Abstract
To investigate the correlation between dynamic changes in serum calcium levels and prognosis in patients with spinal cord injury (SCI), and to provide a reference for clinical assessment of disease severity and prognosis in SCI patients. Data for this study were extracted from version 3.0 of the MIMIC-IV database, including 699 adult patients diagnosed with SCI. Group-based trajectory modeling (GBTM) was used to analyze serum calcium test data, classifying patients into different calcium trajectory types. Baseline characteristics among different trajectory groups were compared using the Kruskal–Wallis test, analysis of variance, and chi-square test. Kaplan–Meier survival curves were plotted with log-rank tests, and a Cox proportional hazards regression model was constructed to analyze the independent impact of calcium trajectories on patient prognosis. Subgroup analyses were further performed to evaluate the robustness of the results. The C-statistic and Net Reclassification Improvement (NRI) were used to evaluate the optimization effect of combining calcium trajectories with conventional prognostic scoring systems on the predictive model for ICU mortality risk in patients with SCI. Trajectory analysis classified serum calcium trajectories into two types: Class 1 (244 cases) characterized by "a steep decline in serum calcium ion levels" and Class 2 (455 cases) characterized by "a slow but persistent decline in serum calcium ion levels." Baseline characteristics showed that Class 1 patients had significantly longer ICU stay, total hospital stay, ICU survival time, and in-hospital survival time compared to Class 2 (P < 0.05). Survival analysis indicated that Class 1 patients had significantly lower 30-day and 60-day all-cause mortality in the ICU than Class 2 (P < 0.05). Cox regression analysis revealed that after adjusting for confounding factors such as age, gender, diabetes mellitus, and hypertension, Class 2 remained an independent risk factor for increased mortality (P < 0.05). Subgroup analyses showed no significant interaction between the results and gender, age, or diabetes mellitus (P > 0.05), but the elevated mortality risk in Class 2 was more pronounced in patients with hypertension (HR = 3.282, P = 0.027). Calcium trajectories can enable 23.6–27.4% of patients to obtain more accurate risk stratification, and in particular, significantly improve the ability to identify high-risk patients among those with the death outcome (Event NRI = 0.185–0.213). The dynamic change trajectory of serum calcium in patients with SCI is closely associated with their prognosis. The trajectory type characterized by "slow but continuous decline in serum calcium ion levels" indicates a significantly increased risk of ICU mortality in patients. Serum calcium trajectory can not only serve as a potential biomarker for evaluating the prognosis of SCI patients but also provide significant incremental predictive value for conventional prognostic scoring systems, thereby offering references for clinical stratified management and personalized treatment in the ICU. However, the results of this study are only applicable to critically ill SCI patients admitted to the ICU.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) is a severe trauma to the central nervous system, often resulting in sensory and motor dysfunction or even lifelong disability in patients, imposing heavy medical and economic burdens on individuals, families, and society1. Epidemiological data show that the annual incidence of SCI is approximately (0.7–1.2) per 900,000 population. In recent years, with the increase in accidental injuries such as traffic accidents and falls from heights, its incidence has been on an annual upward trend2. The prognosis of SCI patients is affected by multiple factors, including injury level, injury severity, timing of treatment, and management of complications. Mortality and survival outcomes of SCI vary by injury characteristics. Studies have found that mortality is extremely high in the first year after injury, which depends on many factors such as the severity of trauma, management of early complications, quality of rescue and medical assistance in the acute phase, and admission to specialized centers3. SCI mortality is also closely associated with the American Spinal Injury Association (ASIA) grade, level of neurological injury, mechanical ventilation, advanced age, and sex4. Thietje et al.5 found that the average survival time of patients with high-level injuries was shorter than that of tetraplegic patients with low-level injuries, and there was a negative correlation between survival rate and higher neurological injury level or complete injury. Charlifue et al.6 identified the need for mechanical ventilation as another important factor affecting the survival rate of SCI patients. Varma et al.7 found through research that advanced age was positively correlated with mortality in SCI patients—the older the patient, the higher the mortality rate. Additionally, studies have shown that the 1-year survival rate of SCI patients ranges from 79 to 100%, the 5-year survival rate from 85 to 96%, and the 10-year survival rate from 81 to 93%. Studies comparing survival rates between males and females usually show that females have a higher survival rate8. Furthermore, several other factors should be considered as potential influences on survival prognosis, including low educational attainment, maladaptive behaviors (substance abuse, suicide attempts), and poor quality of care9.
