Abstract
Reintroduction is an important method for recovering endangered and rare species in their natural habitats. Understanding how these species adapt to new environments is essential for their conservation and population recovery. However, behavioral adaption (particularly circadian activity patterns) of reintroduced Milu deer (Elaphurus davidianus), a special species disappeared in the wild for centuries, remains poorly understood during their initial release into historical range from captivity. We investigated the circadian behavioral rhythms of Milu reintroduced to the East Dongting Lake, using data transmitted by the GPS collars from winter 2020 to autumn 2022. The results showed that Milu’s diurnal activity predominated over nocturnal activity (generally crepuscular pattern), with 1–4 activity peaks within a 24-hour period. From winter 2020 to autumn 2022, there were significant sexual differences in activity rhythms during winter 2020, summer 2021, summer 2022, and autumn 2022 (P < 0.05). Except for summer 2022 of male Milu (U2 = 0.09, P > 0.05), the deer exhibited pronounced activity concentration trends in the eight quarters. Moreover, human disturbance also influenced the circadian rhythm and activity levels of Milu. Our study indicated that Milu gradually adjusted their circadian activity rhythms after reintroduction to their historical habitat and revealed the remarkable behavioral plasticity of Milu. The reintroduction of Milu into nature reserves underscores the significant role of combining ex situ conservation with in situ conservation for the protection of endemic endangered species.
Similar content being viewed by others
Introduction
Reintroduction serves as a vital strategy for safeguarding and reviving endangered species, conservationists have successfully restored numerous species, including the American bison (Bison bison)1, European bison (B. bonasus)2, Eurasian beaver (Castor fiber)3, Eurasian lynx (Lynx lynx)4, European gray wolf (Canis lupus lupus)5, as well as crested ibis (Nipponia nippon)6, and Przewalski’s horse (Equus przewalskii)7 in China. However, reintroduction is a risky endeavor with varying success rates8,9. Animals introduced to new environments—whether rehabilitated and released, translocated from other wild populations, or from captivity—often lack the critical survival skills required in the wild10,11,12,13. Therefore, studying how reintroduced species adapt to local climatic conditions is of great significance14,15. To ensure the reintroduction success, wildlife managers must conduct post-release tracking and monitoring16, particularly behavioral monitoring, to facilitate species conservation and management and to guarantee the effectiveness of these reintroduction projects17,18.
The adaptation of populations to their environment represents a fundamental question in biology19. Animal activity patterns are shaped by behavioral choices that are closely tied to the surrounding environmental conditions20. Behavior reflects the relationship between animals and their environment21, with different species adopting various behavioral adaptation strategies, and often displaying a diurnal and nocturnal 24-hour cycle rhythms13,22,23. Biological rhythms refer to recurring genetic, physiological, and behavioral processes that occur in anticipation or in response to periodic environmental changes24. For instance, mammals display a variety of daily activity patterns, such as being active at night (nocturnal), during the day (diurnal), at dawn and dusk (crepuscular), or irregularly throughout the day and night (cathemeral)25.
Numerous studies focused on the daily activity rhythms of animals to assist wildlife managers and scientists in effectively managing and conserving species13,26. For example, some researchers have investigated the activity patterns to inform hunting management strategies for sika deer (Cervus nippon) in Japan27. Furthermore, the effects of cattle grazing on the diurnal activity patterns of gray brocket (Mazama gouazoubira) and marsh deer (Blastocerus dichotomus) were analyzed in the savannas of northeastern Argentina28. Additionally, the activity patterns of reintroducing medium-sized carnivores in the United States, such as fishers (Pekania pennanti), and the interactions with local mammal species were revealed29. However, investigations on the behavioral rhythms and patterns of captive-bred endangered species reintroduced into new environments are rarely involved.
Milu deer (former name Père David’s deer, Elaphurus davidianus), a large herbivore, is a species endemic to China and listed as Threatened Species on the International Union for Conservation of Nature (IUCN) Red List, once extinct in the wild after the final individual being eradicated in Beijing during the late 19th century9,30. To undercovering the behavioral adaption ability of Milu deer that were reintroduced into the Hunan East Dongting Lake National Nature Reserve in Hunan Province, we monitored 10 individuals using Global Positioning System (GPS) tracking. This study aims to investigate: (1) the circadian behavioral rhythms of reintroduced deer; (2) whether their activity rhythms change over time after their release; (3) whether there are seasonal and sexual differences in the behavioral rhythms of reintroduced deer; and (4) whether their behavioral rhythm patterns change with the habitat alteration.
