14,15,16,17, measurements of interfacial tension between CO₂ and crude oil, ultraviolet–visible (UV–Vis) spectroscopic analysis, and adsorption isotherm experiments, have been employed to evaluate the asphaltene adsorption and the precipitation inhibition capabilities of nanoparticles. For instance, Kazemzadeh et al.18 utilized magnetite nanoparticles to adsorb asphaltene during CO₂ flooding, with asphaltene onset pressure determined through CO₂/oil interfacial tension behavior. Their findings demonstrated a reduction in asphaltene precipitation. According to the results, the addition of 0.5 wt% Fe₃O₄ nanoparticles increased the onset pressure of asphaltene precipitation from 6.2 MPa to 6.55 MPa. The intensity of precipitation was also reduced, evidenced by the bond number slope ratio increasing from 24.74% to 82.77% for B-type asphaltene18.
Significant promise has been demonstrated by nanoparticles, which provide high surface area for adsorption, in conjunction with the biopolymer xanthan gum for stabilization and the biosurfactant eucalyptus oil for solubilization. Through surface interactions, zinc oxide (ZnO) nanoparticles, which have a large surface area and chemical reactivity, stabilize asphaltenes by stopping their aggregation. This occurs primarily via electrostatic and van der Waals attractions between the nanoparticle surfaces and the acid/base functional groups of asphaltene molecules19. In a similar way, the porous structure of silica (SiO2) nanoparticles makes them efficient adsorbents that minimize deposition by capturing asphaltene molecules19. SiO₂ nanoparticles provides a high specific surface area, making them efficient adsorbents that minimize asphaltene deposition by physically capturing and stabilizing asphaltene molecules. This mechanism was demonstrated experimentally by Aghajanzadeh et al.20, who showed that the application of silica nanofluids remediated asphaltene-induced damage in core samples, resulting in three key improvements: enhanced effective permeability, decreased residual oil saturation, and reduced overall core damage. In a related study, Betancur et al.21 further highlighted that optimized injection of silica nanofluids following asphaltene-induced formation damage achieved an additional 11% recovery factor during core flooding tests, underscoring the practical efficacy of SiO₂ nanoparticles in maintaining reservoir productivity.
By creating a barrier around asphaltene particles and postponing precipitation, the biopolymer xanthan gum improves colloidal stability22. Through steric hindrance and solubilization, eucalyptus oil’s natural surfactants break up asphaltene aggregates23. These nanoparticles provide effective and environmentally sustainable alternatives for reservoir flow assurance. The surface chemistry and dispersion of nanoparticles in crude oil determine how well they control asphaltene. While xanthan gum (a biopolymer) and eucalyptus oil (a biosurfactant) offer biodegradable substitutes with a less adverse effect on the environment than the traditionally used polymer- and surfactant-additives, ZnO and SiO2 nanoparticles offer mechanical and chemical inhibition24. Ahmadi et al. developed ZnO/SiO2/xanthan nanocomposites22 with pomegranate seed to mitigate asphaltene-induced damage in shale and carbonate reservoirs. Their study demonstrated that injecting 1–16 pore volumes of the nanofluid significantly reduced asphaltene precipitation from 8.95 to 2.25 wt.% and from 20.06 to 10.25 wt.%, respectively, thereby effectively minimizing its adverse impact on rock permeability and porosity.
According to recent research, functionalized SiO2 nanoparticles perform better than traditional inhibitors because they have a greater capacity for adsorption25. Furthermore, hybrid systems that include polymers and ZnO show synergistic effects that improve asphaltene stability even further1. Through steric hindrance and solubilization, eucalyptus oil’s natural surfactants break up asphaltene aggregates23. These nanoparticles provide effective and environmentally sustainable alternatives for reservoir flow assurance. The surface chemistry and dispersion of nanoparticles in crude oil determine how well they inhibit asphaltene deposition. While xanthan gum and eucalyptus oil offer biodegradable substitutes with less adverse effect on the environment, ZnO and SiO2 nanoparticles offer mechanical and chemical inhibition1. According to recent research, functionalized SiO2 nanoparticles perform better than traditional inhibitors because they have a greater capacity for adsorption26. Furthermore, hybrid systems that include polymers and ZnO show synergistic effects that further improve asphaltene stability27.
Although several studies have proposed methods to assess asphaltene adsorption, integrating multiple analytical techniques such as UV–Vis spectroscopy, AFM, CO₂-oil interfacial tension measurements, adsorption isotherms, and natural depletion tests is essential to optimize results. The novelty of this study is that it conducts such analysis in conjunction with core flooding experiments using nanocomposite to compare asphaltene adsorption on nanocomposite surfaces versus standalone adsorption techniques in carbonate. Furthermore, it conducts permeability reduction tests using NCs as a novel inhibitor under realistic reservoir conditions, distinguishing this work from previous studies that have mainly relied on synthetic oil and core samples. The novelty of this work is threefold: (1) the synthesis and application of the novel ZnO/SiO₂/xanthan/eucalyptus nanocomposite (NCs); (2) a unique multiscale analysis bridging nanoscale characterization (adsorption, AFM) with macroscopic core-flooding; and (3) evaluation under realistic reservoir conditions using live crude oil, providing highly relevant insights for field applications.