Among these factors, metabolic disorders—one of the common complications—have gradually become a key factor affecting patients’ rehabilitation progress and long-term prognosis10,11. As one of the most important minerals in the human body, calcium is not only a major component of bones and teeth, but also plays an irreplaceable role in physiological processes such as nerve conduction, muscle contraction, cellular signal transduction, and regulation of enzyme activity12. Under normal circumstances, the human body maintains the serum calcium concentration within a stable range through the synergistic effect of multiple hormones, including parathyroid hormone, vitamin D, and calcitonin13. After the occurrence of SCI, patients often develop calcium metabolism imbalance due to factors such as prolonged bed rest, neuroregulatory dysfunction, gastrointestinal disorders, and drug interventions14. Srichuachom et al.15 found that the overall prevalence of hypocalcemia in trauma patients upon admission to the emergency department was 56%, and the mortality rate of patients with hypocalcemia was significantly higher than that of patients with normocalcemia. Hypocalcemia is relatively common in adult trauma patients, and the mortality rate in this population is notably higher. Serum calcium levels play a crucial role in SCI repair: calcium ions are involved in nerve injury repair, and long-term hypocalcemia can reduce the calcium ion concentration in the extracellular environment, thereby impairing neuronal function16. Zhang et al.17 observed that the intracellular calcium ion concentration increases significantly after SCI, and there is a significant correlation between changes in intracellular calcium ion concentration and motor function. Intracellular calcium ion overload may play an important role in the pathogenesis of SCI. In addition, long-term hypocalcemia can also affect the bone mineral density (BMD) of SCI patients. It not only leads to reduced bone mass, but also impairs the microstructure of bones, resulting in poor prognosis associated with severe osteoporosis18. Gifre et al.19 followed 35 SCI patients for 12 months and found that 52% of them developed osteoporosis. In recent years, dynamic monitoring of the trajectory of physiological indicators has gradually become an important method for evaluating disease progression and prognosis. As a quantitative indicator reflecting the dynamic changes of calcium metabolism, calcium trajectory can more comprehensively capture the fluctuation characteristics of calcium levels during the course of the disease, and is of greater clinical significance compared with calcium concentration detection at a single time point20,21. In critically ill patients, persistent changes or severe fluctuations in calcium levels often indicate a poor prognosis, and early identification and intervention of abnormal calcium trajectories may improve patients’ clinical outcomes. However, there are currently no relevant studies on the dynamic change pattern of calcium trajectories in SCI patients and its association with prognosis, and systematic observation of the changing trend of calcium metabolism is lacking.
The MIMIC-IV (Medical Information Mart for Intensive Care IV) database is one of the most widely used public databases for intensive care medicine internationally, containing detailed clinical information of a large number of critically ill patients, including demographic data, laboratory test results, medication records, imaging data, and prognostic outcomes22. Therefore, based on the MIMIC-IV database, this study intends to extract serum calcium test data from patients with spinal cord injury, construct a calcium trajectory model, and analyze the correlation between different calcium trajectory types and patient prognosis, aiming to provide new ideas for early clinical identification of high-risk patients and improvement of patients’ clinical outcomes.
Methods
Data source
The data of this study were derived from version 3.0 of the MIMIC-IV (Medical Information Mart for Intensive Care-IV) database, a large-scale public clinical database developed through collaboration between the Laboratory for Computational Physiology at the Massachusetts Institute of Technology and the Beth Israel Deaconess Medical Center. It contains detailed clinical information of patients admitted to the intensive care unit (ICU) of the Beth Israel Deaconess Medical Center from 2008 to 2019, covering multi-dimensional data such as demographic information, diagnosis and treatment records, laboratory test results, vital sign monitoring data, medication information, and prognostic outcomes. The MIMIC-IV database strictly protects patient privacy through de-identification processing; thus, this study did not require approval from the Institutional Review Board (IRB) or informed consent.
Our research team obtained access permissions to the MIMIC-IV database (project number: 68561984), and the researchers have completed and obtained certification for the human research protection training provided by the National Institutes of Health (NIH). During data extraction, Structured Query Language (SQL) was used to link and screen relevant tables in the database, including core tables such as patient basic information (patients), admission records (admissions), ICU stay records (icustays), diagnostic coding (diagnoses_icd), laboratory test results (labevents), and prognostic outcomes (outcomes), ensuring the completeness and accuracy of the extracted data.
Study population
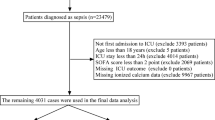
The study population consisted of patients with SCI selected from the MIMIC-IV database. The inclusion criteria were as follows: (1) adult patients aged ≥ 18 years; (2) definite diagnosis of SCI based on the 10th Revision of the International Classification of Diseases (ICD-10) codes (code range: S14.0–S34.9, T09.3, T10.3, T11.3, T12.3, S04.1); (3) ICU stay duration ≥ 24 h to ensure sufficient clinical data records; (4) availability of at least 2 serum calcium test results in laboratory examinations for constructing calcium trajectories; (5) complete clinical data, including demographic information, history of underlying diseases, laboratory test data, and prognostic outcome indicators required for this study. The exclusion criteria were: (1) patients with concurrent severe hepatic or renal failure, end-stage malignant tumors, or other end-stage diseases; (2) patients with congenital calcium metabolism disorders or long-term use of drugs affecting calcium metabolism with unclear medication duration; (3) patients with significant missing clinical data or logical errors that could not be supplemented or corrected after manual verification; (4) patients with repeated ICU admissions, where only the first ICU stay record meeting the inclusion criteria was retained.