Materials and methods
Study sites
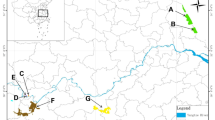
This study was conducted in the Hunan East Dongting Lake National Nature Reserve (HEDL) (112°43′59.5″-113°13′13.4″ E, 29°0′0″-29°37′45.7″ N), one part of Dongting Lake in Hunan Province (Fig. 1). Dongting Lake, the second largest freshwater lake in China, encompasses three internationally significant wetlands. HEDL, a floodplain wetland in the Middle and Lower Yangtze River Basin, covers an area of 1,569 km². The water level in the area fluctuates seasonally. The average annual temperature is 17 °C, with four distinct seasons, and annual precipitation ranges from 1,200 to 1,300 mm. Local vegetation is dominated by species such as Phalaris arundinacea, Carex spp., Polygonum hydropiper, Artemisia selengensis, Phragmites australis, and Miscanthus lutarioriparius31.
Dongting Lake is the historical distribution area of Milu, where there are over 200 milu deer in 2020 that formed a naturally rewilded population in 1998. In this study, the release site (112°50′2.1″ E, 29°20′12.2″ N) of Milu is located at western HEDL, inside the lake embankment dam (Fig. 1). After released Milu can disperse freely in whole Dongting Lake area.
Study area. The data set obtained from Geographic remote sensing ecological network platform (www.gisrs.cn).
Data collection
The Milu individuals (6♂, 4♀) came from Beijing Nanhaizi Milu Park, where all the Milu were bred in captivity, semi-free-ranging and inhabiting a wetland area of approximately 40 ha. It’s difficult to monitor Milu by infrared cameras in wetland habitats, so we used GPS satellite tracking collars (HQAN40L, 800 g, 5 years battery life, solar-powered, Hunan Global Messenger Technology Co., Ltd., China). Because deer need an adaptation period after wearing collars32, they were anesthetized and fitted the collars one month in advance. Milu hard were released to HEDL on 7 Dec. 2020.
Deer activity data were continuously collected using three-axis accelerometer sensors integrated into the collar32,33,34. The sensor monitored real-time acceleration along the X, Y, and Z axes, with a default sensitivity threshold of 0.15 g (gravitational acceleration). When the acceleration on any axis exceeded this threshold, the device recorded an additional count. GPS coordinates and activity data were read one fix per hour, then these data were downloaded using the v3.0401 Hunan Global Messenger Technology Satellite Tracking Data Management System. Seasons were defined based on local climatic conditions: spring (March to May), summer (June to August), autumn (September to November), and winter (December to February)35.
Data analysis
All data were summarized and sorted in Excel 2021. The circadian rhythm graph was plotted using GraphPad Prism 5, activity amounts were presented by mean values and standard error. The home ranges (kernel density estimator (KDE), isopleth 95%) and the distance from the release site during December 2020 to November 2022, and the activity heatmaps of winter 2020 and spring 2021, were used to analyze the initial acclimation period and exploration-dispersion period of reintroduced Milu. Home range data, distances from the release site, and activity heatmaps were obtained from the v3.0401 Hunan Global Messenger Technology Satellite Tracking Data Management System, which employed a Gaussian kernel function for heatmap generation.
To analyze the sexual and seasonal differences in the circadian activity rhythms from winter 2020 to autumn 2022, the circular statistics, Watson one-sample and two-sample tests in R were used to determine whether the activity rhythms were significant. Meanwhile, after assessing the normality of home range area and dispersal distance using Shapiro-Wilk test in R, either the t-test or Wilcoxon test was selected for analysis.
Satellite datasets were obtained from the Landsat ‘T’M image of the United States Land Resources Satellite with a spatial resolution of 30 m and processed using the software ERDAS Image 9.0 remote sensing processing and ArcGIS 9.3.
Results
After data filtering, we had successfully acquired a total of 145,496 valid locations from December 8, 2020 to November 30, 2022 with GPS satellite tracking collars (Table 1).
Circadian activity patterns of Milu
Activity peaks
From December 2020 to November 2022, the daily activity rhythm of Milu exhibited 1–4 peaks (Fig. 2). Activity levels were significantly higher during the day than at night, except in August, October, and November 2022. These results indicated that the reintroduced Milu displayed a notable diurnal rhythm, with typical dawn and dusk activity peaks.
The activity pattern changes after released
Milu exhibited pronounced concentration trends in activity during the eight quarters of both male and female Milu from winter 2020 to autumn 2022 (P < 0.05), except for the summer 2022 of male Milu (U2 = 0.09, P > 0.05). The noticeable changes occurred in the activity patterns of Milu. In the first month of post-release, there are four distinct activity peaks (dawn, dusk, midday, and midnight), and only one significant morning peak was recorded in February and March 2021 (Fig. 2). From December 2020 to June 2021, the activity rhythm of the Milu remained relatively unstable, with considerable fluctuations in the timing of peaks, particularly the evening peak, which lacked consistent concentrated. During July 2021 to November 2022, the rhythm gradually stabilized, and morning and evening peaks became more concentrated. Compared with the first year the diurnal activity rhythm of Milu became more regular, and the timing of activity peaks was more consistent during second year.