Materials and methods
Materials
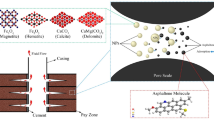
Crude oil and core samples were selected from a carbonate oil reservoir in western Iran. Oil has a viscosity of 8.98 cP (measured at a shear rate of 100 s⁻1) and a density of 0.809 gr/cm3 at 90 °C. Average permeability and porosity of the sampled reservoir were 10 mD, and 14%, respectively. NaCl (2840 ppm), CaCl2 (138 ppm), MgCl2.6H2 (643 ppm), and KCl (80 ppm) were used to form a brine solution for use in the experiments based on Ahmadi et al.28. The eucalyptus plants were puchased from Ilam (Iran). Merck (a commercial company) provided the sodium metasilicate, xanthan, and ZnCl2. Every substance used in this investigation was produced with high purity levels (> 99.5%). The asphaltene fraction was isolated from the crude oil via standard IP143 precipitation, with an obtained average molecular weight of 751 g per mole.
Synthesis procedure
Nanocomposite synthesis details were described in our previous study28. Briefly, eucalyptus leaf extract was made using the aqueous technique. Following the extraction of the eucalyptus leaf extract, 200 mL of the extract were combined with 2 g of zinc chloride and 5 g of sodium metasilicate. The mixture was then well mixed using a thermal stirrer set to 80 °C and 850 rpm for 2 h. After stirring continuously until a black sediment forms, the resultant solution is filtered, the sediment is put in the oven for 12 h, and lastly, the sediment is placed in the oven at 600 °C to totally burn out any remaining impurities. After cleaning the nanomaterials with distilled water and allowing them to dry at room temperature, 10 g of xanthan gum were added to generate the ZnO/SiO2/xanthan/eucalyptus nanocomposite after being refluxed for two hours at 80 °C.
AFM testing
Atomic force microscopy (AFM) analysis was conducted using a 3100 AFM model. Based on the findings of Ahmadi et al.22, a fixed time step of 96 h was selected for asphaltene adsorption studies. Three core samples (final dimensions: 2 × 2 × 0.5 cm) were immersed in 150 mL of a toluene/asphaltene solution (25 mg asphaltene concentration) for 96 h. After drying, AFM measurements were performed. To ensure consistency and eliminate bias, a rigorous protocol was followed. All samples were subjected to a standardized cleaning procedure, rinsed with toluene to remove loosely adsorbed particles, and dried with nitrogen gas before AFM measurements were performed.
Interfacial tension measurement
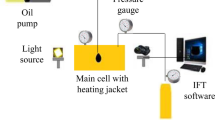
The CO₂/oil interfacial tension (IFT) under reservoir conditions was measured using the experimental setup illustrated in Fig. 1. The IFT behavior of the CO₂/oil system was first investigated as a diagnostic tool to evaluate the fundamental efficacy of NCs in inhibiting asphaltene aggregation before validating its performance in natural depletion core floods.
The entire experimental apparatus was carefully cleaned with organic solvents such as toluene and ethanol prior to purging the high-pressure chamber with nitrogen gas. The glass surfaces were verified as sufficiently clean on both sides, despite being subjected to reservoir conditions. Following the injection of CO₂ gas into the main cell, pressure was monitored at the target temperature using calibrated pressure gauges. Oil droplet formation in the presence of CO₂ was recorded via imaging in the main cell. For each test in this study, the results were averaged across three experimental runs. Liquid and CO₂ densities were determined using hydrometers, in accordance with the National Institute of Standards and Technology (NIST) guidelines and protocols.
Core flooding and natural depletion experiments
The experimental configurations for measuring asphaltene precipitation during natural depletion and core flooding are illustrated in Fig. 2a, b, respectively.
The apparatus for natural depletion tests (Fig. 2a) was used to quantify the weight of asphaltene precipitated due to pressure reduction. The procedure involved gradually depressurizing the live oil–gas mixture in the PVT cell from the initial reservoir pressure to the target pressures (3700, 3500, and 3300 psi) using a hydraulic pump. At each target pressure, the system was stabilized for 24 h to ensure thermodynamic equilibrium. Subsequently, the entire mixture was filtered under pressure through a 0.5-micron metal filter to capture any precipitated asphaltene particles. The residue on the filter was then carefully collected and rinsed with n-heptane to remove any maltenes. The mass of the purified precipitated asphaltenes was measured gravimetrically. The amount of asphaltene precipitation (in weight percent) at each pressure step was calculated using Eq. (1):
This process was repeated for both the baseline crude oil and the crude oil treated with 40 ppm NCs to evaluate the inhibitor’s performance. The IP143 test detected asphaltene precipitation at each stage due to differing asphaltene concentrations between the filtered sample and crude oil. Following the IP143 method, the asphaltene fraction was precipitated through the addition of n-heptane to crude oil in a 40:1 (v/v) ratio following standard procedures. The solution was stored in a dark room for 24 h before filtration through Whatman paper. Pure asphaltene was subsequently isolated using appropriate solvents, as described by Nassar et al.29. The PVT cell pressure was gradually reduced using a hydraulic pump while monitoring pressure gauges.
