Abstract
A Bismuth halide-based perovskite is an emerging, potent material due to its lower toxicity, high chemical stability at ambient temperatures, as well as its outstanding photo-absorbing capability in photovoltaic applications, with properties similar to those of lead-halide-based photovoltaics. The organic hole-transport materials, such as Spiro-OMeTAD and PEDOT: PSS, are used to fabricate perovskite solar cells; however, they are expensive and easily degrade. Therefore, in this study, an attempt was made to fabricate free hole transport layer (HTL) perovskite solar cells with MA3Bi2I9 (MBI) by solvent engineering, which can tune the band gap and surface morphology of the thin film to improve efficiency. Herein, we utilize the solvents Dimethylformamide (DMF) and Dimethyl Sulfoxide (DMSO) to investigate their influence on the band gap and properties of the MBI material. Here, the MBI thin films are fabricated by a simple one-step spin coating method. The hexagonal crystal structure is confirmed by XRD analysis for both films. The UV absorbance of the films is examined, with prominent peaks at 520 nm and 450 nm for the DMF solvent. For the DMSO solvent, the peaks are observed at 510 nm, 431 nm, and 360 nm, respectively. The band gap of DMF and DMSO solvents is 2.25 eV and 2.24 eV, respectively. The photoemission spectra obtained for both films are centered at 474 nm. The formation of perovskite is confirmed by FTIR analysis. The solar cell device is fabricated without a hole transport layer and analyzed using solvent engineering to enhance photovoltaic device performance. DMSO solvent-based solar cell device exhibited 32% more efficient cell performance than the DMF solvent-based solar cell device.
Similar content being viewed by others
Introduction
The commercialization of lead halide perovskite material is a concern due to its toxicity and instability in the presence of heat, light, and humidity. To overcome the hazards and problems, it is indispensable to find an alternate material that is non-toxic and cost-effective. The best material for absorbing solar energy through photosynthesis remains an interesting scenario in the field of photovoltaic applications due to its easy fabrication technique1,2. A power conversion efficiency of 25.2%3,4 was achieved within a short interval of time, making it an interesting topic. Tin, Germanium, Bismuth, and Antimony are the perovskite materials available instead of lead5,6. Among these materials, Tin7 and germanium are not recommended due to their tendency to oxidize, resulting in stable Sn4+ and Ge2+, respectively. Therefore, Bismuth is thermodynamically stable, having a similar electronic configuration to lead [Xe]4f145d106s26p3, with respect to humidity, light, and heat. It also exhibits the effect of screen-charged defects, characterized by rich structural diversity and the absence of radioactivity in the periodic table.
Most investigated and explored for photovoltaics applications with Bismuth halide perovskite materials have the structure of A3Bi2X9, A is CH3NH3+ (MA), NH4, CH(NH2)2+, Cs, Ag, X is Cl, Br, I. Chunfeng et al.8,9 demonstrated that lower band gap (2.19 eV) of formamidinium bismuth perovskite FA3Bi2I9 is obtained than the MA3Bi2I9 (2.25 eV). Also, the solvent engineering would effectively influence the growth of the film FA3Bi2I9. The power conversion efficiency is still lower by 0.022% with an open-circuit voltage of 0.48 V. Cs3Bi2I9 nanosheets exhibit the highest power conversion efficiency of 3.20% with CuI as the Hole Transport Layer (HTL), in which the device architecture has longer-term stability in open air10. For the first time, a power conversion efficiency of 3.6% is achieved by forming a bulk heterojunction absorber layer of Cs3Bi2I9 and Ag3Bi2I9-based bismuth perovskite. By increasing the grain crystal sizes (480 nm on average), the open-circuit voltage of 0.89 V exhibits strong thermal stability and retains its initial power conversion efficiency of up to 90%11. Although bismuth-based perovskite exhibits better stability than lead-based perovskite, the wide band gap of the material and poor film morphology have limited the device’s performance. To increase the efficiency of bismuth halide perovskite, various strategies are employed, including compositional engineering, additive incorporation, coating methods, and solvent engineering, to regulate the film quality. The introduction of thiourea as a Lewis base into the bismuth halide perovskite has slowed down the crystallization and improved the film quality due to strong coordination between thiourea and BiI3. The efficiency of the device is significantly increased due to a higher surface area and wider absorption in the visible region12.