Variable acquisition
Clinical data were retrieved using SQL and PostgreSQL (Version 9.6). The included clinical variables were as follows: gender, age, hospital length of stay (los_hospital_day), ICU length of stay (los_icu_day), ICU survival time (time_icu), in-ICU death status (death_icu_yn), in-hospital survival time (time_hosp), in-hospital death status (death_hosp_yn), presence of diabetes mellitus (Diabetes), presence of hypertension (Hypertension), heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), body temperature (Tem), oxygen saturation (sPO₂), red blood cell count (RBC), white blood cell count (WBC), serum creatinine (Serum_Creatinine), blood glucose (Glu), serum potassium (Potassium), partial pressure of oxygen (PO₂), partial pressure of carbon dioxide (PCO₂), blood pH (pH), serum calcium, lactate, Sequential Organ Failure Assessment score (SOFA), Acute Physiology Score III (APSIII), Simplified Acute Physiology Score Ⅱ (SAPSⅡ), Oxford Acute Severity of Illness Score (OASIS), Glasgow Coma Scale score (GCS), and Charlson Comorbidity Index (CCI).
Outcome indicators
The primary outcomes were all-cause mortality within 30 days and 60 days of ICU admission. Secondary outcomes included ICU length of stay, hospital length of stay, and functional indicators.
Statistical analysis
Data were standardized, and samples with a missing value proportion exceeding 20% were excluded. The Group-Based Trajectory Model (GBTM) was used to identify the unique longitudinal change characteristics of serum calcium levels (Note: “Pmean” in the original text is presumed to be a typo for the core variable "serum calcium levels" based on the study context). To accurately fit the dynamic change trend of serum calcium levels over time, this study first determined the optimal order by comparing the model fitting effects of different polynomial orders (linear, quadratic, and cubic). By comparing the Bayesian Information Criterion (BIC), Akaike Information Criterion (AIC), and residual sum of squares of models with different orders, the quadratic polynomial was finally selected as the trajectory fitting function. Combined with the clinical practical scenario of serum calcium testing in the MIMIC-IV database and data integrity, the time scale unit for GBTM modeling was set to “days”, with the time of ICU admission as the baseline time point (T = 0). Subsequent testing times were standardized as "Day 1, Day 2… Day n after admission". In clinical practice, serum calcium testing time points are affected by factors such as the severity of patients’ conditions, adjustments to treatment plans, and doctors’ diagnosis and treatment decisions, resulting in significant imbalance. Specifically, some patients undergo intensive testing in the first 3 days after admission with extended intervals between subsequent tests, while others have fewer testing points due to early discharge after condition improvement. To address this issue, a two-step approach was adopted in this study: The first step was testing point screening and time alignment. First, all patients’ serum calcium test data were sorted by "time after admission", and abnormal data with ambiguous test time records (e.g., only marked as “during hospitalization” without a specific time) or exceeding the ICU length of stay were excluded. Then, the "time window alignment method" was used to map each patient’s test time to preset standard time nodes. If there was no corresponding test value for a standard node, linear interpolation was used only when the interval between adjacent tests was ≤ 3 days to fill in the value; if the interval between adjacent tests was > 3 days, the node was marked as “missing” to avoid bias caused by excessive interpolation range. The second step was weight assignment and model robustness verification. In GBTM modeling, weights were assigned to each patient’s test data based on "testing point density": a weight of 1.0 was set for patients with ≥ 5 testing points, 0.8 for those with 3–4 testing points, and 0.6 for those with 2 testing points (no samples with 1 testing point existed, as the inclusion criteria required at least 2 tests). The logic behind this weight assignment was that patients with denser testing points had more reliable calcium level change trends and thus should be given higher contribution weight, while patients with fewer testing points had their interference on the overall trajectory classification reduced by lowering their weights. Meanwhile, sensitivity analysis was conducted to verify the robustness of the processing method: trajectory classification was performed based on the “unweighted model” and “weighted model” respectively, and the number of trajectories, sample proportion of each trajectory group, and entropy value (classification accuracy) of the two models were compared. The results showed that the entropy value of the weighted model (0.89) was higher than that of the unweighted model (0.82), and the consistency of trajectory classification results between the two models reached 91.3% (Kappa = 0.85, P < 0.001), indicating that the method for addressing the imbalance of testing time points in this study was reliable and did not introduce significant bias.