Dynamic core flooding experiments were conducted to assess permeability reduction in reservoir cores (Fig. 2b). The carbonate core was aged at 90 °C and 3700 psi for three weeks. Initial water permeability was determined using Darcy’s law at a flow rate of 0.1 cc/min, followed by injection of brine. Temperature and pressure were controlled using a heating jacket and pump. After introducing crude oil into the core holder, effluent volumes were recorded to determine residual water saturation (Swir), while maintaining an overburden pressure of 2500 psi. Absolute oil permeability was measured at Swir. Permeability and differential pressure were then evaluated under natural depletion conditions at 90 °C and pressures of 3700, 3500, and 3300 psi. The experimental conditions (90 °C, pressures from 3300 to 3700 psi) were selected to be representative of moderate-to-deep carbonate reservoir environments in the Azar field. The same procedure was repeated with NCs, where 40 ppm28 nanoparticles were introduced into the crude oil during the recombination process (recombination well volume is 2000 cc). The system was allowed to stabilize for 96 h to ensure maximum adsorption before comparing permeability and differential pressure to non-nanocomposite cases.
Batch adsorption studies
Batch experiments were performed to evaluate asphaltene adsorption on NCs nanoparticles in toluene. Nanoparticles (5–900 ppm) were added to asphaltene-toluene solutions and stirred at 200 rpm for 4 h. The concentration of asphaltenes was determined by ultraviolet–visible spectroscopy at a wavelength of 410 nm, and nanoparticle-asphaltene aggregates were separated via centrifugation (3000 rpm, 30 min). The adsorption capacity, Q (mg/g), was calculated using Eq. (2) 18.
where V is the volume in liters of the solution, m is the mass of the nanoparticles, Co is the starting asphaltene concentration, and Ce is the equilibrium asphaltene concentration.
Results and discussion
Characterization of NCs
The morphological and structural properties of the NCs were analyzed using field emission scanning electron microscopy (FESEM), Fourier transform infrared spectroscopy (FTIR) (Thermo VERTEX70 model), and X-ray diffraction (XRD). FESEM imaging (Fig. 3a) revealed a well-defined micropore structure of the NCs, displaying a porous morphology with distinct grain boundaries. Furthermore, the micrographs confirm the uniform and random dispersion of silica and zinc nanoparticles across the xanthan surface. FTIR spectroscopy (Fig. 3b) was used to characterize the functional groups existing in the nanoparticles. The spectrum exhibits characteristic absorption bands, including stretched C-H bonds at 2924 and 2860 cm⁻1, and a broad peak at 3436 cm⁻1 attributed to O–H stretching vibrations from hydroxyl groups in alcohols and carboxylic acids. Additional peaks were observed at 1024 cm⁻1 (bending C–H₂), 1425 cm⁻1 (acidic C–O stretching), and 1645 cm⁻1 (C=O ester carbonyl stretching). The broad bands at 3400–3500 cm⁻1 are associated with O–H stretching from adsorbed water molecules30. Furthermore, peaks at 479 and 520 cm⁻1 correspond to Zn–O stretching vibrations, while the Si–O-Si bending vibration appears at 877 cm⁻1. The absence of extraneous peaks confirms the successful synthesis of the nanocomposite without impurities31,32.
Characterization tests: (a) SEM, (b) FTIR, and (c) XRD, reprinted with permission from28.
The crystallinity and phase composition of the NCs were examined via powder XRD using a Philips PW1820 diffractometer (Fig. 3c). The XRD pattern, recorded between 10° and 80° (2θ), exhibits characteristic diffraction peaks with reduced intensity and increased peak broadening, indicative of the nanoscale dimensions of the material. Distinct peaks at 2θ = 29.6°, 31.9°, 35.1°, and 36.4° correspond to the crystalline planes of ZnO and SiO₂ nanoparticles. The absence of secondary phases confirms the high purity of the synthesized nanocomposite33. The concordance of the results from FESEM, FTIR, and XRD analyses provides a high degree of certainty regarding the successful preparation, composite nature, and purity of the synthesized NCs.
The adsorption of asphaltenes on NCs was evaluated through both linear and nonlinear regression of Langmuir and Freundlich adsorption isotherm models34. The corresponding fitting parameters for the experimental data are presented in Table 1.
The equilibrium concentration of asphaltenes in the solution phase, denoted as Ce, is measured in milligrams per liter (mg/L). The Freundlich constants, KF [(mg/g)(L/mg)] and 1/n (unitless), represent the adsorption capacity and intensity factor, respectively, for asphaltene adsorption onto nanoparticles, where the adsorbed amount is expressed in mg/g. Additionally, KL (L/mg) is the Langmuir equilibrium constant, and Qm (mg/g) denotes the maximum asphaltene adsorption capacity of the nanoparticles. Figure 4 illustrates the adsorption behavior of asphaltenes onto the nanoparticle surfaces for a concentration range of 5 to 900 ppm. In this study, all experiments were conducted in replicate.