Seasonal differences
Milu had the lowest activity levels in winter mornings, with the lowest point occurring at 6:00–7:00, 1–2 h later than the lowest points in the mornings of the other three seasons (Fig. 3). Morning activity peaks occurred at 8:00–9:00 in winter, 7:00–8:00 in spring, 6:00–7:00 in summer, and 7:00–8:00 in autumn, indicating a delayed pattern in winter. Evening activity peaks occurred at 18:00–19:00 in winter, 19:00–20:00 in spring, 20:00–21:00 in summer, and 18:00–20:00 in autumn, showing an advanced pattern in winter.
Sexual differences
The activity rhythm patterns of males and females were consistent in most months (Fig. 2). Females generally exhibited more variable and numerous activity peaks compared to males, though there was no consistent trend in the timing of peaks between sexes. However, there were significant sexual differences in activity rhythms during four distinct quarters: winter 2020 (U2 = 0.12, P < 0.001), summer 2021 (U2 = 0.09, P < 0.001), summer 2022 (U2 = 0.47, P < 0.001), and autumn 2022 (U2 = 0.09, P < 0.05), and female activity levels were significantly higher than males in summer 2022 (Fig. 3).
Dispersal distances and home ranges during initial acclimation period and exploration-dispersion period
In the first quarter of post-release, the average distance between the 10 Milu and their release site was 5.59 km (SD = 0.03 km, CV = 0.50%) (Fig. 4a), and the home range size was 50.36 km² (SD = 3.03 km², CV = 6.02%) (Fig. 4b). From the second to the eighth quarter, the average distance from the release site increased to 5.60–11.16 km (SD = 0.64–9.96 km, CV = 9.82–139.00%), and the home range size expanded to 96.24–249.77 km² (SD = 41.61–317.85 km², CV = 38.33–159.79%). During the first quarter (winter 2020), the deer’s activities were concentrated around the release site, while in the second quarter (spring 2021), their activity range began to expand (Fig. 5).
There were significant differences in dispersal distances among Winter 2020 with Winter 2021 (t = -2.71, P < 0.05)/ Spring 2022 (t = -2.39, P < 0.05)/ Autumn 2022 (t = -3.27, P < 0.05), among Spring 2021 with Winter 2021 (t = -2.35, P < 0.05) / Spring 2022 (t = -2.71, P < 0.05), between Summer 2021 and Spring 2022 (t = -2.71, P < 0.05), and between Winter 2021 and Summer 2022 (t = 3.05, P < 0.05), while no significant differences were found among other seasons. Meanwhile, for home range size, significant differences occurred among Winter 2020 with Spring 2021 (t = -5.80, P < 0.001)/Summer 2021 (t = -2.44, P < 0.05), between Spring 2021 and Summer 2021 (t = 3.38, P < 0.05). The difference of home range size between males and females was significant only during autumn 2021 and the dispersal distance varied during winter 2021 (t = 2.75, P < 0.05).
These results suggested that the first quarter represented an initial acclimation period, during which the deer remained close to the release site. Starting from the second quarter, they entered an exploration-dispersion period, gradually adapting to the local environment and expanding their range.
Circadian rhythms of activity level in different habitats
From December 8, 2020 to November 30, 2022, five male and one female Milu crossed the dam of the lake area into farmland. A total of 7823 valid activity loci were recorded from May 11 to October 28 in 2021 and 2022. Male Milu exhibited a distinct diurnal-nocturnal activity rhythm in both the lake areas and farmlands, with activity peaks during dawn and dusk (Fig. 6). Moreover, activity levels were significantly higher in the lake area than farmland.
Discussion
Diurnal activity rhythms of Milu
The circadian behavioral rhythm in mammals is regulated by the light-dark cycle and exhibits diverse activity patterns, including diurnal, nocturnal, crepuscular, or even cathemeral strategy. Notably, the variations among mammal species reflect differing degrees of behavioral plasticity, and that intraspecific variations are evidenced both between and within populations11,36. Our study found Milu exhibited primarily crepuscular activity pattern, consistent with northern deer species like sika deer27, red deer (Cervus elaphus)37, and others23,37,38,39,40,41. Some African ungulates (e.g., Hippotragus niger, Equus quagga) show same peaks with midday rest42. The reintroduced Milu also follows this bimodal pattern during behavioral stability period, with peaks at 6:00–9:00 and 18:00–21:00, due to their natural timidity similar to other deer. The previously rewilding Milu population in Dongting Lake displayed pattern of resting in morning and feeding in afternoon (14:00–17:00) during adaptive phase43, and another semi-wild population in Shishou Nature Reserve showed dawn/dusk feeding peak44.