A two-stage iterative model fitting method was used for analysis: first, a polynomial function was applied to the trajectories to determine the number of groups, with the number of groups in the model ranging from 1 (corresponding to trajectories with no obvious characteristics) to 6; after selecting the appropriate number of groups, the model further determined the specific structure of each trajectory. The model fitting effect was evaluated using BIC, entropy value (> 0.7), and the minimum group size criterion (> 5%). For missing covariate data, multiple imputation was used to fill in missing values for continuous variables with a small amount of missing data, while the most frequent category was used for categorical variables. Additionally, the consistency between the predicted group probabilities and the established population proportions was calibrated. Furthermore, this study also balanced the operational convenience of the model and its intuitiveness in clinical scenarios.
Based on the different classifications of calcium trajectories, the Kruskal–Wallis test or analysis of variance (ANOVA) was used to determine the basic characteristics of continuous variables, while the chi-square test or Fisher’s exact test was applied to examine the basic characteristics of categorical variables. The Kaplan–Meier method was used to plot survival curves for different groups, and the log-rank test was employed to assess differences in survival rates between groups. A Cox proportional hazards regression model was constructed to further analyze the independent effect of calcium trajectories on the prognosis of patients with SCI after adjusting for potential confounding factors such as demographic characteristics, clinical indicators, and comorbidities. Additionally, subgroup analysis was conducted to evaluate the robustness of the results. To verify the incremental predictive value of serum calcium trajectories as prognostic biomarkers, the C-statistic and Net Reclassification Improvement (NRI) were further used to assess the optimization effect of combining calcium trajectories with conventional prognostic scoring systems on the predictive model for ICU mortality risk in SCI patients. All statistical analyses were performed using SPSS 26.0 or R 4.2.0 software, with a two-tailed test significance level (α) set at 0.05.
Results
Results of trajectory analysis
Model fitting indices for different numbers of categories (ranging from 1 to 6), including Log Likelihood, Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), and Scaled Bayesian Information Criterion (SABIC), are presented in Fig. 1A. The log likelihood value increases with the increasing number of categories. However, in the two-category model, AIC and BIC reach the lowest levels, indicating an optimal balance between model fit and complexity. Additionally, an entropy value close to 1 enhances the classification accuracy, guiding us to select the two-category model as the optimal solution (Fig. 1B,C).
(A) presents the model selection based on scaled and ranked values of log-likelihood, AIC, BIC, and SA-BIC; (B) shows the changes in predicted mean values over time for the two categories along with their corresponding confidence intervals; (C) depicts the predicted probability density distributions of the two categories under different time and value conditions.
Baseline characteristics of patients
A total of 699 patients diagnosed with SCI were included in this study. Based on the pattern of serum calcium ion levels, they were divided into two groups: Class 1 (n = 244) and Class 2 (n = 455). Class 1 was characterized by a "steep decline in serum calcium ion levels", while Class 2 was characterized by a "slow but persistent decline in serum calcium ion levels". Differences between the two groups were analyzed and compared. In terms of clinical indicators, there were no statistically significant differences between the two groups in sex, age, in-ICU death status (death_icu_yn), in-hospital death status (death_hosp_yn), presence of diabetes mellitus (Diabetes), presence of hypertension (Hypertension), heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), body temperature (Tem), age group (age_group), SOFA group (sofa_group), Sequential Organ Failure Assessment (SOFA) score, Acute Physiology and Chronic Health Evaluation III (APS III) score, Simplified Acute Physiology Score II (SAPS II), Oxford Acute Severity of Illness Score (OASIS), Charlson Comorbidity Index (CCI), injury level, ASIA classification, or timing of surgery (all P > 0.05). However, Class 1 had significantly longer hospital length of stay (los_hospital_day), ICU length of stay (los_icu_day), ICU survival time (time_icu), and in-hospital survival time (time_hosp) compared with Class 2 (all P < 0.05). Regarding blood biochemical indicators, a significant difference was observed in oxygen saturation (sPO₂) between the two groups (P < 0.05), while no significant differences were found in red blood cell count (RBC), white blood cell count (WBC), serum creatinine (Serum_Creatinine), blood glucose (Glu), serum potassium (potassium), partial pressure of oxygen (PO₂), partial pressure of carbon dioxide (PCO₂), blood pH value, serum calcium (calcium), or lactate between the two groups (all P > 0.05). (Table 1).
