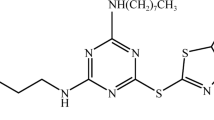
Abstract
In order to provide long-term anti-corrosion properties of the coatings on the substrate, a microcapsule self-healing coatings system was designed in this paper. Microcapsules were synthesized with ethyl cellulose and octadecyl amine, which were added to epoxy resin to prepare self-healing coatings. The shape of microcapsules was spherical, the average particle size of microcapsules was about 100–120 μm, and the average thickness of microcapsules was 4.43 μm, which was conducive to the preparation of the coatings. The initial evaporation temperature of the microcapsules was 165℃. The ethyl cellulose organic crosslinked shell enhances the strength of the microcapsules and improves the thermal stability of the microcapsules. Electrochemical impedance spectroscopy showed that the impedance of the coatings with 6 wt% microcapsules was about 2.68 × 106 Ω/cm2, which was 2 orders of magnitude higher than that of the coatings without microcapsules (5.23 × 104 Ω/cm2). Scanning electron microscope was used to observe the artificial scratches of the coatings for 24 h, and it was found that the microcapsule could repair the artificial scratches of the coatings well. The results showed that the addition of microcapsules can improve the anti-corrosion properties of the coatings.
Similar content being viewed by others
Introduction
The corrosion behavior of carbon steel in the complex oceanic environment has caused huge economic losses1,2,3. However, now people enhance the awareness of environmental protection and pay more attention to the eco-friendly coatings. Epoxy coatings are explored as a promising replacement for the standard anti-corrosive treatments as it is corrosion-resistant materials with good adhesion in various metallic substrates4,5,6. However, these films are still susceptible to corrosive media permeation due to epoxy micropores and cracks7. Therefore, corrosion inhibitor is widely used to improve the coatings corrosion resistance8,9,10,11. The direct addition of corrosion inhibitor compounds in the coatings will have some adverse effects, such as premature leakage, unnecessary chemical reactions with the coatings and metal matrix, and eventually lead to the destruction of the coatings7,12,13,14. Faced with this challenge, the development of functional coatings with the ability to automatically repair micro-damage has become a key research direction of researchers15.
The self-healing coatings is an effective anti-corrosion film at present. The carbon steel coated self-healing coatings can still be protected when the coatings surface is damaged16,17,18, and the service life of the metal is prolonged. Therefore the studies on self-healing coatings has attracted more attention. Wang19 made a flexible molecular chain and used the four-heavy hydrogen key (UPy) to achieve the dynamic reversible repair of the polymer coatings, and introduced it into epoxy resin, which gives the coatings the ability to repair at least 30 °C. Bhardwaj sushant20 et al. USES an in-place polymerization method to combine the average outside diameter of approximately 100 m to be covered with the microcapsules of flaxseed oil. EIS showed that the addition of microcapsules increased the pore resistance of polyester paint and decreased the coatings capacitance. Sun et al.21 has prepared bi-functional composite coatings on magnesium alloy AZ31 by embedding urea-formaldehyde microcapsules with phenolphthalein/epoxy resin.
It is reported that the adsorption corrosion inhibitors mostly contain polar groups of O, N, S, P or organic compounds with unsaturated bonds, and such corrosion inhibitors include quinolines, alkyne alcohols, amines, etc. Octadecyl amine(ODA) is an organic long-chain compound containing nitrogen atoms with high surface activity. When in contact with a metal surface, its polar group is chemically adsorbed on the active metal surface. The lone pair electrons of nitrogen atoms combine with the empty orbitals of iron atoms to form covalent bonds, while its non-polar group is physically adsorbed on the metal surface, consequently a hydrophobic protective film was formed on the steel surface to isolate the direct contact between corrosive medium and steel22. Li et al.23 used attapulgite as a nanocontainer to load octadecyl amine and prepared octadecyl amine-attapulgite-graphene(ODA-ATP-G) nanocomposites. The results showed that the prepared coatings could still achieve excellent anti-corrosion properties after soaking in 3.5 wt % brine for 60 days. And as H2O and O2 continuously penetrate the coatings/substrate interface through the pores and defects of the coatings, ODA is slowly released from ODA-ATP-G along with H2O and O2. Ethyl cellulose(EC) is a cellulose derivative, which has the advantages of high biocompatibility, degradability, degradability and good film formation24, so it has been widely concerned. Zhang25 et al. prepared self-healing microcapsules with corrosion inhibition effect using EC as the wall material and glycerol as the core material. Wang26 et al. successfully prepared self-healing microcapsules by wrapped expoy resin with EC. However, the development of economical, environmentally friendly and bio-friendly coatings remains a huge challenge.