Studies revealed complex activity rhythm variations among deer species. For example, Tufted deer, Reeves’ muntjac (Muntiacus reevesi), Alpine Musk Deer (Moschus chrysogaster) and Forest Musk Deer (Moschus berezovskii) showed crepuscular activities23,48, while Sambar was nocturnal in China’s Southwest Mountains23. Circadian rhythms become disrupted without light/dark cycles45, like Arctic reindeer Rangifer tarandus during continuous darkness/daylight10,46,47, displayed more nocturnal behavior than most ruminants10. While most deer show crepuscular or multimodal rhythms23,27,42,43,44, exceptions including unimodal/nocturnal patterns exist10,23,48. Although unimodal rhythms are uncommon in deer, observed in Tufted deer and Reeves’ muntjac (peak dusk)48, Milu also displayed unimodal patterns during February/March/September 2021 (both sexes) and May-June 2022 (males only), revealing specially seasonal behavioral flexibility.
Circadian rhythm changes of reintroduced Milu
Reintroduced captive-bred animals must adapt behaviorally to new environments, adjusting activity rhythms while exploring unfamiliar territories2. Giant anteaters (Myrmecophaga tridactyla) demonstrate behavioral plasticity by seasonally modifying their activity patterns in response to environmental conditions11, while reintroduced fishers (Pekania pennanti) develop diurnal activity peaks as a likely predator avoidance strategy29. Similarly, released giant pandas (Ailuropoda melanoleuca) show initially low activity levels (20–40 days post-release) that gradually increase, eventually stabilizing with 1–2 daytime peaks and one nighttime peak51. In this study Milu exhibited comparable adaptive patterns: initially remaining within 5.59 km of the release site (50.39 km² home range) before entering an exploration phase where their range expanded 2–5 times, ultimately developing stable diurnal rhythms with distinct dawn and dusk activity peaks.
Seasonal variation of circadian rhythms
Milu deer exhibited obvious seasonal variations in their circadian activity rhythms, characterized by shifting peak activity levels that appear influenced by both environmental conditions and physiological adaptations. After released, Milu demonstrated notable adaptability with 3–4 activity peaks across December 2020-February 2022, indicating the rhythm plasticity. There are also seasonal differences in other species, such as sika deer exhibiting crepuscular patterns in spring/summer but shifting to trimodal peaks (dawn, dusk, midnight) in autumn27, and elk and white-lipped deer are the same49,50.
The thermoregulatory hypothesis provides a framework for understanding these patterns, proposing that animals minimize activity during extreme cold conditions (such as winter months) to conserve energy reserves12,52. This situation occurred to released Milu populations in Dongting Lake area, with significantly reduced activity levels correspond to low temperatures in winters. Such seasonal behavioral adjustments are consistent with patterns of other ungulates, reindeer show decreased winter activity in response to cold temperatures and increased precipitation10, while moose shift their activity peaks to cooler dawn/dusk periods during snow-free seasons but become more diurnal in snowy conditions to avoid cold stress39.
Multiple factors contribute to these seasonal activity modifications. Temperature serves as a primary driver of rhythmic changes22, evidenced by earlier dusk activity peaks in Tufted deer and sambar during cold seasons23, and temperature-dependent reproductive rhythms in reintroduced anteaters (Myrmecophaga tridactyla)11. Desert mule deer (Odocoileus hemionus crooki) exemplify thermoregulatory behavioral plasticity, switching between diurnal winter foraging/nocturnal resting and summer shade-seeking/activity reduction53. The interaction between physiological mechanisms and environmental cues shapes activity patterns during energetically demanding life stages13,54, resulting in the diverse seasonal rhythms observed across species.
Sexual differences of circadian rhythms
Sexual dimorphism has been widely recognized as a key driver of behavioral variation in animals55, particularly in sexually dimorphic species like Milu12. These large social animals exhibit distinct sexual differences in both morphology and behavior that significantly influence their circadian rhythms. During the rutting season, male Milu demonstrate increased exploratory behavior and focus on securing harems, directly impacting their activity patterns. Sexual behavior differences occur also in male sika deer, which show elevated activity during mating seasons25, and male roe deer that exhibit greater sensitivity to high temperatures compared to females40. The larger body size of males may provide adaptive advantages under extreme weather conditions56.
Contrastive analysis revealed largely overlapping but distinct activity differences between male and female Milu (Figs. 2 and 3). Females displayed significantly higher activity levels during the 2022 fawn-rearing season (May-July). This behavioral pattern is similar to female brown bears (Ursus arctos), which increase daytime activity to protect cubs57. While male Milu rarely moved around with fawn, the females’ heightened activity reflects their maternal investment and vigilance. These findings underscore how sexual dimorphism manifests in both reproductive strategies and environmental adaptations.