Results of survival analysis
Kaplan–Meier survival curves were used to analyze the 30-day and 60-day in-ICU mortality rates between the two groups of patients. The results showed significant differences in mortality rates between the two groups. Regarding the 30-day in-ICU mortality, the survival probability of Class 1 within the first 30 days was significantly higher than that of Class 2 (P < 0.05), and the survival curve indicated a survival benefit in Class 1 (Fig. 2A). For the 60-day in-ICU mortality, the 60-day survival probability of Class 1 was significantly higher than that of Class 2 (P < 0.05), with significant differences in survival curves between the two groups at all stages (P < 0.05) (Fig. 2B). Throughout the curve, the survival rate of patients in Class 1 remained stable overall, while that of patients in Class 2 decreased significantly, suggesting that the risk of in-ICU death in Class 2 was higher than in Class 1. This phenomenon may be related to the disease status, clinical diagnosis and treatment, and treatment response of the two groups.
Cox regression analysis
Univariate and multivariate Cox regression analyses were performed in this study to explore the relationship between different serum calcium ion trajectory categories and 30-day and 60-day in-ICU mortality. Compared with serum calcium ion trajectory Class 1, trajectory Class 2 was significantly associated with an increased risk of in-ICU death at both time points (P < 0.05). After adjusting for multiple confounding factors such as age, sex, diabetes mellitus, hypertension, and various physiological parameters, the hazard ratio (HR) for Class 2 ranged from 1.969 to 2.122, with all P-values < 0.05 (Table 2). These findings suggest that Class 1 may present more favorable clinical manifestations and is associated with improved clinical prognosis in patients with spinal cord injury. The consistently lower risk of death associated with trajectory Class 1 highlights the application value of serum calcium ion trajectory analysis in the stratified management and personalized treatment of spinal cord injury.
Subgroup analysis
Different subgroups stratified by sex, age, hypertension, and diabetes mellitus were correlated with trajectory categories. In males, Class 2 showed a non-significant trend toward an increased risk of death (HR = 2.090, P = 0.064), while no significant difference was observed in females (HR = 1.772, P = 0.305), with no interaction between sexes (P > 0.05). Older patients exhibited a non-significant trend of elevated death risk, whereas no significance was noted in younger patients (HR = 2.011, P = 0.320), and no interaction was found between different age groups (P > 0.05). Hypertensive patients faced an increased risk of mortality (HR = 3.282, P = 0.027), but no significance was observed in non-hypertensive patients (HR = 1.449, P = 0.362), with no significant interaction related to hypertension (P > 0.05). Neither diabetic patients (HR = 2.525, P = 0.251) nor non-diabetic patients (HR = 1.734, P = 0.119) showed significant differences, and no significant interaction was found regarding diabetes mellitus (P > 0.05). (Table 3).
Results of predictive performance
The C-statistic ranges from 0.5 to 1.0, with values closer to 1.0 indicating a stronger ability of the model to distinguish between “death” and “survival” outcomes. The C-statistic of Model 4 (fully adjusted combined model) was 0.782 (95% CI: 0.721–0.843), which represented an increase of 0.059 compared with Model 1 (P = 0.018) and a slight improvement compared with Model 3 (ΔC = 0.017, P = 0.049). This suggests that combining calcium trajectories with conventional indicators can significantly enhance the discriminatory ability of the model.
The NRI is used to evaluate the degree of optimization in the prognostic risk stratification of patients by the model after the inclusion of a new indicator (calcium trajectories). It is divided into Overall NRI, Event NRI (for patients with the death outcome), and Non-event NRI (for patients who survived). An NRI > 0 indicates that the new indicator can improve the accuracy of risk classification. In this study, "high risk (mortality probability ≥ 20%)" and "low risk (mortality probability < 20%)" were used as stratification thresholds to calculate the NRI of Model 3 and Model 4 relative to Model 1. The Overall NRI of Model 3 compared with Model 1 was 0.236 (95% CI: 0.089–0.383, P = 0.002), among which the Event NRI was 0.185 (95% CI: 0.052–0.318, P = 0.006) and the Non-event NRI was 0.051 (95% CI: − 0.012–0.114, P = 0.108). This indicates that after adding calcium trajectories, 18.5% of patients who died were correctly reclassified as high-risk, and 5.1% of patients who survived were correctly reclassified as low-risk, resulting in a significant improvement in the overall accuracy of risk classification. The Overall NRI of Model 4 compared with Model 1 was 0.274 (95% CI: 0.118–0.430, P < 0.001), with an Event NRI of 0.213 (95% CI: 0.075–0.351, P = 0.002) and a Non-event NRI of 0.061 (95% CI: − 0.008–0.130, P = 0.082). The NRI was further improved after full adjustment, and the statistical significance of the Event NRI was stronger, suggesting that the incremental predictive value of calcium trajectories is more stable after controlling for confounding factors. (Table 4).