In this study, Ethyl cellulose (EC) was used as the wall material and Octadecyl amine (ODA) as the core material, and the microcapsules was synthesized by solvent evaporation. Because of its water-insoluble nature, EC is mainly used as tablet adhesive and film coating material, etc., and is usually used as a coating excipient to prepare corrosion inhibition capsules, which is a continuous release of drug effect to prevent the premature effect of water-soluble drugs; ODA has a good anti-corrosion effect on iron and copper, and is usually used as a surfactant to modify some organic compounds. In this paper, we innovatively synthesized self-healing microcapsules with corrosion inhibition effect on metal substrates by using EC-encapsulated ODA. The self-healing coatings was prepared by adding microcapsules to epoxy resin, and the effects of different microcapsules dosages on the corrosion resistance of the coatings were discussed. The self-healing coatings prepared not only has the advantages of wide traditional coatings substrate, simple process and environmental protection, but also can make the coatings have a certain degree of self-healing ability. Figure 1 is the Graphical abstract.
Experimental part
Materials
The metal matrix material is AH 36 carbon steel, and the specific composition is shown in Table 1. The chemical reagents used are epoxy resin (Shanghai Aotun Chemical Technology Co., LTD.), 593 curing agent (Shanghai Aotun Chemical Technology Co., LTD.), Octadecyl amine (90%, Shanghai Maclin Biochemical Co., LTD.), Ethyl cellulose (98%, Shanghai Maclin Biochemical Co., LTD.), polyvinyl alcohol (PVA, Shanghai Maclin Biochemical Co., LTD.), Gelatin (Tianjin Oppsen Chemical Co., Ltd.), emulsifier OP-10 (Tianjin Oppsen Chemical Co.), anhydroethanol (Tianjin Opson Chemical Co., LTD.), acetone (Tianjin Opson Chemical Co., Ltd.), dichloromethane (Liaoning Quanrui Reagent Co., LTD.). The above chemicals do not need to be purified twice, no further treatment in experiment. AH36 steel (10 mm×10 mm×6 mm) substrates were polished by 180, 400, 800, 1200, 1500 grit sandpapers and then rinsed using ethanol and acetone for several times.
The experimental equipments used are the Electrochemical Workstation (CHI 760E, Shanghai Chenhua Instrument Co., Ltd.), Fourier transform infrared spectrometer (IS10 (Nicolet), USA), Scanning electron microscope (HITACHI S-3400 N, Hitachi Analyzer Shanghai Co., Ltd.), Thermal weight analyzer (STA-8000, Perkin Elmer Instruments).
Preparation of EC@ODA microcapsules
Ethyl cellulose microcapsules containing ODA were obtained by the solvent evaporation method. 1 g of EC was dissolved in 50 mL of methylene chloride. 1.5 g ODA was added to the mixture of EC and methylene chloride. The previous solutions were added to 125 mL of a mixture of 0.8 wt % PVA and 1 wt % gelatin as slowly as possible, which were mechanically emulsified at 550 rpm and reacted at 42 °C for 4 h until methylene chloride was completely evaporated. After the reaction, the ODA-loaded EC microcapsules were separated by filtering and washing them with deionized water. Finally, the ODA-loaded EC microcapsules were dried in a drying vacuum oven at 50 °C25. The schematic diagrams of the microcapsules is shown in Fig. 2.