Human disturbance impacts on the behavioral rhythms of Milu
Mammals often adjust activity patterns in response to human disturbances57,58,59, demonstrating behavioral flexibility as an adaptive strategy60,61. Reintroduced Pyrenean brown bears became predominantly nocturnal to avoid human activity57, while gray brocket and marsh deer reduced daytime activity when coexisting with cattle but reverted to diurnal patterns after cattle removal25,28.White-tailed deer in farmland showed bimodal morning/dusk activity peaks (for abundant food resources), while forest/wetland populations exhibited only dusk peaks20. Hunting pressure drove sika deer toward nocturnal activity (crepuscular in spring/summer, trimodal in autumn)27 and red deer toward twilight/nocturnal patterns37. White-tailed deer in recreational areas shifted activity to pre-dawn hours (avoiding human presence), unlike stable patterns in protected zones38. Sika deer in protected area displayed stronger morning than dusk peaks—a deviation from typical crepuscular rhythms likely caused by human disturbance25.
This study revealed that the activity patterns of Milu during October-November 2021 were abnormal, with daytime activity levels in October-November 2022 being lower than those at night. This shift was mainly due to the reed harvesting period in East Dongting Lake during these months. Although the circadian rhythms of Milu in farmland and those in the East Dongting Lake wetland were generally consistent, the activity levels of deer in the lake area were higher across all time periods within a 24-hour cycle. This suggested that Milu reduced their activity levels to minimize exposure and avoid potential risks in farmland .
Conclusions and implications
Our long-term tracking and monitoring research revealed that captive bred species reintroduced into their historical distribution areas adjusted their circadian behavioral rhythms over time to adapt to the local natural environment. Specifically, we found that Milu exhibited a crepuscular behavioral rhythm characterized by a bimodal pattern, with activity peaks occurring from 6:00 to 9:00 and from 18:00 to 21:00. These rhythms were different between sexes and varied seasonally. Additionally, while the circadian activity patterns of male Milu were similar in farmland and East Dongting Lake wetland, their activity levels were significantly higher in the lake area. This study provides an important ecological perspective for understanding how animals adjust their behavior patterns during the reintroduction process and offers scientific evidence for optimizing species conservation and reintroduction strategies.
Data availability
Data is provided within the manuscript and site information is provided in supplementary material.
References
Martin, J. M. et al. Integrated evidence-based extent of occurrence for North American Bison (Bison Bison) since 1500 CE and before. Ecology 104 (1), e3864. https://doi.org/10.1002/ecy.3864 (2022).
Schmitz, P., Caspers, S., Warren, P. & Witte, K. First steps into the wild – Exploration behavior of European Bison after the first reintroduction in Western Europe. PLoS ONE. 10 (11), e0143046. https://doi.org/10.1371/journal.pone.0143046 (2015).
Needham, R. J., Zabel, R. W., Roberts, D. & Kemp, P. S. The impact of reintroduced Eurasian beaver (Castor fiber) dams on the upstream movement of brown trout (Salmo trutta) in upland areas of great Britain. PLoS ONE. 20 (2), e0313648. https://doi.org/10.1371/journal.pone.0313648 (2025).
Iannella, M., Biondi, M. & Serva, D. Functional connectivity and the current arrangement of protected areas show multiple, poorly protected dispersal corridors for the Eurasian Lynx. Biol. Conserv. 291, 110498. https://doi.org/10.1016/j.biocon.2024.110498 (2024).
Biró, Z., Katona, K., Szabó, L., Sütő, D. & Heltai, M. Grey Wolf (Canis lupus) recolonization in hungary: does the predation risk affect the red deer (Cervus elaphus). Population? Animals. 14 (24), 3557. https://doi.org/10.3390/ani14243557 (2024).
Zou, Y., Jiang, Y., Song, Z., Fang, X. & Ding, C. The crested ibises expanding to plain areas exhibit a higher tolerance of human proximity. Avian Res. 15, 100165. https://doi.org/10.1016/j.avrs.2024.100165 (2024).
Turghan, M. A., Jiang, Z. & Niu, Z. An update on status and conservation of the przewalski’s horse (Equus ferus przewalskii): captive breeding and reintroduction projects. Animals 12 (22), 3158. https://doi.org/10.3390/ani12223158 (2022).
Taylor, G. et al. Is reintroduction biology an effective applied science? Trends Ecol. Evol. 32 (11), 873–880. https://doi.org/10.1016/j.tree.2017.08.002 (2017).
Cheng, Z. B. et al. Reintroduction, distribution, population dynamics and conservation of a species formerly extinct in the wild: A review of thirty-five years of successful Milu (Elaphurus davidianus) reintroduction in China. Glob Ecol. Conserv. 31, e01860. https://doi.org/10.1016/j.gecco.2021.e01860 (2021).