As the asphaltene concentration approached 100 ppm, NCs exhibited increased asphaltene adsorption. The nanoparticle adsorption stabilized as the asphaltene concentration in the solution increased. The Langmuir model provided a better fit to the recorded NCs experimental adsorption data compared to the Freundlich model, as illustrated in Figs. 5 and 6. The corresponding Freundlich and Langmuir isotherm parameters corresponding to these displayed models are presented in Table 2. The experimental findings revealed that the NCs adsorbents exhibited a high adsorption capacity for asphaltenes, as indicated by the maximum adsorption quantity (Qm) per unit mass of nanoparticles. Specifically, the Langmuir Qm value was 185.1852 mg/g, whereas the Freundlich constant (KF) for NCs was 9.0302 ([mg/g][L/mg]). These values confirm the NCs adsorption capacities. These findings align with previous observations reported by Dudasova et al.35.
IFT of CO2/oil with NCs
Fig. 7 illustrates the pressure-dependent interfacial tension between CO₂ and oil without NCs (base) and with NCs (blue curve). The change in IFT slope is attributed to the adsorption of asphaltene molecules onto the NCs in the bulk oil phase. This sequestration prevents the asphaltenes from migrating to and stabilizing the CO₂-oil interface, resulting in an interfacial behavior that more closely resembles a ‘clean’ system and thus an altered IFT-pressure relationship18.
As the pressure increased from 200 to 3700 psi, the base case, and the case with NCs exhibited two distinct response patterns, with slope transitions occurring beyond 2200 psi. The rate of IFT decline was slower in the second pressure zone due to asphaltene precipitation, while the introduction of NCs altered (increased) the interfacial-tension slope in these secondary zones. These pattern modifications were attributed to the adsorption of asphaltene molecules onto nanoparticle surfaces. Notably, NCs achieved a substantial improvement in slope stability within the second pressure zone (> 2200 psi).
UV–Vis spectrophotometry confirmed high asphaltene adsorption on NCs over a 96-h period. NCs enhanced the IFT slope, though the effect was more pronounced in the second pressure region than in the first. This suggests that nanoparticle addition slows but does not entirely prevent asphaltene agglomeration. These findings are consistent with the study by Kazemzadeh et al.18, which examined the effect of nanoparticles on asphaltene precipitation during CO₂ injection through interfacial tension (IFT) analysis of the CO₂-crude oil system. While their study did not directly measure asphaltene adsorption on nanoparticle surfaces, our research expands upon their results by examining this effect through core flooding and natural depletion experiments. The experiments were conducted at three different end pressures (3700, 3500, and 3300 psi) with a 96-h interval to assess nanoparticle performance. Table 3 presents the influence of nanoparticle type on CO₂/oil IFT as a function of pressure. As previously noted, each scenario (base case and NCs) follows two distinct correlation trends.
As pressure increased, asphaltene precipitation occurred in the second portion, leading to a decline in the slope. Under normal conditions (with no NCs present), the ratio of the second to the first portion was 31.43%. The introduction of NCs nanoparticles into the crude oil resulted in an increased slope in the second region, while the first zone’s slope remained largely unchanged. At a concentration of 40 ppm, NCs increased the second/first ratio to 45.71%. The findings indicate that NCs exhibit a high asphaltene absorption capacity and significantly improve the rate of change for the IFT slope.
Core flood and natural depletion evaluations
This research systematically evaluates the role of nanoparticles in modulating asphaltene precipitation under natural reservoir depletion conditions. The experimental results demonstrate that asphaltene adsorption onto NCs significantly modifies interfacial properties, as evidenced by pressure-induced variations in the interfacial tension slope at elevated pressures. To assess the pressure-dependent adsorption effects, experiments were conducted at 3700, 3500, and 3300 psi. These tests evaluated precipitation changes during natural depletion with 40 ppm NCs. Figure 8 reveals that the presence of NCs affected asphaltene precipitation. Experimental results demonstrated that asphaltene precipitation increased with pressure reduction from 3700 to 3300 psi in both the baseline case and NCs-treated systems. However, the NCs-modified system exhibited significantly lower precipitation rates across all three evaluated pressure points compared to the untreated control. At 3700 psi, asphaltene precipitation decreased in the presence of NCs from 3.89 to 1.12 wt.%, and at 3300 psi, asphaltene precipitation decreased in the presence of NCs from 8.12 to 4.12 wt.%. This trend is attributed to declining fluid power and density. Notably, NCs demonstrated a more substantial reduction in asphaltene precipitation. The results suggest that NCs effectively mitigate asphaltene precipitation over a 96-h period, with NCs exhibiting superior asphaltene inhibition performance. The observed reduction in precipitation aligns with the IFT behavior of the CO₂-oil system (as it was discussed there, although nanoparticles enhanced the second slope, the first segment retained a steeper gradient).