Preparation of coatings
The synthesized microcapsules were added to the epoxy resin according to the mass fraction of 0 wt%, 3 wt%, 6 wt%, 9 wt%, 12 wt% and 15 wt% respectively, and the curing agent was added (the ratio of epoxy resin to curing agent was 4:1). The microcapsules were dispersed in the epoxy resin uniformly for 5 min by ultrasonic dispersion. The carbon steel matrix (10 mm×10 mm×6 mm) was sanded to 1500 # with waterproof sandpaper, then the sample was polished. The sample was cleaned with acetone in ultrasonic cleaning machine, then cleaned with deionized water, and dried in drying oven. A self-healing coatings (EC@ODA/EP coatings) was prepared by covering the above prepared epoxy resin containing microcapsules on the surface of the sample. The coatings was placed in a drying oven and cured at a constant temperature of 25 °C for 4 h, and then continued to be cured at a constant temperature of 80 °C for 6 h. Among them, the coatings with 0 wt% microcapsules addition is epoxy coatings(EP coatings), which is in contrast with other coatings with microcapsules addition.
Results and discussion
Chemical composition analysis of microcapsules
FT-IR spectra were used to determine whether ODA were encapsulated in EC. FT-IR spectra of microcapsules, EC and ODA are shown in Fig. 3.
From the Fig. 3, the characteristic peaks of EC are located at 3 475 cm− 1 (-OH telescopic vibration), 2 975 cm− 1 (telescopic vibration of -CH2), 2 871 cm− 1 (symmetric telescopic vibration of -CH2), peaks at 1 377 cm− 1 belong to -CH3 bending vibration, 1 112 cm− 1 (strong peak caused by mixing of C-O-C bond and C-OH bond); at the same time, the characteristic peaks of EC and ODA all appear on the characteristic peaks of the microcapsules; the characteristic peaks of EC@ODA and ODA at -CH2 (2 916 cm− 1), -CH3 (2 852 cm− 1), bending vibration of N-H (1 571 cm− 1), expansion vibration of C-N (1 463 cm− 1), N-H (721 cm− 1), confirmed that the ODA was completely embedded by the EC.
Thermal stability analysis and encapsulation rate calculation of the microcapsules
As shown in Fig. 4(a), the initial decomposition temperature of the EC is 310 °C, and the weight hardly changes after 500 °C. At 600 °C, the residue was 37.45 wt%, which may have formed a thermally stable material of carbon. The initial decomposition temperature of ODA was 130 °C, and its terminal decomposition temperature was 265 °C. The microcapsules began to decompose at about 100 °C, because of the moisture on the surface of the material, and the heating water evaporation caused the thermal weight curve of the microcapsules to decline. After removing the weight of water evaporation, the first thermal weight curve drops because of the decomposition of ODA, and the second drop was when the EC begins to decompose. The temperature at which the greatest mass change occurs in Fig. 4 (b) corresponded to the temperature at which the curve dropped in Fig. 4 (a). As shown in Fig. 4(b), at 96.33 °C, the DTG curve showed a quality loss rate of about 0.49/(%/min), at 268.62 °C, the DTG curve showed a quality loss rate of about 2.78/(%/min), and in the range of 285–552 °C, the DTG curve showed a higher quality loss rate, with a maximum value of 10.85/(%/min) at 356.54 °C. The thermal decomposition temperature of the microcapsules was 165 °C, and the slope of this curve was less than that of EC. The curve of EC@ODA microcapsules initially decomposes at a lower temperature than EC, which was because the microcapsules contains ODA and ODA was heated by decomposition at lower temperatures. The results showed that the EC was successfully wrapped around the ODA. Meanwhile, according to formula (1) and Fig. 3, the encapsulation rate of microcapsules is 24.56 wt %.
where Zx is the mass percentage of the encapsulated ODA, Zw is the mass percentage of the decomposed microcapsules, and Zb is the mass percentage of the decomposed EC.
The morphology of the microcapsules and the coatings
Figure 4 showed the surface topography of the EC@ODA microcapsules. Figure 5(a) showed the enlarged SEM images of a single microcapsules, where the synthesized microcapsules were spherical and had a smooth and dense surface, which allowed the EC to better wrap the ODA. According to Fig. 5(b), the capsule wall thickness of the synthesized microcapsules was about 4.43 μm. As can be seen from Fig. 5(c), the particle size of the synthesized microcapsules was mainly concentrated from 100 ~ 120 μm. Figure 5(d) showed the SEM map of the microcapsules and Fig. 5(e) showed the element distribute of the microcapsules.