The most common technique used to deposit MA3Bi2I9 film on FTO or glass substrates is the solution method, specifically spin coating. One-step spin coating13,14,15 2. Two-step spin coating. Zheng et al.16 introduced the deposition of (CH3NH3)3Bi2I9 film using a two-step process, high-low vacuum, to yield a large grain size (500 nm for a 300 nm thick MBI film), a pinhole-free, and highly compact layer of the film. The solar cell device exhibits a power conversion efficiency which is 1.64% with an external quantum efficiency of 60%. Device configurations are also contributing to the high PCE of organic-inorganic perovskite solar cells, with mesoporous13 and planar14 structures being used in both normal (n-i-p) and inverted (p-i-n) configurations to achieve high device efficiency. Among them, the inverted (p-i-n) planar structure shows better performance due to its free hysteresis behavior, low-temperature fabrication process, and good reproducibility. In general, organic Hole Transport Layer (HTL) materials (Spiro-OMeTAD, PEDOT: PSS, PTAA) are used to fabricate the perovskite solar cells in order to increase the device performance. Hao et al.15 reported that the perovskite thin film coated at low relative humidity (RH) obtained a needle-like structure with enhanced device performance. CH3NH3PbI3 perovskite solar cells are fabricated with composites of spiro-MeOTAD, Li-TFSI, and tert-butylpyridine HTL materials, which reflects on the efficiency of the device. Cristina et al.17 investigated, for the first time, the optoelectronic properties, surface morphology, and structural properties of methylammonium bismuth iodide via the thermal co-evaporation method. Enhancing the homogeneous, compact layers of perovskite thin films increases the device’s performance. The perovskite solar cell device is fabricated with spiro-MeOTAD as HTL, which increases the device performance. However, these organic HTL materials are costly and easily degrade in an ambient atmosphere, which hinders large-scale production. Therefore, researchers are seeking alternative materials for organic HTL with high stability and cost-effectiveness.
Herein, we attempted to fabricate a photovoltaic device with a free hole-transport layer, achieved through a planar device configuration, such as FTO/TiO2/MA3Bi2I9/Carbon/FTO. MA3Bi2I9 film has been deposited on the FTO substrate by the one-step spin coating method to obtain a compact thin film and to understand the optoelectronic properties of the MBI thin film-based solar cell performance. A one-step spin coating method is employed due to its simplicity, ease of film fabrication, and cost-effectiveness. There are other methods available to coat the materials, such as vapor-assisted deposition and chemical vapor deposition. However, these methods need highly equipped instruments and maintain a high vacuum, which are very expensive and require facile instrument handling. Therefore, we are choosing the spin coating method for fabricating the solar cell device. To manufacture the perovskite solar cell device, instead of using expensive organic HTL material, cost-effective and highly chemically stable (at ambient atmosphere) carbon was used, which could act as both HTL and contact layer. In addition, we investigated opto-electronic properties and surface morphology of the MA3Bi2I9 thin film via solvent engineering. DMF and DMSO are two different solvents used to study the MA3Bi2I9 thin films. Solvent engineering is used to tune the morphology of the MBI thin film, resulting in a reduced band gap, which in turn decreases the rate of charge recombination reflected in the open-circuit voltage (Voc) and efficiency of the device.
Experimental
Materials and methods
Fluorine-doped Tin Oxide (FTO) with a surface resistivity of ~ 7 Ω/sq was purchased from Sigma Aldrich. All Bismuth perovskite precursors, including Bismuth (III) Iodide BiI3 (purity > 98.0%), Methylamine Hydroiodide CH5N.HI (purity > 99.0%) was purchased from Tokyo Chemical Industry (TCI). N, N-Dimethyl Formamide C3H7NO (DMF purity ≥ 99% Emplura), and Dimethyl sulfoxide (DMSO purity ≥ 99% Emplura) were purchased from Merck. Titanium (IV) oxide powder (TiO2, purity 99.7%), Carbon paste (purity 99.9%), Ethanol, and Acetone (Emsure) were purchased from Sigma-Aldrich. All the above chemicals were used without further purification.
Solvent engineering of MA3Bi2I9 film fabrication
The precursor solution of MA3Bi2I9 was prepared by dissolving methyl ammonium iodide and bismuth iodide in a 3:2 molar ratio in 1 mL of DMSO (Dimethyl Sulfoxide) and 1 mL of DMF (N, N-dimethylformamide), respectively. The solution was stirred continuously for 1 h, and a red-colored solution was obtained. It was subsequently spin-coated on the FTO substrate at 2000 rpm for 30 s. Then, the films were annealed at 120 °C for 1 h to obtain an orange-colored film (FTO/MA3Bi2I9).