Atomic force microscopy (AFM) analysis of carbonate sheets using NCs
Fig. 9 and Table 4 present the AFM results for carbonate sheets with NCs and without NCs (Base case). Each test was conducted at 96-h intervals. The asphaltene content in the rock samples remained constant at 25 mg before and after the addition of 40 ppm of NCs. Prior to AFM analysis, the carbonate sheets were submerged in asphaltene for 96 h in their base condition, followed by rinsing with toluene and drying with nitrogen. A consistent methodology was applied for AFM characterization of the NCs-treated carbonate sheets.
The surface roughness parameters average roughness (Ra), root mean square roughness (Rq), and peak-to-valley roughness (Rt) were measured over 96 h for carbonate rocks treated with NCs, as well as for the base condition. The results (Fig. 9 and Table 4) indicate that NCs significantly reduced Ra, Rq, and Rt while exhibiting high asphaltene adsorption capacity. Morphological analysis confirmed that NCs facilitated asphaltene adsorption on carbonate sheets, and NCs demonstrate superior performance. After 96 h, the roughness values (Ra, Rq, Rt) for the base condition were 56.70 ± 1.42 nm, 67.21 ± 1.39, and 369.71 ± 2.63 nm, respectively. Incorporation of NCs led to substantial reductions in surface roughness. Specifically, decreased Ra, Rq, and Rt values of 11.42 ± 0.25, 12.52 ± 0.56, and 13.23 ± 1.74, respectively. These findings demonstrate that NCs exhibit enhanced efficiency in asphaltene adsorption and surface roughness mitigation compared to untreated carbonate systems. The significant reduction in all surface roughness parameters (Ra, Rq, Rt) upon treatment with NCs is a direct result of inhibited asphaltene deposition. The NCs adsorb asphaltene particles onto their surfaces, preventing the formation and growth of large aggregates that would otherwise coat the carbonate substrate. Consequently, the rock surface remains significantly smoother, closely resembling its original state.
Permeability and porosity reduction
The optimal conditions for core flooding experiments included a concentration of 40 ppm NCs, a duration of 96 h, and a reservoir temperature of 90 °C. The tests were conducted under three different pressure conditions: 3700, 3500, and 3300 psi. Figure 10 illustrates the permeability reduction ratio (k/ki) for carbonate samples with and without the application of NCs. These results demonstrate that at pressures of 3700, 3500, and 3300 psi, the addition of 40 ppm NCs causes a measurable increase in permeability. The porous media exhibited a favorable response within these pressure ranges. This improvement in k/ki and the observed reduction in differential pressure may be attributed to the adsorption of asphaltenes on the nanocomposite surfaces33.
Core flooding tests revealed asphaltene precipitation, as illustrated in Fig. 11. The extent of precipitation was determined by subtracting the initial asphaltene content from the measured asphaltene concentration under the given test conditions. Pressures above 3000 psi were selected based on bubble points of 3300, 3500, and 3700 psi. At 90 °C, Fig. 11 demonstrates that 40 ppm reduced asphaltene deposition (this optimum concentration was selected based on Ahmadi et al.28). The presence of carbonate sheets facilitated the adsorption of asphaltene onto nanocomposite, resulting in lower asphaltene formation over the 96-h interval. Furthermore, NCs nanofluids exhibited superior performance in suppressing asphaltene precipitation as pore capacity increased from 2 to 9.5. A similar effect was reported by Kashefi et al.36, who found that zeolite beta nanoparticles effectively reduced asphaltene deposition.
Figure 12 illustrates the porosity reduction ratio (φ/φi) for carbonate and carbonate-nanocomposite samples subjected to 90 °C for a 96-h period. The results demonstrate that asphaltene deposition contributes more significantly to porosity reduction. Additionally, the figure reveals that NCs mitigate porosity reduction. At 9.5 pore volumes (PV), the measured porosity reduction ratio was reduced to 0.875%. This mitigation of permeability and porosity impairment translates directly to significant operational benefits, including higher sustainable production rates, a reduced frequency of costly well stimulation workovers, and improved ultimate oil recovery.
Table 5 presents a comparative analysis of experimental results from this study and prior literature37,38,39. The data indicate that NCs nanocomposite exhibit superior performance as nano-additives for inhibiting asphaltene precipitation in carbonate reservoirs compared to alternative nanomaterials. Specifically, the synthesized nanocomposites achieved asphaltene reduction efficiencies of 4.00 wt.% (3300 psi), 2.24 wt.% (3500 psi), and 2.77 wt.% (3700 psi), demonstrating significantly higher effectiveness than previously reported results 37,38,39. This enhanced performance suggests that the NCs system offers a promising solution for mitigating asphaltene-related challenges in high-pressure reservoir conditions.
Future studies and limitations
While this study demonstrates the efficacy of the novel ZnO/SiO₂/xanthan/eucalyptus nanocomposite (NCs) in suppressing asphaltene precipitation in a specific carbonate rock, its scope presents limitations that also define avenues for future research. The findings are primarily confined to a single type of carbonate rock and crude oil under a specific set of thermodynamic conditions. To fully establish the nanocomposite’s broad applicability, future work should validate these results across a wider range of reservoir lithologies, including different carbonate types and shale formations, which exhibit distinct pore structures and geochemical interactions40,41,42. Furthermore, investigating the performance of NCs under different fluid-rock interaction scenarios, such as those involving CO₂ impurities like N₂8 or alternative saturating fluids like bitumen43, would be highly valuable. Expanding the experimental work to include different water compositions, such as low-salinity brines, could also reveal synergistic effects44.