Discussion
As one of the most critical electrolytes in the human body, serum calcium plays a vital role in maintaining physiological functions such as nerve conduction, muscle contraction, cell signal transduction, and bone metabolism through its concentration homeostasis23. SCI, as a severe central nervous system trauma, is often accompanied by systemic stress responses and multisystem metabolic disorders, among which calcium metabolism abnormalities are a typical manifestation24. Studies have shown that SCI patients frequently experience fluctuations in serum calcium levels during the acute phase of injury, which may be associated with the following mechanisms: on the one hand, sympathetic nerve dysfunction caused by trauma can inhibit osteoblast activity and enhance osteoclast function, leading to increased release of bone calcium, which may result in transient hypercalcemia in the early stage; on the other hand, disuse osteoporosis due to long-term bed rest after SCI, increased renal calcium excretion, and reduced intestinal calcium absorption may lead to subsequent hypocalcemia25,26,27. In addition, calcium ions exert a dual role in the nerve repair process after SCI: an appropriate influx of calcium ions is necessary for nerve regeneration, but excessive influx can activate apoptotic pathways and exacerbate nerve cell damage28,29. Although previous studies have focused on calcium metabolism abnormalities in critically ill patients, there is currently a lack of reports on the dynamic trajectory of serum calcium levels in SCI patients and its relationship with prognosis30. Based on large-sample data from the MIMIC-IV database, this study is the first to reveal the longitudinal change characteristics of serum calcium in SCI patients through trajectory model analysis, providing a new perspective for understanding the relationship between calcium metabolism and SCI prognosis.
In this study, GBTM was used to classify the serum calcium trajectories of 699 SCI patients into two categories: Class 1, characterized by "a steep decline in serum calcium ion levels," and Class 2, characterized by "a slow but persistent decline in serum calcium ion levels." Baseline characteristic analysis showed that the duration of ICU stay, total hospital stay, ICU survival time, and in-hospital survival time in Class 1 patients were significantly longer than those in Class 2 (P < 0.05). Survival analysis and Cox regression results revealed that the 30-day and 60-day all-cause mortality rates in the ICU were significantly lower in Class 1 patients than in Class 2 (P < 0.05). After adjusting for confounding factors such as age, sex, diabetes mellitus, and hypertension, Class 2 remained an independent risk factor for increased mortality (30-day HR = 2.064, 60-day HR = 2.122). These results suggest that the dynamic change pattern of serum calcium may better reflect the severity and prognosis of SCI patients than a single calcium level measurement. Mechanistically, the rapid decline in calcium levels in Class 1 patients may be related to the “compensatory regulation” of the acute stress response. Traumatic stress in the acute phase of SCI can activate the hypothalamic–pituitary–adrenal axis, prompting the temporary release of bone calcium to meet the body’s needs. The subsequent rapid decline may reflect the body’s rapid restoration of calcium homeostasis through pathways such as renal excretion and bone redeposition, indicating that these patients have stronger metabolic regulation capabilities and a more timely stress response to injury. In contrast, the persistent slow decline in calcium levels in Class 2 patients may reflect long-term metabolic disorders: on the one hand, long-term suppression of sympathetic nerve function after SCI can lead to sustained enhancement of osteoclast activity, with bone calcium loss exceeding the body’s compensatory capacity; on the other hand, persistent inflammatory responses can damage renal tubular function, affecting calcium reabsorption and resulting in a gradual decline in serum calcium levels31,32. This long-term hypocalcemic state may further trigger a cascade of reactions, such as weakened myocardial contractility, increased risk of arrhythmias, and suppressed immune function, ultimately exacerbating multiple organ dysfunction and increasing the risk of death33. Furthermore, the NRI analysis confirmed that calcium trajectories can enable 23.6%-27.4% of patients to obtain more accurate risk stratification, and in particular, significantly improve the ability to identify high-risk patients among those with the death outcome (Event NRI = 0.185–0.213). Serum calcium trajectories are not only a potential biomarker for the prognosis of patients with SCI but also can provide significant incremental predictive value for conventional prognostic scoring systems.
Furthermore, subgroup analysis revealed that the association between Class 2 trajectory and increased mortality risk was more pronounced in hypertensive patients (HR = 3.282, P = 0.027), whereas no significant difference was observed in non-hypertensive patients (HR = 1.449, P = 0.362). This may suggest that serum calcium trajectories could also reflect systemic stress and vascular risk. Similar abnormalities in calcium metabolism have been shown to be associated with prognosis in non-SCI ICU populations. The systemic stress response activates the hypothalamic–pituitary–adrenal axis, triggering the release of bone calcium and subsequent compensatory regulation of calcium homeostasis. Persistent inflammatory responses impair renal tubular function, affecting calcium reabsorption. Additionally, vascular endothelial dysfunction caused by underlying conditions such as hypertension further exacerbates abnormal calcium channel regulation. These processes are unrelated to SCI but represent common pathological changes in critically ill patients30,33,34,35. The subgroup results of hypertensive patients in this study may precisely reflect this shared mechanism. Hypertensive patients already have pre-existing calcium signaling disorders in vascular endothelial cells. Calcium metabolism abnormalities induced by SCI overlap with underlying vascular lesions, forming a vicious cycle of "calcium metabolism disorder—vascular contractile dysfunction—tissue hypoxia," which ultimately amplifies the risk of death34,35. In this case, changes in serum calcium trajectories are more likely to reflect systemic vascular/metabolic vulnerability rather than mechanisms solely related to SCI. This finding suggests that monitoring calcium trajectories may have greater prognostic value for SCI patients with comorbid hypertension.