Solar cell fabrication
The Fluorine-doped Tin Oxide (FTO) transparent conductive glass substrate was cleaned sequentially with deionized water (DI), Ethanol, and Acetone in a sonication bath, each for 30 min. TiO2 was used as an electron transport layer, which was coated directly on an FTO glass substrate. 0.2 g of Titanium(IV) oxide powder was mixed with 5 drops of Nitric acid and 8 drops of Acetic acid, and stirred continuously for 2 h. The obtained TiO2 paste was directly coated onto the FTO glass substrate using the doctor blade method, followed by annealing of the thin film in a muffle furnace at 450 °C for 30 min. The MA3Bi2I9 precursor solution was spin-coated onto a TiO2-coated FTO glass substrate and annealed at 120 °C for 1 h. The counter electrode is made by coating carbon paste over another FTO glass substrate using the doctor blade method and then dried at 150 °C (FTO/TiO2/MA3BiI9/Carbon/FTO). They were sandwiched and subjected to J-V characterization.
Characterization
UV-visible absorption analysis of the MA3Bi2I9 thin film was investigated using a Jasco UV-750 series with DRS over the range of 200 nm to 800 nm. The crystallinity of the film was confirmed using a Bruker ECO D8 Advance diffractometer with CuKα1 radiation (λ = 0.15406 nm). FTIR determined the functional group of MA3Bi2I9 thin film – Spectrum Two with a PerkinElmer in the range of 550 cm−1 to 4000 cm−1. Molecular stretching was confirmed using a LabRam HR Evolution Horiba spectrometer with excitation at 532 nm from a He-Ne laser. The morphology and microstructure were studied by Scanning Electron Microscope (SEM) – ZEISS EVO 18, which was equipped with Energy–dispersive X-ray spectroscopy. J-V characterization was performed using a Keithley Electrometer 6517B with a portable solar simulator, which consisted of a Xenon lamp (100 mW/cm²). The photoluminescence of the thin film was examined using a Horiba Fluoromax plus model with FluorEssence (Version 9.0) software (Link: https://www.horiba.com/ind/scientific/products/detail/action/show/Product/fluoromax-1576/).
Results and discussion
Mechanism of MA3Bi2I9 thin film on substrate
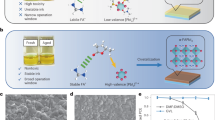
The mechanism of forming the MA3Bi2I9 thin film by spin coating using the precursors is represented in a schematic diagram in Fig. 1. MAI and BiI3 were dissolved in the solvents of DMF and DMSO and introduced over the FTO substrate, which was spinning at 2000 rotations per minute. It forms an intermediate phase of BiI3 over FTO after the evaporation of the solvents. This was due to the hindrance of rapid reaction between MAI and BiI3, leading to the homogeneous coating of orange-colored MA3Bi2I9 perovskite crystallites, which were annealed at 120 °C. Figure 2 shows the thin film of MA3Bi2I9 coated over the TiO2/FTO substrate before and after the annealing process.
XRD analysis
The XRD patterns for the synthesized MA3Bi2I9 thin film deposited on FTO are depicted in Fig. 3. The intense peaks for DMF-MBI are obtained at 16.35° (004), 24.63° (006), 29.74° (204) and DMSO-MBI peaks at 12.50° (011), 16.23° (004), 24.44° (006), 31.58° (205) and 32.28° (2–16) were an index of MA3Bi2I9 film respectively and belonged to typical hexagonal space group P63/mmc which were confirmed by JCPDS: 01-058-613417. The firm diffraction peaks indicate that the perovskites are highly crystalline in nature with larger crystallite sizes. This finding aligns with previous results from literature reports17, which indicate a preferred crystal growth orientation along the c-axis, orthogonal to the FTO plane. Ran et al. investigated a step-evaporation-spin coating method to achieve the preferred orientation of BiI3 in the MA3Bi2I9 thin film. It was found that the calculated values in XRD were not consistent with the experimental values, leading to the formation of a thin film of MA3Bi2I9 through multiple layers. As a result, the orientation of BiI3 layers does not affect the growth of MA3Bi2I9 due to the evaporation of the second and third layers, and the surface morphology was different from that of the first layer on the ITO substrate18. Thus, the growth of MA3Bi2I9 thin films without preferred orientation is advisable for the fabrication of thin films.
There was no evidence for secondary phase formation or any other impurities, however in DMF-MBI film has two little humps at 9° and 19.48°, these peaks were assigned to MBI crystal19, and in DMSO-MBI peak at 26.35° (113) assigned to BiI3 which revealed that incomplete conversion of MBI from precursor solution. The crystallite size was calculated for a full-width half maximum of (006) plane of ~ 79.68 nm for DMF-MBI film and ~ 54.20 nm for DMSO-MBI film by using the Debye-Scherrer equation20.