Figure 6 showed the surface morphology of coatings with different microcapsules addition amounts. The EP coatings in Fig. 6(a) shows some easily detected micro-defects, which are typical of EP coatings. The EP coatings is inherently brittle and micro-defects are formed in its structure due to solvent evaporation. The presence of these micro-defects in the structure of the EP coatings undoubtedly reduces the corrosion resistance because they open the way to corrosive media. The Fig. 6(b) and (c) showed that the micro-defects gradually disappear by dispersing the microcapsules in the epoxy coatings. This may be due to the chemical reaction of the hydroxyl group in the structure of the EC with the epoxy group in the epoxy resin, formed a new bond. With the increasing of microcapsules addition amounts, the micro defects on the coatings become less and less. According to Fig. 6(d)~(f), new micro-defects were generated after the addition amounts of microcapsules gradually increased. This is due to the accumulation of excessive microcapsules, which causes the surface of the coatings to raise, re-opening the way to the corrosive medium, and once again reducing the corrosion resistance of the coatings. Figure 7 showed the cross-sectional SEM and EDS element mapping of the EP coatings and the EC@ODA/EP coatings. Compared with EP coatings, the EC@ODA/EP coatings had a more even distribution of carbon elements, and the coatings binded to the substrate more closely, indicating that the addition amounts of microcapsules can make the coatings more dense.
The corrosion resistance of the self-healing coatings
Figure 8 showed the Nyquist diagram of the corrosion resistance of the self-healing coatings with different microcapsules addition amounts. The larger the diameter of the semicircle in the corrosion solution, the higher the impedance and the better the corrosion resistance. As shown in Fig. 8, it was not difficult to find that the self-healing coatings coated with 6 wt% microcapsules had better corrosion resistance than the coatings coated with other microcapsules, which was mainly related to the relatively flat coatings (as shown in Fig. 6(c)). In the low frequency region, the impedance modulus of the coatings with 3 wt% microcapsule addition amounts were lower than that of the coatings with 6 wt% microcapsule addition amounts. Due to the insufficient addition amounts of the microcapsules, there were also holes on the surface of the epoxy coatings (as shown in Fig. 6(a) and (b)), causing that the corrosion medium can infiltrate from the holes. Therefore, its corrosion resistance was not as good as that of the self-healing coatings with a microcapsule addition amounts of 6 wt%. However, the low-frequency region impedance value of 9 wt%, 12 wt% and 15 wt% was lower than the impedance mode value of 6 wt% microcapsules. Due to the increasing amount of microcapsules, when the amount of microcapsules exceeded a certain limit, caused the presence of particles on the coatings surface (as shown in Fig. 6(d), (e), (f)). This opened the path for the corrosive medium to contact the metal matrix and re-accelerated the corrosion of the metal matrix. Figure 9 showed the bode diagram of the electrochemical impedance spectrum of the EC@ODA microcapsules coatings with different contents. All coatings that the microcapsules were added, the impedance modulus |Z| can still reach more than 1 × 105Ω /cm2, which indicated that the prepared self-healing coatings can effectively delay the corrosion time of the metal matrix.
Two equivalent circuit diagrams are in Fig. 10, and the electrochemical parameters obtained from the fitting are listed in Table 2. Rs is the electrolyte resistance. CPE is a constant phase element, which is related to the diffusion behavior of electrolyte solution in the coatings, and CPE reflects the penetration resistance of the coatings. Rct is the resistance of charge transfer, the greater the value indicates that the less the anions on the passivation film, the slower corrosion rate, and improves the corrosion resistance. The charge transfer resistance Rct of samples coatings with microcapsules addition amounts of 0 wt%, 3 wt%, 6 wt%, 9 wt%, 12 wt% and 15 wt% are as follows: 2.68 × 106, 8.17 × 105, 5.83 × 105, 3.52 × 105, 2.17 × 104, 5.23 × 104 Ω/cm2. The larger the charge transfer resistance, the better the corrosion resistance of the coatings, with the largest arc diameter of the coatings electrochemical impedance spectrum of the 6 wt% microcapsules added amount, which matched the fitting results. Therefore, the corrosion resistance of EC@ODA coatings was better when the amount of microcapsules was 6 wt%.