This study has several limitations. First, the data were derived from the MIMIC-IV database, which only includes patients admitted to the ICU between 2008 and 2019. This may have led to the SCI patients with milder conditions who did not require ICU admission, resulting in selection bias. Second, the timing of serum calcium measurements was determined by clinical practice, with no standardized testing frequency, which may have affected the accuracy of the trajectory model in capturing dynamic changes in calcium levels. Third, the sample size of some subgroups in the subgroup analysis was relatively small, potentially leading to insufficient statistical power. Fourth, as a retrospective observational study, it cannot establish a causal relationship between calcium trajectories and mortality risk; prospective studies will be needed to validate this relationship in the future. Finally, due to limitations in data sources, this study did not include a non-SCI critically ill control group, making it impossible to verify the specificity of calcium trajectories through direct comparison.
Conclusion
This study confirms that the dynamic change trajectory of serum calcium in patients with SCI can serve as a potential biomarker for prognostic assessment. Among these trajectories, the "slow but continuous decline" type (Class 2) indicates a higher risk of ICU mortality. Furthermore, calcium trajectories can provide significant incremental predictive value for conventional prognostic scoring systems. However, the results of this study are only applicable to critically ill SCI patients admitted to the ICU. Clinicians can identify high-risk populations by dynamically monitoring the serum calcium levels of SCI patients and take targeted intervention measures to improve prognosis. Future studies may further combine the specific characteristics of spinal cord injury to explore the association between calcium trajectories and neural repair mechanisms, thereby providing a theoretical basis for the precise treatment of SCI.
Data availability
The data that support the findings of this study are openly available in the Medical Information Mart for Intensive Care (MIMIC)-IV database at https://doi.org/10.13026/kpb9-mt58.
References
Yu, T. et al. Exosome-mediated repair of spinal cord injury: A promising therapeutic strategy. Stem Cell Res. Ther. 15(1), 6 (2024).
Guan, B. et al. Global, regional and national burden of traumatic brain injury and spinal cord injury, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. BMJ Open 13(10), e75049 (2023).
Sabre, L. et al. Mortality and causes of death after traumatic spinal cord injury in Estonia. J. Spinal Cord Med. 36(6), 687–694 (2013).
Zadra, A. et al. Life expectancy and long-term survival after traumatic spinal cord injury: A systematic review. Eur. J. Phys. Rehabil. Med. 60(5), 822–831 (2024).
Thietje, R. et al. Long-term survival and causes of death in patients below the age of 60 with traumatic spinal cord injury in Germany. J. Clin. Med. 34, 482–487 (2021).
Charlifue, S. et al. Mechanical ventilation, health, and quality of life following spinal cord injury. Arch. Phys. Med. Rehabil. 92(3), 457–463 (2011).
Varma, A. et al. Predictors of early mortality after traumatic spinal cord injury: A population-based study. Spine 35(7), 778–783 (2010).
Chamberlain, J. D. et al. Mortality and longevity after a spinal cord injury: Systematic review and meta-analysis. Neuroepidemiology 44(3), 182–198 (2015).
Stroud, M. W. et al. Preinjury alcohol and drug use among persons with spinal cord injury: Implications for rehabilitation. J. Spinal Cord Med. 34(5), 461–472 (2011).
Bykowski, E. A. et al. Identification of serum metabolites as prognostic biomarkers following spinal cord injury: A pilot study. Metabolites 13(5), 605 (2023).
Chen, J. et al. Metabolic reprogramming: A new option for the treatment of spinal cord injury. Neural Regen. Res. 20(4), 1042–1057 (2025).
Matikainen, N. et al. Physiology of calcium homeostasis: An overview. Endocrinol. Metab. Clin. N. Am. 50(4), 575–590 (2021).
Young, K. et al. Regulation of 1 and 24 hydroxylation of vitamin D metabolites in the proximal tubule. Exp. Biol. Med. (Maywood) 247(13), 1103–1111 (2022).
Dora, I. E. et al. Basal metabolic rate versus dietary vitamin D and calcium intakes and the association with body composition and bone health after chronic spinal cord injury. Inquiry 61, 1418095550 (2024).
Srichuachom, W., Krintratun, S., Chenthanakij, B., et al. Prevalence and outcomes of hypocalcemia on ED arrival in traumatic patients before blood transfusions a systematic review and meta-analysis. Scand. J. Trauma Resusc. Emerg. Med. 33(1), 43 (2025).