Raman spectroscopy
Raman spectroscopy was performed on both DMF-MBI and DMSO-MBI thin films to obtain the vibrational modes of the compounds, which are shown in Fig. 4. Spectroscopy was taken in the range from 50 cm−1 to 400 cm−1. I-Bi-I, Bi-I-Bi scissoring, and Bi-I stretching modes were commonly assigned to low wavenumbers (below 200 cm−1). The weak and strong bands were observed at 87 cm−1 and 94 cm−1, respectively, belonging to the I-Bi-I scissoring mode of DMF-MBI and DMSO-MBI. Bi-I stretching mode was noticed at 142 cm−1 and 150 cm−1 for DMF-MBI and DMSO-MBI thin films, respectively. These bands were well matched with previous literature. Senol et al. reported the calculated values obtained using DFT analysis and experimental Raman data for the MBI thin film. It is revealed that Bi-I-Bi scissoring modes were not observed in the experimental band but were presented in the DFT-calculated data. The present analysis of Raman Spectroscopy confirmed the Bi-I-Bi scissoring modes. In addition, a Bi-I stretch vibration was observed at 142 cm−1, accompanied by a small hump and a strong band at 150 cm−1, for both DMF-MBI and DMSO-MBI thin films. The small hump at 229 cm−1 corresponds to a combination of vibrational bands, specifically 87 cm−1 + 142 cm−1 and 94 cm−1 + 150 cm−1, which is caused by LO-TO splitting. The band at 377 cm-1 observed in DMF-MBI is uncertain.
FTIR analysis
Functional groups of the MA3Bi2I9 thin film were investigated by transmittance frequency using Fourier Transform Infrared analysis. The functional groups were scanned in the range of 550 cm−1 to 4000 cm−1 as depicted in Fig. 5. The presence of N-H stretching vibration in DMF-MBI and DMSO-MBI was confirmed at 2993.93 cm−1 and 3004.17 cm−1, respectively. The sharp band at 1729.70 cm−1 in DMF-MBI and 1642.96 cm−1 in DMSO-MBI was attributed to the C = O ester fatty acid group. The sharp band at 1124.05 cm−1 confirmed the presence of C-N stretching vibration, which was caused by DMF solvents in DMF-MBI thin film.
Similarly, C-S and S-O stretching vibration originating from DMSO was confirmed by a sharp peak at 1011.39 cm−1 in DMSO-MBI thin film. However, typical vibrational bonds for the formation of MBI perovskite thin film were noticed in both films, such as N-H bending at 1490.41 cm−1 in DMF-MBI and 1531.94 cm−1 in DMSO-MBI, respectively21. The presence of N-H wagging was confirmed in both films, which was 1024.04 cm−1 for DMF-MBI and 890 cm−1 for DMSO-MBI thin films. C-H bending caused by methylammonium is also clearly seen at 1377.79 cm−1 in DMF-MBI and 1442.53 cm−1 in DMSO-MBI, respectively. From this, it was clearly proved in solvents of DMF and DMSO that the MBI thin film was formed, and the presence of solvent in the film was confirmed.
Scanning electron microscope
Solvent engineering influences the morphology of the thin film, as investigated by Scanning Electron Microscope (SEM). The images of the films are shown in Figs. 6 and 7. SEM images of DMF-MBI thin film in Figs. 6(a)-(c) revealed that the isolated large grain size of a hexagonal-like structure has poor surface coverage and possesses lots of pin holes over the substrate surface. A hexagonal structure is composed of rods and spherical shapes of particles. The cross-section image of DMF-MBI film on the FTO substrate is shown in Fig. 6(d), and the thickness of DMF-MBI is 0.98 μm. Figures 7(a)-(c) show the images of the DMSO-MBI thin film on an FTO substrate, which is similar to the DMF film, featuring a large area of hexagonal structure accompanied by non-uniformity of the surface and coverage of the hexagonal structure composed of spherical particles. Therefore, the solvent may influence the morphology of the film, leading to a decrease in the recombination rate of charge carriers and resulting in a higher open-circuit voltage for the device with DMSO-MBI than for the device with DMF-MBI film. The cross-section of DMSO-MBI thin film is shown in Fig. 7(d), and the thickness of DMSO-MBI is 0.83 μm.