As can be seen from Fig. 11, the impedance value of the coatings of different microcapsules decreased to a certain extent after 7 d, and the low-frequency impedance valueof the self-healing epoxy coatings was one quantity higher than that of the epoxy coatings. As shown in Fig. 12, the impedance mode value of the self-healing epoxy coatings with 6 wt% addition amounts of microcapsules still reached 3.7 × 104 Ω/cm2, while the impedance mode value of the coatings with 15 wt% addition amounts of microcapsules also reached 4.1 × 103 Ω/cm2, which was still higher than the coatings without microcapsules. After 7 days of corrosion soaking, the resistance value of the coatings with microcapsules was higher than that of the coatings without microcapsules, indicating that the addition amounts of the microcapsules can effectively improve the corrosion resistance of the coatings. The self-healing coatings can effectively isolate the electrolyte solution from the metal matrix. After the coatings was damaged, ODA in the microcapsules is released, which adsorbed on the substrate surface and reacted with epoxy resin to form a cross-linked structure to repair defects, restore the coating’s ability to block corrosive media, and effectively protect the metal matrix.
In general, the corrosion resistance is mainly judged by the corrosion current density. The lower the current density is, the lower the corrosion rate is, and the better the corrosion resistance effect is. Figure 13 shows the polarization curves of substrates without coatings and coated with different types of coatings in 3.5% NaCl solution. Table 3 showed the electrochemical parameters of the potentiodynamic polarization curves of the uncoated metal matrix and metal matrix coated with coatings of different microcapsules addition amounts. The results showed that the self-corrosion current density of two coatings were 8.94 × 10− 7 and 2.83 × 10− 8 A/cm2, which were less than the self-corrosion current density of the metal matrix, which can effectively reduce the corrosion rate and have a good protective effect on the metal matrix. Moreover, the data in Table 3 can prove that the corrosion resistance of the epoxy coatings was further improved after microcapsules are added to the coatings.
The corrosion rate is obtained from the following formula:
Vp represents the corrosion rate, Ecorr the corrosion potential, Icorr the self-corrosion current density, K, Mm and ρm represent the constant, relative atomic mass of the metal matrix, and density, respectively.
The corrosion suppression rate is obtained from the following formula:
where, PE represents the protection efficiency. Icorr, o and Icorr, i indicate the self-corrosion current density of the metal matrix in the absence and presence of coatings, respectively.
Salt immersion test
\ Figure 14 showed the appearance of the coatings sample after 192 h immersion in sodium chloride solution. In Fig. 14(a) the scratch area of the pure EP coatings had be corroded, and the longer the time, the more serious the corrosion; Fig. 14(b) showed the EC@ODA/EP coatings, with the length of time, there was no serious corrosion phenomenon at the scratch, showed the much better corrosion resistance. When cracks such as scratches appear on the coatings, the wall of the microcapsules at the scratch location cracks, and a large amount of ODA corrosion inhibitor percolates to the scratch location. As shown in Fig. 15(b), after 7 days, new products appeared in the scratches of the self-healing coatings, while in contrast, the pure epoxy coatings in Fig. 15(a) remained in its original form. ODA can form a layer of single molecule or multi-molecule hydrophobic film on the metal surface, effectively protecting the metal matrix at the scratch location, preventing the occurrence of local corrosion reaction, and realizing the self-healing effect of the coatings27, as shown in Fig. 16.
Contact angle analysis of ODA
To explore the corrosion resistance mechanism of ODA, we tested the hydrophobicity, and the test results were shown in Fig. 17. According to Fig. 17, the contact angle increases continuously as the concentration of ODA solution increases. This is because when the concentration of ODA increases, the ODA adsorbed on the surface of the metal matrix increases, and the amino group is adsorbed on the metal matrix as a hydrophilic group, while the alkyl group is exposed as a hydrophobic group in the outer layer, which isolates the contact between water and the metal matrix, making the collective water contact Angle continuously larger. This can effectively prevent the water from contacting the metal matrix, and prevent the metal matrix from being corroded.
Analysis of the corrosion resistance mechanism of the microcapsules
ODA is an adsorption corrosion inhibitor, which has polar genes and can combine with the surface structure of the metal to form a mono layer membrane in the whole anode and cathode region28,29, so as to inhibit and reduce the rate of the electrolysis process. For example, some nitrogen-containing or hydroxyl-containing and having surface activity organic compounds, its molecules have two opposite properties: hydrophilic and hydrophobicity. This type of compound constructed from hydrophilic groups (such as amino groups) can effectively cover the metal surface layer, form a dense water repellent film and protect the metal surface from water corrosion.