To enhance the predictive power and fundamental understanding of the inhibition process, future efforts should integrate advanced numerical modeling. This could include employing three-dimensional peridynamic simulations to model the mechanical failure of rocks under asphaltene-induced stress45 or using numerical characterization methods to understand fluid flow dynamics in complex pore networks46. The experimental methodology itself can be refined by implementing more robust calibration and error evaluation methods for measurement systems to improve data accuracy47, and by employing advanced AFM techniques, such as ultra-large scale stitchless AFM, to achieve higher-resolution, artifact-free analysis of surface roughness over larger areas48,49.
Finally, to truly assess the practical value of this technology, long-term studies simulating the entire lifecycle of a well, including thermal-induced stresses and their impact on well integrity and formation damage over time, are essential50. Conducting such comprehensive and multi-faceted research will be crucial for transitioning this promising laboratory-scale solution into a reliable and widely deployed technology for flow assurance in the oil and gas industry. Future studies, including detailed biodegradability and ecotoxicity assays, are recommended to fully validate the environmental credentials of the NCs.
Conclusions
In this study, a potentially more environmentally friendly ZnO/SiO2/xanthan/eucalyptus nanocomposite (NCs) was synthesized and employed as an asphaltene inhibitor, and its performance was evaluated for that purpose. The inhibitory mechanism is attributed to a synergistic effect: ZnO/SiO₂ nanoparticles provide primary adsorption sites, xanthan gum stabilizes the dispersion and provides steric hindrance, and eucalyptus oil acts as a natural solubilizer and dispersant. The comprehensive evaluation, spanning from the nanoscale to the core scale, confirmed its high efficacy. Atomic force microscopy revealed NCs’ remarkable effectiveness, reducing surface roughness parameters significantly (Rₐ from 56.70 to 11.42 nm). This was complemented by interfacial tension measurements, which showed a substantial 45.71% change in the CO₂-oil IFT slope, directly indicating the suppression of asphaltene migration to the interface. Adsorption isotherm studies further confirmed monolayer coverage following the Langmuir model with a high capacity of 185.2 mg/g. Under realistic reservoir conditions (90 °C, 3700 psi), core flooding demonstrations showed NCs reduced permeability impairment by over 50% and limited asphaltene precipitation to 4.12 wt.% compared to 8.12 wt.% in untreated systems. These consistent, multiscale results robustly demonstrate NCs’ strong potential for practical application in mitigating asphaltene-related formation damage and flow assurance challenges in carbonate formations.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Hemmati, M. & Ahmadi, Y. Combining zinc oxide nanoparticles with green and novel nanocomposites for enhanced oil recovery in porous media by considering asphaltene deposition and production parameters. J. Pet. Explor. Prod. Technol. 15, 15 (2025).
Quainoo, K. A., Baojun, B. & Mingzhen, W. Review on asphaltene precipitation and deposition kinetics and CO2 interactions. Adv. Coll. Interface Sci. 341, 103488 (2025).
Alhumaidan, F. S., Hauser, A., Rana, M. S., Lababidi, H. M. S. & Behbehani, M. Changes in asphaltene structure during thermal cracking of residual oils: XRD study. Fuel 150, 558–564 (2015).
Milad, B., Tinni, A., Lo, P. A. & Eljadi, R. Extended review and experimental setup development for continuous solvent and oil coinjection for asphaltene deposition in porous media. Energy Fuels 38, 11562–11581 (2024).
Talebi, A., Shafiei, M., Kazemzadeh, Y., Escrochi, M. & Riazi, M. Asphaltene prevention and treatment by using nanomaterial: A comprehensive review. J. Mol. Liq. https://doi.org/10.1016/j.molliq.2023.121891 (2023).
Afekare, D., Garno, J. & Rao, D. Enhancing oil recovery using silica nanoparticles: Nanoscale wettability alteration effects and implications for shale oil recovery. J. Pet. Sci. Eng. 203, 108897 (2021).
Jagadisan, A. & Banerjee, S. Asphaltene adsorption on solid surfaces investigated using quartz crystal microbalance with dissipation under flow conditions. ACS Omega 9, 15982–15995 (2024).
Shirazi, M., Kord, S. & Tamsilian, Y. Novel smart water-based titania nanofluid for enhanced oil recovery. J. Mol. Liq. 296, 112064 (2019).
Naik, S., You, Z. & Bedrikovetsky, P. Productivity index enhancement by wettability alteration in two-phase compressible flows. J. Nat. Gas Sci. Eng. 50, 101–114 (2018).
Sun, L., Wang, G. & Zhang, C. Experimental investigation of a novel high performance multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid damper. J. Intell. Mater. Syst. Struct. 35, 661–672 (2024).