Badarni, K. et al. Association between admission ionized calcium level and neurological outcome of patients with isolated severe traumatic brain injury: A retrospective cohort study. Neurocrit. Care 39(2), 386–398 (2023).
Zhang, Y., Hou, S. & Wu, Y. Changes of intracellular calcium and the correlation with functional damage of the spinal cord after spinal cord injury. Chin. J. Traumatol. 5(1), 40–42 (2002).
Mun, C., Sho, K. & Kim, O. Long-term changes in bone mineral density and associated risk factors in individuals with spinal cord injury: A retrospective study. Medicine (Baltimore) 103(39), e39790 (2024).
Gifre, L. et al. Risk factors for the development of osteoporosis after spinal cord injury. A 12-month follow-up study. Osteoporos. Int. 26(9), 2273–2280 (2015).
Wang, K., Lu, D. & Wang, F. Subphenotypes of platelet count trajectories in sepsis from multi-center ICU data. Sci. Rep. 14(1), 20187 (2024).
Ghosh, S. et al. Calcium imaging: A technique to monitor calcium dynamics in biological systems. Physiol. Mol. Biol. Plants 29(12), 1777–1811 (2023).
Johnson, A. E. W. et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data 10(1), 1 (2023).
Yuan, S. et al. Health effects of high serum calcium levels: Updated phenome-wide Mendelian randomisation investigation and review of Mendelian randomisation studies. EBioMedicine 76, 103865 (2022).
Hu, X. et al. Spinal cord injury: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target Ther. 8(1), 245 (2023).
Maimoun, L. et al. Changes in osteoprotegerin/RANKL system, bone mineral density, and bone biochemicals markers in patients with recent spinal cord injury. Calcif. Tissue Int. 76(6), 404–411 (2005).
Del Rivero, T. & Bethea, J. R. The effects of spinal cord injury on bone loss and dysregulation of the calcium/parathyroid hormone loop in mice. Osteoporos. Sarcopenia 2(3), 164–169 (2016).
Zhuang, D. et al. Low serum calcium promotes traumatic intracerebral hematoma expansion by the response of immune cell: A multicenter retrospective cohort study. Sci. Rep. 15(1), 8639 (2025).
Kang, K. et al. BAPTA, a calcium chelator, neuroprotects injured neurons in vitro and promotes motor recovery after spinal cord transection in vivo. CNS Neurosci. Ther. 27(8), 919–929 (2021).
Duarte, V. N. et al. Calcium plays an essential role in early-stage dendrite injury detection and regeneration. Prog. Neurobio.l 239, 102635 (2024).
Meng, K., Lei, X. & He, D. Association between serum calcium and in-hospital mortality in intensive care unit patients with cerebral infarction: A cohort study. Front. Neurol. 15, 1428868 (2024).
Metzger, C. E. et al. Inflammaging and bone loss in a rat model of spinal cord injury. J. Neurotrauma 40(9–10), 901–917 (2023).
Metzger, C. E. et al. A moderate spinal contusion injury in rats alters bone turnover both below and above the level of injury with sex-based differences apparent in long-term recovery. Bone Rep. 21, 101761 (2024).
Bove-Fenderson, E. & Mannstadt, M. Hypocalcemic disorders. Best Pract. Res. Clin. Endocrinol. Metab. 32(5), 639–656 (2018).
Benitez-Albiter, A. et al. Contributing factors to endothelial dysfunction in individuals with spinal cord injuries. Pulse (Basel) 12(1), 49–57 (2024).
Dai, C. & Khalil, R. A. Calcium signaling dynamics in vascular cells and their dysregulation in vascular disease. Biomolecules 15(6), 892 (2025).
Acknowledgements
The authors would like to express their gratitude to the MIMIC database for providing data support.
Funding
This work was supported by the Clinical Evidence-Based Research Special Project for the Construction of High-Level Traditional Chinese Medicine Hospitals of Wangjing Hospital, China Academy of Chinese Medical Sciences (WJYY-XZKT-2023-14).
Author information
Authors and Affiliations
Contributions
WCL and LXL were responsible for the study design and manuscript revision. LXM, LF, LZJ and LR performed data curation and statistical analysis. LXM completed the manuscript drafting.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study complies with the tenets of the Declaration of Helsinki, and the need for informed consent was waived due to the use of anonymized data. The institutional review board of Beth Israel Deaconess Medical Center (BIDMC) waived the need for informed consent due to the use of anonymized data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, X., Liu, F., Liu, Z. et al. Association between prognosis and calcium trajectories in patients with spinal cord injury based on MIMIC-IV data. Sci Rep 15, 35941 (2025). https://doi.org/10.1038/s41598-025-22280-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-22280-z