Loe, L. E. et al. Activity pattern of Arctic reindeer in a predator-free environment: no need to keep a daily rhythm. Oecologia 152, 617–624. https://doi.org/10.1007/s00442-007-0681-7 (2007).
Di Blanco, Y. E., Spørring, K. L. & Di Bitetti, M. S. Daily activity pattern of reintroduced giant anteaters (Myrmecophaga tridactyla): effects of seasonality and experience. Mammalia 81 (1), 11–21. https://doi.org/10.1515/mammalia-2015-0088 (2017).
Carvalho, F., Galantinho, A., Somers, M. J., Do, L. & San, E. Influence of season, sex, and interspecific interactions on the diel activity patterns of two sympatric African small carnivores. Sci. Rep. 14 (1), 29701. https://doi.org/10.1038/s41598-024-80619-4 (2024).
Shero, M. R., Costa, D. P., Burns, J. M. & Goetz, K. T. Breath-hold capacities and circadian dive rhythmicity shape optimal foraging strategies in a Polar marine mammal, the Weddell seal (Leptonychotes weddellii). Commun. Biol. 7 (1), 1394. https://doi.org/10.1038/s42003-024-07029-0 (2024).
Smedley, D. C. et al. Movements, space use and site fidelity of translocated and resident mule deer (Odocoileus hemionus). Wildl Res. 46 (7), 509–517. https://doi.org/10.1071/WR19043 (2019).
Whiting, J. C. et al. Timing and synchrony of births in Bighorn sheep: implications for reintroduction and conservation. Wildl Res. 39 (7), 565–572. https://doi.org/10.1071/WR12059 (2012).
Whiting, J. C., Stewart, K. M., Bowyer, R. T. & Flinders, J. T. Reintroduced Bighorn sheep: do females adjust maternal care to compensate for late-born young? Eur. J. Wildl. Res. 56 (3), 349–357. https://doi.org/10.1007/s10344-009-0323-y (2010).
Berger-Tal, O. & Saltz, D. Using the movement patterns of reintroduced animals to improve reintroduction success. Curr. Zool. 60 (4), 515–526. https://doi.org/10.1093/czoolo/60.4.515 (2014).
Davies, A. B., Tambling, C. J., Kerley, G. I. & Asner, G. P. Limited Spatial response to direct predation risk by African herbivores following predator reintroduction. Ecol. Evol. 6 (14), 5048–5058. https://doi.org/10.1038/s41598-024-80619-4 (2016).
Harrison, J. O. et al. Local genetic adaptation to habitat in wild chimpanzees. Science 387, eadn7954. https://doi.org/10.1126/science.adn795 (2025).
Delisle, Z. J., Sample, R. D., Caudell, J. N. & Swihart, R. K. Deer activity levels and patterns vary along gradients of food availability and anthropogenic development. Sci. Rep. 14 (1), 10223. https://doi.org/10.1038/s41598-024-60079-6 (2024).
Guiden, P. W. et al. Reintroduced megaherbivores indirectly shape small-mammal responses to moonlight. Ecology 104 (2), e3884. https://doi.org/10.1002/ecy.3884 (2023).
Farsi, H. et al. Entrainment of circadian rhythms of locomotor activity by ambient temperature cycles in the dromedary camel. Sci. Rep. 10 (1), 19515. https://doi.org/10.1038/s41598-020-76535-y (2020).
Li, Q. et al. Daily activity rhythms of animals in the Southwest mountains, china: influences of interspecific relationships and seasons. Animals 14, 2842. https://doi.org/10.3390/ani14192842 (2024).
Thoré, E. S. et al. Time is of the essence: the importance of considering biological rhythms in an increasingly polluted world. PLoS biol. 22 (1), e3002478. https://doi.org/10.1371/journal.pbio.3002478 (2024).
Kawamura, K. et al. Diel and monthly activity pattern of brown bears and Sika deer in the Shiretoko Peninsula, Hokkaido, Japan. J. Vet. Med. Sci. 84 (8), 1146–1156. https://doi.org/10.1292/jvms.21-0665 (2022).
Kohl, M. T. et al. Diel predator activity drives a dynamic landscape of fear. Ecol. Monogr. 88 (4), 638–652. https://doi.org/10.1002/ecm.1313 (2017).
Ikeda, T. et al. Effects of culling intensity on diel and seasonal activity patterns of Sika deer (Cervus nippon). Sci. Rep. 9 (1), 17205. https://doi.org/10.1002/wlb3.01263 (2019).
Di Bitetti, M. S., Iezzi, M. E., Cruz, P., Varela, D. & De Angelo, C. Effects of cattle on habitat use and diel activity of large native herbivores in a South American rangeland. J. Nat. Conserv. 58, 125913. https://doi.org/10.1016/j.jnc.2020.125900 (2020).