Karimi, A. et al. Wettability alteration in carbonates using zirconium oxide nanofluids: EOR implications. Energy Fuels 26, 1028–1036 (2012).
Sircar, A., Rayavarapu, K., Bist, N., Yadav, K. & Singh, S. Applications of nanoparticles in enhanced oil recovery. Pet. Res. https://doi.org/10.1016/j.ptlrs.2021.08.004 (2022).
Hama, S. M., Manshad, A. K. & Ali, J. A. Experimental investigation of new derived anionic natural surfactant from peanut oil: Application for enhanced oil recovery. J. Mol. Liq. 395, 123876 (2024).
Manshad, A. K. et al. Performance evaluation of the green surfactant-treated nanofluid in enhanced oil recovery: Dill-hop extracts and SiO2/bentonite nanocomposites. Energy Fuels 38, 1799–1812 (2024).
Shojaati, F., Riazi, M., Mousavi, S. H. & Derikvand, Z. Experimental investigation of the inhibitory behavior of metal oxides nanoparticles on asphaltene precipitation. Coll. Surf A Physicochem. Eng. Asp. 531, 99–110 (2017).
Ezeonyeka, N. L., Hemmati-Sarapardeh, A. & Husein, M. M. Asphaltenes adsorption onto metal oxide nanoparticles: A critical evaluation of measurement techniques. Energy Fuels 32, 2213–2223 (2018).
Li, X., Guo, Y., Sun, Q., Lan, W. & Guo, X. Effect of nanoparticles on asphaltene aggregation in a microsized pore. Ind. Eng. Chem. Res. 57, 9009–9017 (2018).
Kazemzadeh, Y., Malayeri, M. R., Riazi, M. & Parsaei, R. Impact of Fe3O4 nanoparticles on asphaltene precipitation during CO2 injection. J. Nat. Gas Sci. Eng. 22, 227–234 (2015).
Ahmadi, S., Khormali, A. & Kazemzadeh, Y. A critical review of the phenomenon of inhibiting asphaltene precipitation in the petroleum industry. Processes 13, 212 (2025).
Aghajanzadeh, M. R. & Sharifi, M. Stabilizing silica nanoparticles in high saline water by using polyvinylpyrrolidone for reduction of asphaltene precipitation damage under dynamic condition. Chin. J. Chem. Eng. 27, 1021–1029 (2019).
Betancur, S., Carmona, J. C., Nassar, N. N., Franco, C. A. & Cortés, F. B. Role of particle size and surface acidity of silica gel nanoparticles in inhibition of formation damage by asphaltene in oil reservoirs. Ind. Eng. Chem. Res. 55, 6122–6132 (2016).
Ahmadi, Y., Akbari, A., Mansouri, M., Alibak, A. H. & Vaferi, B. Innovative xanthan gum-based nanocomposites for asphaltene precipitation prevention in shale and carbonate rocks. Int. J. Biol. Macromol. 280, 136331 (2024).
Ahmadi, Y. & Fatahi, M. Synthesis of novel green nanocomposites by considering enhanced oil recovery and asphaltene adsorption effectiveness in calcite and dolomite formations. Fuel 381, 133410 (2025).
Bayat, A. E. & Shams, R. Appraising the impacts of SiO2, ZnO and TiO2 nanoparticles on rheological properties and shale inhibition of water-based drilling muds. Coll. Surf A Physicochem. Eng. Asp. 581, 123792 (2019).
Elizondo-Villarreal, N. et al. Synthesis and characterization of SiO2 nanoparticles for application as nanoadsorbent to clean wastewater. Coatings 14, 919 (2024).
Kazemi, S. et al. Recent advances in green synthesized nanoparticles: From production to application. Mater. Today Sustain. https://doi.org/10.1016/j.mtsust.2023.100500 (2023).
Ghanem, A., Nessim, M. I., Khalil, N. A. & El-Nagar, R. A. Imidazolium-based ionic liquids as dispersants to improve the stability of asphaltene in Egyptian heavy crude oil. Sci. Rep. 13, 17158 (2023).
Ahmadi, Y., Mousavifard, S. R. & Mansouri, M. Low salinity brine injection using a new green nanocomposite by considering the interfacial tension, contact angle, and spontaneous imbibition tests. J. Pet. Explor. Prod. Technol. 15, 95 (2025).
Nassar, N. N., Hassan, A. & Pereira-Almao, P. Metal oxide nanoparticles for asphaltene adsorption and oxidation. Energy Fuels 25, 1017–1023 (2011).
Kumar, H. & Rani, R. Structural and Optical Characterization of ZnO Nanoparticles Synthesized by Microemulsion Route. Int. Lett. Chem. Phys. Astron. 19, (2013).
Latthe, S., Liu, S., Terashima, C., Nakata, K. & Fujishima, A. Transparent, adherent, and photocatalytic SiO2-TiO2 coatings on polycarbonate for self-cleaning applications. Coatings 4, 497–507 (2014).
Bhattacharjee, S. DLS and zeta potential—What they are and what they are not?. J. Control. Release https://doi.org/10.1016/j.jconrel.2016.06.017 (2016).