SEM images for DMF-MBI and DMSO-MBI thin films were coated on TiO2/FTO for fabricating solar cell devices (hence TiO2 acts as an electron transport layer (ETL)), and were examined. Figures 8(a) and (b) represent TiO2 coated on an FTO substrate, composed of particles that were spherical in nature and uniform coverage over the substrate with a pin-hole-free thin film obtained. DMF-MBI and DMSO-MBI thin films were coated on TiO2/FTO substrate, as shown in Figs. 8(c) and (d), and it was noticed that the bare MBI/FTO thin film possessed pin holes with non-uniformity of coverage; meanwhile, MBI coated on TiO2/FTO, the pin holes of the MBI film were covered with TiO2. The cross-section images of DMF-MBI/TiO2/FTO and DMSO-MBI/TiO2/FTO were depicted in Figs. 8(e) and (f), respectively.
In general, the crystallization process occurs during the spin coating and annealing process in two stages: the evaporation of selected solvents to induce nucleation sites, followed by growth stages. Various methods are employed to obtain uniform and dense films. Synthesized engineering is one of the efficient ways to achieve smooth and uniform films by (i) obtaining high solubility of Bismuth precursors into the solvent prior to the precipitate formation, (ii) quickly initiating the nucleation sites by rapid solubility of Bismuth precursors. However, the solubility of the bismuth precursor in the DMF solvent is very low; therefore, MBI was crystallized quickly and formed at an early stage of spinning due to its weak Lewis basicity and high vapor pressure (2.7 Torr), which led to low supersaturation. In XRD, we also confirmed the crystallization of MBI at 9° and 29°. As a result, the film is formed with poor surface coverage and the presence of pin holes. In the case of DMSO, with strong Lewis’s basicity due to the donation of electrons to the methyl group, it enhances the solubility of the Bismuth precursor and low vapor pressure (0.42 Torr)22. Therefore, there is an increase in supersaturation by increasing nucleation sites during spin coating. However, we still obtained incomplete conversion of BiI3, as confirmed by XRD due to the low annealing temperature.
In addition, poor surface coverage and large grain size are caused by the high humidity present at ambient conditions, which allows the film to absorb moisture from the surroundings. This leads to an increase in the rate of residual solvents a result of decreased supersaturation of the solution15. This is another crucial reason for increasing the grain size and possible pin holes of the film (DMF-MBI, DMSO-MBI). These pinholes weaken the film’s light absorption and increase the rate of charge recombination. Therefore, the coating environment influences the morphology of the film, so it is a critical component for fabricating compact Bi-based thin films.
UV vis absorption
Figure 9 shows the UV-visible spectroscopy of the deposited DMF and DMSO solvents of the MA3Bi2I9 film on FTO glass substrate over the range varying from 300 nm to 700 nm. The prominent absorbance spectra were obtained at 520 nm and one weak peak at 450 for DMF solvent (labeled as DMF-MBI) (Fig. 9(a)) and for DMSO solvent (labeled as DMSO-MBI) at 510 nm, 431 nm, and 360 nm were obtained which shows the typical film formation of MBI (Fig. 9(b)). These peaks are attributed to an electron transition from the ground state of Bi3+ (1S0) to excited states (3P1, 3P2, 1P1) of Bi2I9, which is faced with bi-octahedrons23. The sharp peak around 500 nm confirmed the formation of MBI thin film for both solvents. The pattern obtained is similar to the results obtained by Momblona et al. (2020) for MBI thin films by the co-evaporation method. There was one addition hump noted at 640 nm in DMF solvent revealed that good absorption in the visible region meanwhile no addition peaks were noted for DMSO solvent from 600 nm to 690 nm. So, an annealing temperature of 120ᵒC for DMF-MBI thin film was not sufficient to complete the conversion of MBI from precursor solution, and a higher temperature was greater than 120ᵒC required, which was due to the lower evaporation rate of DMF solvent. However, MBI-DMSO significantly shows higher absorption than MBI-DMF, due to the decrease in light scattering of incident UV light. Thus, the reduction in band gap of MBI-DMSO compared to MBI-DMF, as ashown in Table 18.
The band gap of the MA3Bi2I9 film was calculated using the Tauc plot, which plots the energy of light (hν) versus the linear absorption edge as a function of (αhν)0.5, as depicted in Figs. 9(c) and (d). MBI-DMSO shows a lower absorption edge than MBI-DMF, so the calculated band gap of the DMF-MBI film was ∼ 2.25 eV, and the DMSO-MBI film was 2.24 eV. Indirect band-to-band transition was confirmed by a steep absorption edge, as shown in the UV absorption spectra. DMSO solvent reduced the band gap of the 0.01 eV MBI thin film than the DMF solvent, which revealed that the solvent influences the band gap to a certain extent. Zhang et al. also reported highly smooth and compact, pinhole-free with large-grain-sized (500 nm) thin film was synthesized by a two-step spin-coating method and obtained a band gap of 2.26 eV with direct band-to-band transition16,18. Table 1 presents a comparison of band gap values for existing perovskite materials.