Parsons, M. A. et al. Habitat selection and Spatiotemporal interactions of a reintroduced mesocarnivore. J. Wildl. Manage. 83 (4), 1–12. https://doi.org/10.1002/jwmg.21670 (2019).
Jiang, Z. & Harris, R. B. Elaphurus davidianus. IUCN red list threat. Species E. https://doi.org/10.2305/IUCN.UK.2016-2.RLTS.T7121A22159785.en (2016). T7121A22159785.
Hou, Z. et al. Life forms and ecotypes of wetland plants at lake Dongting wetlands. J. Lake Sci. 28, 1095–1102. https://doi.org/10.18307/2016.0520 (2016).
Stiegler, J. et al. Mammals show faster recovery from capture and tagging in human-disturbed landscapes. Nat. Commun. 15 (1), 8079. https://doi.org/10.1038/s41467-024-52381-8 (2024).
Ensing, E. P. et al. GPS based daily activity patterns in European red deer and North American elk (Cervus elaphus): indication for a weak circadian clock in ungulates. PLoS ONE. 9 (9), e106997. https://doi.org/10.1371/journal.pone.0106997 (2014).
Sarout, B. N. M. et al. Assessment of circadian rhythm of activity combined with random regression model as a novel approach to monitoring sheep in an extensive system. Appl. Anim. Behav. Sci. 207, 26–38. https://doi.org/10.1016/j.applanim.2018.06.007 (2018).
Wang, Z., Zhi, R., Feng, G. L. & Li, S. P. The impacts of the typical fields on the season identification using the multi-factors climate state similarity measurement. Clim. Change Res. 14, 350–361. https://doi.org/10.12006/j.issn.1673-1719.2017.223 (2018).
Tattersall, I. The concept of cathemerality: history and definition. Folia Primatol. 77, 7–14. https://doi.org/10.1159/000089692 (2006).
Kamler, J. F., Jędrzejewska, B. & Jędrzejewski, W. Activity patterns of red deer in Białowieża National Park, Poland. J. Mammal. 88, 508–514. https://doi.org/10.1644/06-MAMM-A-169R.1 (2007).
Millien, V., Truchon, F. & St-Laurent, M. H. White-tailed deer limit their spatio-temporal overlap with hikers in a protected area. Sci. Rep. 14 (1), 32143. https://doi.org/10.1371/journal.pone.0313648 (2024).
Bao, H. et al. Effects of inter-and intra-specific interactions on moose habitat selection limited by temperature. Remote Sens. 14 (24), 6401. https://doi.org/10.3390/rs14246401 (2022).
Rigoudy, N. et al. Agricultural land use and reproductive behaviour constrain responses to summer thermal stress in a large herbivore. Biol. Conserv. 302, 110888. https://doi.org/10.1016/j.biocon.2024.110888 (2025).
Krop-Benesch, A., Berger, A., Hofer, H. & Heurich, M. Long-term measurement of roe deer (Capreolus capreolus) activity using two-axis accelerometers in GPS-collars. Ital. J. Zool. 80 (1), 69–81. https://doi.org/10.1080/11250003.2012.725777 (2013).
Owen-Smith, N. & Goodall, V. Coping with savanna seasonality: comparative daily activity patterns of African ungulates as revealed by GPS telemetry. J. Zool. 293 (3), 181–191. https://doi.org/10.1111/jzo.12132 (2014).
Wang, S. et al. Behavioural rhythms during the adaptive phase of introduced milu/Père david’s deer (Elaphurus davidianus) in the Dongting lake wetland. China Pak J. Zool. 49 (5), 1657–1664. https://doi.org/10.17582/journal.pjz/2017.49.5.1657.1664 (2017).
Yang, D., Li, Z., Li, P. & Jiang, Z. Diurnal activity time budget of Père david’s deer in Hubei Shishou Milu National nature Reserve, China. Acta Ecol. Sin. 33, 1397–1404. https://doi.org/10.5846/stxb201206100830 (2013).
Arnold, W. et al. Circadian rhythmicity persists through the Polar night and midnight sun in Svalbard reindeer. Sci. Rep. 8 (1), 14466. https://doi.org/10.1038/s41598-018-32778-4 (2018).
van Oort, B. et al. Circadian organization in reindeer. Nature 438, 1095–1096. https://doi.org/10.1038/4381095a (2005).
Meier, S. A. et al. Uncoupling of behavioral and metabolic 24-h rhythms in reindeer. Curr. Biol. 34 (7), 1596–1603. https://doi.org/10.1016/j.cub.2024.02.072 (2024).
Xiang, D. et al. Daily activity rhythm of sympatric ungulate species in Fanjingshan Reserve, China. Glob Ecol. Conserv. 56, e03271. https://doi.org/10.1016/j.gecco.2024.e03271 (2024).