ODA is formed by forming a complex with ions on the metal surface and forming a thin film by chemisorption. At the same time, the epoxy group in the epoxy resin can carry out ring-opening reaction in the amino group of ODA to form a secondary hydroxyl group, producing a stable compound, and in the non-heating reaction process, the generated secondary hydroxyl group almost does not react30. The reaction mechanism is shown in Fig. 18.
Conclusion
In this paper, microcapsules with ethyl cellulose as wall material and octadecyl amine as core material were synthesized by solvent evaporation method. The self-repairing coatings was successfully prepared by adding the synthesized microcapsules to epoxy resin.
-
1.
Microcapsules of EC coated ODA were synthesized. The surface of the microcapsule was smooth and dense, and the size was about 100 to 120 μm and the wall thickness was about 4.43 μm.
-
2.
FT-IR and TG test results showed that ODA was effectively wrapped in the EC. The weight-loss curve of the microcapsules showed that the EC shell contained about 24.56 wt% of the ODA.
-
3.
The SEM diagrams of the coatings can be prove that the addition of microcapsules can make the coatings more dense.
-
4.
The electrochemical test results showed that when the microcapsule dosage was 6 wt%, the impedance modulus of the coatings can reach 2.68 × 106 Ω/cm2, and the impedance modulus of the coatings was still 3.7 × 104 Ω/cm2 after soaking in 3.5% NaCl solution for 7 days, which was higher than that of the EP coatings.
Data availability
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
References
Zhang, M. et al. Smart self-healing coating with multiple synergistic effects based on ZIF-11 for corrosion protection of carbon steel. Colloids Surf. A. 684, 133186 (2024).
Zhou, C. et al. Epoxy composite coating with excellent anticorrosion and self-healing performances based on multifunctional zeolitic imidazolate framework derived nanocontainers. Chem. Eng. J. 385, 123835 (2020).
Liu, C., Cheng, L., Cui, L., Qian, B. & Zeng, R. Corrosion self-diagnosing and self-repairing polymeric coatings based on zeolitic imidazolate framework decorated hydroxyapatite nanocontainer on steel. Chem. Eng. J. 431, 133476 (2022).
Cheng, L., Liu, C., Zhao, H. & Wang, L. Hierarchically self-reporting and self-healing photothermal responsive coatings towards smart corrosion protection. Chem. Eng. J. 467, 143463 (2023).
Hao, J. et al. Smart Polymer with rapid self-healing and early corrosion reporting capabilities: Design, performance and mechanism. Chem. Eng. J. 456, 141159 (2023).
Maria, B. et al. Phosphate glass doped with niobium and bismuth oxides as an eco-friendly corrosion protection matrix of iron steel in HCl medium: Experimental and theoretical insights. J. Alloys Compd. 938, 168570 (2023).
Miri, T., Seifzadeh, D. & Rajabalizadeh, Z. Smart epoxy coating containing inhibitor-loaded cellulose nanocrystals for corrosion protection of steel. Surf. Coat. Technol. 477, 130241 (2024).
Gerardo, L. H. et al. The ZrC and Ti[sbnd]Ni nanostructures in epoxy coatings: An anticorrosion and tribological study. Surf. Coat. Technol. 469, 129816 (2023).
Zheng, W. et al. Enhancing chloride ion penetration resistance into concrete by using graphene oxide reinforced waterborne epoxy coating. Prog. Org. Coat. 138, 105389 (2020).
Guo, Y. et al. Preparation and performance of a composite epoxy coating based on modified hydroxyapatite. Surf. Coat. Technol. 443, 128614 (2022).
Li, C. et al. Benzotriazole corrosion inhibitor loaded nanocontainer based on g-C3N4 and hollow polyaniline spheres towards enhancing anticorrosion performance of waterborne epoxy coatings. Prog. Org. Coat. 174, 107276 (2023).
Zhu, Q. et al. pH-responsive mesoporous silica nanocontainers based on Zn-BTA complexes as stoppers for controllable release of corrosion inhibitors and application in epoxy coatings. Prog. Org. Coat. 181, 107581 (2023).