Photoluminescence spectra
Photoemission spectra for MBI are depicted in Fig. 10. In general, photoluminescence response was observed for small compounds, resulting from the radiative recombination of charge carriers occupying localized states within the band gap. The strong photoluminescence spectra exhibited at 474 nm for both DMF-MBI and DMSO-MBI, with an excited energy of 320 nm, showed weak but broad emission. This result was similar to that obtained by Senol et al. for high excitation spectra (488 nm), which also exhibited weak and broad emission spectra (1.65 eV). It was obtained due to insufficient energy to excite electrons from the valence band to the conduction band16,27. The presence of additional band gap states or indirect band gaps of the radiative recombination process caused this scenario. The large band gap and weak emission spectra influenced the device’s efficiency. DMSO-MBI exhibits higher intensity than DMF-MBI thin film, as expected due to its higher Voc and PCE, resulting from the lower band gap and suppressed non-radiative recombination of charge carriers compared to DMF-MBI.
J-V characteristics
Finally, we examined the performance of solar cell devices based on two different solvents of MBI thin films. Figure 11 shows the J-V characteristics of the fabricated MA3Bi2I9 solar cell, with the device inserted in the top left corner. Table 2 presents solar cell parameters, including open-circuit voltage (Voc), short-circuit current density (Jsc), Power conversion efficiency, and fill factor, based on DMF and DMSO solvent-based MBI thin films. Herein, we attempted a hole-transport layer-free photovoltaic device structure: FTO/TiO2/MBI/Carbon/FTO, with a device area of 0.5 cm × 0.5 cm. Hence, TiO2 is used as an Electron Transport Layer (ETL) in photovoltaic devices. The ETL layer plays a significant role in the device’s efficiency because it transfers the charge carriers generated in the active layer. It is crucial to separate the photoelectrons before the recombination process occurs. The thickness of the ETL layers plays a significant role in charge separation. The optimized thickness enhanced the device’s performance.
Figure 12 shows the current density-voltage (J-V) curve for DMF-MBI and DMSO-MBI thin films. It was noted that the DMSO-MBI solar cell device exhibited a significantly higher power conversion efficiency of 0.439% compared to the DMF-MBI device, which had an efficiency of 0.143%. The open circuit voltage (Voc) of the DMSO-MBI thin film was increased to 0.252 V, whereas the thin film of the DMF-MBI device was given only 0.170 V. Two factors were causes for the low efficiency of the device: (i) Incomplete conversion of perovskite precursor in DMF solvent, and (ii) More pin holes of MBI thin film both in DMF and DMSO solvent. SEM images showed that the presence of pinholes in both DMF-MBI and DMSO-MBI thin films was reflected in a low open-circuit voltage value, leading to leakage current in solar cell devices and a high rate of recombination. Even though the efficiency of the device was too low, solvent engineering made a clear difference in efficiency was clearly confirmed. Moreover, enhancing PCE depends on pinhole-free, smooth, and uniformly coated thin films, which should be addressed in future work. Table 3 shows a comparison of existing work based on MA3Bi2I9 perovskite for solar cell application parameters. The stability of perovskite was investigated in an open atmosphere. We found that for up to 3 months, the color of the perovskite remains unchanged, and after that color changes. Therefore, we can conclude that the stability of our fabricated perovskite coated on TiO2 is approximately 3 months.