Khorsand Zak, A., Razali, R., Abd Majid, W. H. & Darroudi, M. Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. Int. J. Nanomed. 6, 1399–1403 (2011).
Khormali, A., Sharifov, A. R. & Torba, D. I. Experimental and modeling study of asphaltene adsorption onto the reservoir rocks. Pet. Sci. Technol. 36, 1482–1489 (2018).
Dudášová, D., Simon, S., Hemmingsen, P. V. & Sjöblom, J. Study of asphaltenes adsorption onto different minerals and clays. Part 1. Experimental adsorption with UV depletion detection. Coll. Surf A Physicochem. Eng. Asp. 317, 1–9 (2008).
Kashefi, S., Lotfollahi, M. N. & Shahrabadi, A. Investigation of asphaltene adsorption onto zeolite beta nanoparticles to reduce asphaltene deposition in a silica sand pack. Oil Gas Sci. Technol. 73, 2 (2018).
Ahmadi, Y. & Aminshahidy, B. Inhibition of asphaltene precipitation by hydrophobic CaO and SiO2 nanoparticles during natural depletion and CO2 tests. Int. J. Oil Gas Coal Technol. 24, 394–414 (2020).
Mahmoudi Alemi, F., Mousavi Dehghani, S. A., Rashidi, A., Hosseinpour, N. & Mohammadi, S. Potential application of Fe2O3and functionalized sio2nanoparticles for inhibiting asphaltene precipitation in live oil at reservoir conditions. Energy Fuels 35, 5908–5924 (2021).
Ahmadi, Y. & Mansouri, M. An experimental investigation of using Ni-doped ZnO–ZrO2 nanoparticles as a new asphaltene deposition inhibitor in ultra low carbonate porous media. Energy Sour. Part A Recovery Util. Environ. Eff. 44, 9429–9447 (2022).
Dong, Z. et al. Analysis of pore types in lower cretaceous qingshankou shale influenced by electric heating. Energy Fuels 38, 20577–20590 (2024).
Wang, L., Zhang, Y., Han, R. & Li, X. LA-ICP-MS analyses of trace elements in zoned sphalerite: A study from the maoping carbonate-hosted Pb-Zn(-Ge) deposit, southwest China. Ore Geol. Rev. 157, 105468 (2023).
Zhang, L., Yuan, X., Luo, L., Tian, Y. & Zeng, S. Seepage Characteristics of broken carbonaceous shale under cyclic loading and unloading conditions. Energy Fuels 38, 1192–1203 (2024).
Peng, Y. et al. Evaluation framework for bitumen-aggregate interfacial adhesion incorporating pull-off test and fluorescence tracing method. Constr. Build. Mater. 451, 138773 (2024).
Li, L., Jin, H., Tu, W. & Zhou, Z. Study on the minimum safe thickness of water inrush prevention in karst tunnel under the coupling effect of blasting power and water pressure. Tunn. Undergr. Space Technol. 153, 105994 (2024).
Yang, J. et al. Three-dimensional peridynamics based on matrix operation and its application in rock mass compression failure simulation. Comput. Geotech. 185, 107354 (2025).
Qian, Y. et al. Numerical characterization and formation process study of rail light bands in high-speed turnout areas. Eng. Fail Anal. 168, 109083 (2025).
Jiang, T., Tang, Y., Xu, C. & Liu, W. A calibration and error evaluation method of a combined tracking-based vision measurement system for meter-scale components. IEEE Trans. Industr. Inform. 21, 4958–4967 (2025).
Liu, Y., Li, X., Ge, L. & Zhang, Z. Ultralarge-area stitchless scanning probe lithography and in situ characterization system using a compliant nanomanipulator. IEEE/ASME Trans. Mechatron. 29, 924–935 (2024).
Liu, Y. et al. Ultra-large scale stitchless AFM: Advancing nanoscale characterization and manipulation with zero stitching error and high throughput. Small 20, 2303838 (2024).
Yu, H., Zhao, Z., Dahi Taleghani, A., Lian, Z. & Zhang, Q. Modeling thermal-induced wellhead growth through the lifecycle of a well. Geoenergy Sci. Eng. 241, 213098 (2024).
Author information
Authors and Affiliations
Contributions
Yaser Ahmadi was responsible for methodology development, experimental investigation, and formal data analysis, while also providing research resources and supervision throughout the study. Yaser Ahmadi prepared the original manuscript draft and coordinated the research activities. David A. Wood contributed to the conceptual foundation of the research and participated in methodological design and investigative processes. David A. Wood authored portions of the original draft and led the critical review and editing of the manuscript. Additionally, Wood managed project administration including funding oversight and timeline management. The authors confirm that all listed contributions accurately reflect their respective involvement in the research and manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests or personal relationships that could have influenced the work reported in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ahmadi, Y., Wood, D.A. A green nanocomposite suppresses asphaltene precipitation in carbonates via multiscale evaluation. Sci Rep 15, 34653 (2025). https://doi.org/10.1038/s41598-025-20820-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-20820-1