Wichrowski, M. W. et al. Activity and movements of reintroduced elk in southeastern Kentucky. Southeast. Nat. 4, 365–374. https://doi.org/10.1656/1528-7092(2005)004[ (2005). 0365:AAMORE]2.0.CO;2.
Li, B. et al. Diurnal time budgets and behavioral rhythms of white-lipped deer (Cervus albirostris) in the Qilian mountains of Qinghai, China. Pak J. Zool. 46, 1557–1563. https://doi.org/10.5555/20163232764 (2014). https://www.cabidigitallibrary.org/doi/pdf/
He, L. et al. Assessing the health status of released, captive-bred giant pandas (Ailuropoda melanoleuca) through activity patterns. Folia Zool. 68 (2), 72–78. https://doi.org/10.25225/fozo.054.2019 (2019).
Camps, D., Villero, D., Ruiz-Olmo, J. & Brotons, L. Niche constraints to the Northwards expansion of the common genet (Genetta genetta, Linnaeus 1758) in Europe. Mamm. Biol. 81, 399–409. https://doi.org/10.1016/j.mambio.2016.03.003 (2016).
Hayes, C. L. & Krausman, P. R. Nocturnal activity of female desert mule deer. J. Wildl. Manage. 57, 897–904. https://doi.org/10.2307/3809095 (1993).
Pettigrew, G. W., Di Vita, V., Pettigrew, M. & Gilchrist, J. S. The diel activity pattern of mountain hare (Lepus timidus) on managed Heather moorland in Scotland. Ecol. Evol. 11 (12), 7106–7113. https://doi.org/10.1002/ece3.7512 (2021).
Jędrzejewski, W. et al. Effect of sex, age, and reproductive status on daily activity levels and activity patterns in Jaguars (Panthera onca). Mamm. Res. 66, 531–539. https://doi.org/10.1007/s13364-021-00589-0 (2021).
Marcelli, M., Fusillo, R. & Boitani, L. Sexual segregation in the activity patterns of European polecats (Mustela putorius). J. Zool. 261, 249–255. https://doi.org/10.1017/S0952836903004151 (2003).
Parres, A. et al. Activity patterns in the reintroduced pyrenean brown bear population. Mamm. Res. 65, 435–444. https://doi.org/10.1007/s13364-020-00507-w (2020).
Gaynor, K. M., Hojnowski, C. E., Carter, N. H. & Brashares, J. S. The influence of human disturbance on wildlife nocturnality. Science 360, 1232–1235. https://doi.org/10.1126/science.aar712 (2018).
Edwards, S., Noack, J., Heyns, L. & Rodenwoldt, D. Are camera traps a reliable method for estimating activity patterns? A case study comparing technologies for estimating brown hyaena activity curves. Remote Sens. Ecol. Conserv. 7, 129–138. https://doi.org/10.1002/rse2.175 (2021).
Carter, N. H., Shrestha, B. K., Karki, J. B., Pradhan, N. M. B. & Liu, J. Coexistence between wildlife and humans at fine spatial scales. Proc. Natl. Acad. Sci. 109, 15360–15365. (2012). https://doi.org/10.1073/pnas.1210490109
Donatelli, A., Mastrantonio, G. & Ciucci, P. Circadian activity of small brown bear populations living in human-dominated landscapes. Sci. Rep. 12 (1), 15804. https://doi.org/10.1038/s41598-022-20163-1 (2022).
Acknowledgements
We would like to thank Dr. Yucheng Song, Mr. Yiwei Fu and Mr. Yunfei Li for their assistance with data collection, and extend special thanks to Dr. Yuqi Cao and Dr. Qinguo Wei for their expert guidance in statistical analysis. We gratefully acknowledge the journal’s editors and anonymous reviewers for their valuable comments on our manuscript.
Funding
The present study was supported by Beijing Municipal Fiscal Project [No.25CA005; No.25CD008, No.25CD010] and the Key research and development project of China’s Ministry of Science and Technology [grant number: 2024YFF1306404-1], 2024 Central Forestry and Grassland Ecological Protection and Restoration Fund.
Author information
Authors and Affiliations
Contributions
J.B, K.C: conceptualization. Z.C, H.Z: software, data collection, and writing—original draft preparation. J.M., C.F., W.L., Z.Z.: data analysis. Q.G., Q.Z., P.Z, S.Z, C.Z: data collection and field survey. All authors were committed to improving this paper and are responsible for the viewpoints mentioned in this work. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cheng, Z., Zhang, H., Ma, J. et al. Behavioral shifts of reintroduced Milu deer Elaphurus davidianus in East Dongting lake of China. Sci Rep 15, 34833 (2025). https://doi.org/10.1038/s41598-025-21345-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-21345-3