Cheng, M. et al. New valve-free organosilica nanocontainer for active anticorrosion of polymer coatings. Compos. Part. B. 224, 109185 (2021).
Attarzadeh, N., Kazemi, A. & Molaei, M. Fattah Arash. Multipurpose surface modification of PEO coatings using tricalcium phosphate addition to improve the bedding for apatite compounds. J. Alloys Compd. 877, 160275 (2021).
Hu, J., Ji, C. & Wei, Y. Research progress on external self-healing coating based on microcapsule technology. Paint Coat. Ind. 370, 1–7 (2024).
Chen, Y. et al. Smart micro/nano container-based self-healing coatings on magnesium alloys: A review. J. Magnesium Alloys. 11, 2230–2259 (2023).
Tiaw, Y. & Daniel, C. Corrosion-responsive self‐healing coatings (Adv. Mater. 47/2023). Adv. Mater. 35, 70343 (2023).
Ali, D., Ghasem, B. & Bahram, R. Designing a novel targeted-release nano-container based on the silanized graphene oxide decorated with cerium acetylacetonate loaded beta-cyclodextrin (β -CD-CeA-MGO) for epoxy anti-corrosion coating. Chem. Eng. J. 400, 125860 (2020).
Wang, Q. Preparation and performance of epoxy resin self-healing anti-corrosion coating (2023).
Bhardwaj, S., Rani, N., Singh, A., Chauhan, A. & Rishabh Self-healing properties of microencapsulated linseed oil-poly(urea formaldehyde) additives embedded polyester coating on Galvalume steel sheet. Trans. IMF. 102(2), 67–76 (2024).
Sun, C. et al. Corrosion sensing and self-healing composite coatings on magnesium alloy AZ31 enabled by stimuli-responsive microcapsules loaded with phenolphthalein and epoxy resin. Prog. Org. Coat. 186, 108043 (2023).
Liu, X. et al. Preparation and characterization of polyelectrolyte-modified attapulgite as nanocontainers for protection of carbon steel. J. Eng. 165, 871813 (2018).
Li, Y. & Nan, F. Achieving long term anti-corrosion waterborne epoxy coating by attapulgite loaded octadecylamine/graphene nanocomposite. Polym. Test. 129, 108290 (2023).
Zhang, R. et al. Construction and characterization of microcapsule carrier of linseed oil based on ethyl cellulose particles. Food Res. Dev. 43(20), 29–37 (2022).
Zhang, S. et al. Research on the corrosion resistance of an epoxy resin-based self-healing propylene glycol-loaded ethyl cellulose microcapsule coating. Coatings 13(9), 1514 (2023).
Wang, X., Xia, L., Fu, X., Xu, W. & Wang, X. Particle characteristics and sustained release properties of EpoxyResin/Ethyl cellulose microcapsule. J. Building Mater. 23(02), 396–400 (2020).
Dong, H. et al. Preparation of hexamethylene diisocyanate microcapsules and their application in self-healing coatings. Surf. Technol. 52(04), 272–284 (2023).
Wang, Y., Cao, Z., Sun, W. & Zhang, F. Classification and development direction of corrosion inhibitors. Total Corros. Control. 23(02), 24–26 (2009).
Yang, J. Design and application of pipeline anticorrosion technology in oil and gas storage and transportation. Chem. Eng. Manage. 34, 150–151 (2018).
Bai, R., Wei, M., Kang, H. & Gao, Y. A discussion on the influence of amino resin on the properties of high solids epoxy primer. China Coat. 36(12), 40–43 (2021).
Acknowledgements
Natural Science Foundation of Liaoning Province, Liaoning province (2022-MS-355).
Author information
Authors and Affiliations
Contributions
C.Q.D: Conceptualization, Formal analysis, Software, Validation, Writing - original draft, Writing- review & editing; K.S.M: Funding acquisition, Supervision; L.C.S: Data curation; S.D.P: Methodology; L.J.H: Software, Validation; C.H: Investigation; L.X.T: Formal analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cao, Q., Kang, S., Lu, C. et al. Properties and corrosion resistance mechanism of a self-healing octadecyl amine loaded ethyl cellulose microcapsule coatings loaded with epoxy resin. Sci Rep 15, 1386 (2025). https://doi.org/10.1038/s41598-025-85282-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85282-x