Conclusion
In this study, we fabricated MA3Bi2I9 thin films by a simple one-step spin coating method using two different solvents (1 mL of DMF and DMSO) and subjected them to an annealing temperature of 120 °C to obtain compact MBI thin films. In DMSO-MBI, the band gap of 0.01 eV is tuned, and enhancing device performance is found to be due to the control of nucleation sites during spinning, rather than in DMF-MBI. The hexagonal crystal structure with space group P63/mmc is confirmed by XRD analysis for both thin films. The prominent absorption peaks in the visible region are noticed for both films, which enhances the device performance. The formation of the perovskite bond is confirmed via FTRI analysis for both MA3Bi2I9 thin films. DMSO solvent-based solar cell device exhibited 32% more efficient cell performance than the DMF solvent-based solar cell device. The DMSO-MBI photovoltaic device exhibited a power conversion efficiency of ~ 0.439% and a short-circuit current density of ~ 0.4815 mA/cm². An additional rate of charge recombination is effectively controlled in the DMSO-MBI film, which is reflected in an open-circuit voltage (Voc) of ~ 0.252 V, rather than the DMF-MBI, which is ~ 0.170 V. Solvent engineering could be significantly affected by the morphology of the thin film; however, high humidity in an ambient environment severely affects the film morphology, therefore efficiency of the device drastically decreases. Smooth and uniform homogeneous surface coverage over the substrate, without pinholes, will effectively tackle humidity, which is a crucial factor influencing the efficiency of the solar cell device. The optimistic approach towards the fabrication of lead-free bismuth halide perovskite solar cells with homogeneous, smooth surfaces that are free from pinholes, and are also non-toxic, has a significant impact, resulting in higher stability.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Toe, M. Z., Kumar, R., Tan, W. K., Matsuda, A. & Pung, S. Y. ‘Effect of reduced graphene oxide on the structural, optical, and power conversion efficiency of ZnO thin film-based dye-sensitized solar cells’, Thin film, vol. 803, p. 140468, (2024).
Abdel-Galeil, M. M., Kumar, R., Cheong, K. Y. & Shater, R. E. I. Investigation on influence of thickness variation effect of TiO2 film, spacer and counter electrode for improved dye-sensitized solar cells performance. Optik (Stuttg) 227, 166108 (2021).
Zhang, H. et al. Prof. Yongzhen Wu, ‘Formamidinium lead Iodide-Based inverted perovskite solar cells with efficiency over 25% enabled by an amphiphilic molecular Hole-Transporter’. Angew. Chem. Int. Ed. 63, e202401260 (2024).
Yang, T. et al. One-stone-for-two-birds strategy to attain beyond 25% perovskite solar cells. Nat. Commun. 14, 839 (2023).
Mehrabian, M., Akhavan, O., Rabiee, N., Afshar, E. N. & Zare, E. N. Lead-free MAGeI3 as a suitable alternative for MAPbI3 in nanostructured perovskite solar cells: A simulation study. Environ. Sci. Pollut. Res. 30, 57032–57040 (2023).
Hamanshu Gehlot, S., Murugan, S. & Pokhriyal Influence of layer thickness on the power conversion efficiency of Tin halide-based planar heterojunction solar cells. Environ. Sci. Pollut. Res. 30, 98647–98654 (2023).
Jagdish Nehra, M., Kilak, R. K. & Singhal Effect of surface termination on electronic and optical properties of lead-free tin-based eco-friendly perovskite solar cell: a first principal study. Environ. Sci. Pollut. Res. 30, 98796–98804 (2023).
Chunfeng Lan, G. et al. Lead-free formamidinium bismuth perovskites (FA)3Bi2I9 with low bandgap for potential photovoltaic application. Sol. Energy. 177, 501–507 (2019).
Sarita Kumari, A., Meena, A., Singh, A. S. & Verma Calculation of electronic and optical properties of Methylammonium lead iodide perovskite for application in solar cell. Environ. Sci. Pollut. Res. 28, 25382–25389 (2021).
Fan, B. et al. Lead-free, air-stable ultrathin Cs3Bi2I9 perovskite nanosheets for solar cells. Sol. Energy Mater. Sol. Cells 184, 15–21 (2018).
Wanpei, H. et al. Liming Ding, Shangfeng Yang ‘Bulk heterojunction gifts bismuth-based lead-free perovskite solar cells with record efficiency’. Nano Energy. 68, 104362 (2020).
Qiaoying Jia, C. et al. Large-grained all-inorganic bismuth-based perovskites with narrow band gap via Lewis acid – base adduct approach. ACS Appl. Mater. Interfaces. 12, 43876–43884 (2020).
Yao, K. et al. -.Jen, ‘A copper-doped nickel oxide bilayer for enhancing efficiency and stability of hysteresis-free inverted mesoporous perovskite solar cells’. Nano Energy. 40, 155–162 (2017).
Hou, Y. et al. Cesar Omar Ramirez Quiroz, et at. ‘Overcoming the Interface Losses in Planar Heterojunction Perovskite-Based Solar Cells’, Advanced Materials, vol. 28, pp. 5112–5120, (2016).
Hao Gao, C. et al. Zhigang Zou ‘Nucleation and crystal growth of organic-inorganic lead halide perovskites under different relative humidity’. ACS Appl. Mater. Interfaces. 7, 9110–9117 (2015).
Zhang, Z. et al. High-quality (CH3NH3)3Bi2I9 film-based solar cells: Pushing efficiency up to 1.64%. J. Phys. Chem. Lett. 8, 4300–4307 (2017).
Momblona, C. et al. Co-evaporation as an optimal technique towards compact methylammonium bismuth iodide layers. Sci. Rep. 10, 10640 (2020).
Chenxin Ran, Z. et al. Construction of compact Methylammonium bismuth iodide film promoting Lead-Free inverted planar heterojunction Organohalide solar cells with Open-Circuit voltage over 0.8 V. J. Phys. Chem. Lett. 8, 394–400 (2017).
Sagar, M. et al. An effective approach of vapour assisted morphological tailoring for reducing metal defect sites in lead-free, (CH3NH3)3Bi2I9 bismuth-based perovskite solar cells for improved performance and long-term stability. Nano Energy. 49, 614–624 (2018).
Wenderott, J. K. et al. ‘Local Optoelectronic Characterization of Solvent-Annealed, Lead-Free, Bismuth-Based Perovskite Films’, Langmuir, vol. 34, pp. 7647–7654, (2018).
Murugan Vigneshwaran, T. et al. Kenji Yoshino, Takashi Minemoto, Shuzi Hayase, ‘Facile synthesis and characterization of sulfur doped low bandgap bismuth based perovskites by soluble precursor route’. Chem. Mater. 28, 6436–6440 (2016).
Shin, S. S. et al. Solvent-engineering method to deposit compact bismuth-based thin films: Mechanism and application to photovoltaics. Chem. Mater. 30, 336–343 (2018).
Muhd Sahrul Nizam Sahul Hameed & Aziz, F. ‘Solvent engineering of lead-free bismuth-based perovskite material for potential application of solar cell’, in Materials Today: Proceedings, Elsevier Ltd, vol. 46, pp. 1837–1842, (2021).
Ryosuke Nishikubo, H. et al. Mohammad Khaja Nazeeruddin, Akinori Saeki, ‘Optoelectronic and energy level exploration of bismuth and Antimony-Based materials for Lead-Free solar cells’. Chem. Mater. 32, 6416–6424 (2020).
Jiang, Y. et al. Yabing Qi, ‘Reduction of lead leakage from damaged lead halide perovskite solar modules using self-healing polymer-based encapsulation’. Nat. Energy. 4, 585–593 (2019).
Serhan, M. et al. Erica Forzani, ‘Total iron measurement in human serum with a smartphone’, in AIChE Annual Meeting, Conference Proceedings, American Institute of Chemical Engineers, vol. 26, p. 2800309, (2019).
Senol Öz, J. C. et al. Zero-dimensional (CH3NH3)3Bi2I9 perovskite for optoelectronic applications. Sol. Energy Mater. Sol. Cells. 158, 195–201 (2016).
Kulkarni, A., Singh, T., Ikegami, M. & Miyasaka, T. Photovoltaic enhancement of bismuth halide hybrid perovskite by N-methyl pyrrolidone-assisted morphology conversion. RSC Adv. 7, 9456–9460 (2017).
Acknowledgements
The authors wish to acknowledge the Karpagam Academy of Higher Education for funding this work with fellowship money for a research project (KAHE/RS/Ph.D/Fellowship/2023-24/1930). The authors wish to acknowledge the Centre for Material Chemistry (CMC), Karpagam Academy of Higher Education, Coimbatore, for utilizing the instruments (UV-visible spectroscopy, PL, FTIR). The authors wish to thank the International Research Centre, Kalasalingam Academy of Research and Education, for providing a characterization facility. The authors extend their appreciation to Taif University, Saudi Arabia for supporting this work through project number TU-DSPP-2024-19.
Funding
The research was funded by Taif University Saudi Arabia project number TU-DSPP-2024-19.
Author information
Authors and Affiliations
Contributions
S. Kumaresan, N. D. Jayram, H. Ramalingam, and J. Balasundaram: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, and Writing – review & editing; A. Dinesh, M. Ayyar, and V. Mohanavel: Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, and Writing – review & editing; S. Rajendran, M. Santhamoorthy, S. Santhoshkumar, A.M.S. Alhuthali, M.H. Abdellattif, R. Balachandran, and M.K. Hossain: Project administration, Funding acquisition, Validation, Visualization, and Writing – review & editing; All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kumaresan, S., Jayram, N.D., Ramalingam, H. et al. Fabrication and characterization of MA₃Bi₂I₉ Bismuth-based perovskite material for potential solar cell applications. Sci Rep 15, 34659 (2025). https://doi.org/10.1038/s41598-025-21078-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-21078-3