Abstract
Identification of lesion demarcation during thoracoscopic anatomical lesion resection is fundamental for treating children with congenital lung malformation. Existing lesion demarcations do not always meet the needs of clinical practice. This study aimed to explore the safety and efficacy of near-infrared fluorescence imaging with nebulized inhalation of indocyanine green for thoracoscopic anatomical lesion resection in children with congenital lung malformation. Under the fluorescence scope, the area of uniform green fluorescence was produced when indocyanine green was distributed into normal lung tissue, and lesions showed little or no green fluorescence, thus delineating a clear fluorescent lesion demarcation. Under the guidance of fluorescent demarcation, all the patients except one case of extralobar sequestration underwent thoracoscopic anatomical lesion resection successfully.
Similar content being viewed by others
Introduction
Thoracoscopic anatomical lesion resection (TALR) is a novel surgical method for congenital lung malformation (CLM) that completely removes the lesion and retains all normal lung tissue1. We identify the lesion demarcation based on the turtle shell-like lobuli structure. Specifically, we deemed the parenchyma with lobuli structure on the surface of the lobe as normal lung and the tissues without lobuli structure as lesion, thus forming a clear borderline between them. We believe that this may be the best strategy for the treatment of CLM. Furthermore, Matthew and Benedict DTD2 expressed keen interest in TALR and stated that this novel approach follows a less traveled road from a segmental resection that follows the bronchus. With more experience, this procedure may be appropriate for a larger group of patients. In spite of this, clinical conditions can sometimes be encountered: 1) the existing demarcation of the lesion is not always complete; 2) some lesions are not close to the pulmonary veins (the pulmonary veins cannot be used as the internal demarcation directly); 3) the beginner’ lack of experience in lesion demarcation judgement discourages them from attempting TALR. The above limits the application of TALR to some extent, which prompted us to look for more effective methods for demarcation judgement.
Near infrared (NIR) fluorescence imaging with indocyanine green (ICG) delivery via intravenous injection has been widely used in adult cancer surgery in recent decades and has achieved good results in intraoperative navigation. Whether this technique can be used in patients with CLM should be fully taken into consideration. CLM means that there may be bronchial and pulmonary malformation within the lesion. As a result, a ventilation difference is supposed to exist between the lesion and the normal lung. We hypothesized that differences in ventilation exist in patients with CLM. Taking advantage of these differences, we attempted to administer ICG by nebulized inhalation in children with different types of lung malformations and retrospectively analyzed the feasibility of fluorescence imaging for surgical navigation.
Methods
Patients
This study was conducted between February 1 and July 30, 2023. The inclusion criteria were 1) patients with CLM from 6 months to 1 year undergoing fluorescence imaging-assisted TALR.
The exclusion criteria were as follows: (1) those for whom single-lung ventilation was not established during surgery, (2) cases in which mass consolidation from CT scan image and (3) cases with residual lesion due to past surgery. The flow chart for patients exclusion could be seen in Fig. 1.
Details of ICG inhalation
For the sake of safety, some measures such as (1) closely monitoring vital signs and (2) preparing anti-allergic drugs (diphenhydramine, epinephrine, etc.) ahead of time should be carried out. One to two hours prior to anesthesia, ICG was dissolved in 2 ml sterilized water for injection, and adequate ICG (0.25 mg/kg)3 was atomized for inhalation by high-frequency vibration atomization machine in the wards for about 5 min.
Operative technique for TALR
After endotracheal intubation, the infant was placed in lateral decubitus position. Single-lung ventilation was then established. For congenital pulmonary airway malformation (CPAM), the peripheral part of the lesion is first split along the fluorescent demarcation (the external one), and the hilar part of the lung is dissociated along the plane of the pulmonary veins (as the internal one) to separate the hilar part of the lesion. The vascular and bronchial structures of the lesions were sealed and divided separately. The lesion was completely removed as the internal and external boundaries were finally approximated, and met1. For intralobar sequestration, the nourishing vessels from the aorta were first sealed and divided, and then the demarcation between the lesion and normal lung tissue was mobilized following the same step as CPAM procedure. A chest tube was routinely placed during surgery. CT scan on postoperative d 1 and 3 month after surgery was arranged, respectively. Informed consent was obtained from the children’ guardians. This study was approved by Ethics committee of West China hospital, Sichuan University. Besides, we declare all methods were performed in accordance with the relevant guidelines and regulations.
NIR fluorescence imaging system
A fluorescence endoscopic imaging system (Optomedic Technique Inc., Guangdong, China) was used in this study. There are three modes of fluorescence imaging: standard, black-white, and pseudo-color. Standard mode was used because the color contrast was more significant. After near-infrared imaging, the normal lung tissue showed fluorescent green, whereas the color of the lesion tissue remained unchanged, and the diseased tissue could be clearly delineated. The fluorescence imaging window and white-light window were displayed on the same screen and compared. The area of uniform fluorescent green was produced when ICG was distributed into normal lung tissue, and the demarcation adjacent to the lesion was defined as the fluorescence demarcation.
Results and discussion
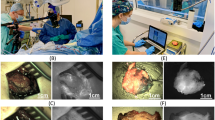
All 12 cases of CLM, including extralobar bronchopulmonary sequestration (case 1), intralobar bronchopulmonary sequestration (case 4), and 5 cases of CPAM (type 1,2 or hybrid of them), which were verified by pathological findings, were successfully treated with atomized ICG inhalation. Intraoperative near-infrared imaging significantly showed fluorescent green in normal lung parenchyma (Figs. 2B and D and 3B and D), while lung lesions showed little or no fluorescent green, thus forming a clear demarcation between the normal lung and lesion under the circumstance of single-lung ventilation which was highly consistent with the structural boundary (Fig. 2A, C and 3A). Fluorescence imaging time was defined as operative time and the median time was 130 min (range: 106–143.3 min). When single-lung ventilation was switched to double-lung ventilation (Fig. 4), the fluorescent green on the normal lung became lighter and lighter and finally disappeared (Fig. 5 and Video 1). There was an incomplete external demarcation (Fig. 3C) in two patients (cases 6 and 9) before NIR fluorescence imaging. In Case 2, the lesion was not close to the pulmonary vein. The fluorescence demarcation was highly consistent with the existing one (except for cases 6 and 9) in terms of external boundaries. However, the fluorescence boundaries and the pulmonary vein plane (internal demarcation) were not totally matched (Fig. 4A and B). Therefore, intraoperative navigation for TALR can be achieved by using fluorescent boundaries to some degree. No intraoperative ICG-related allergic reactions were observed. Basic data, such as intra- and post-operative parameters, are included in Table 1.
(A) and (B) displayed the existing lesion demarcation (yellow dotted line) and fluorescent one delineated by NIR imaging on eBPS patient in which the lesion joins with the normal lung (case 1), respectively; 2 C and 2D shown lesion demarcation identified by naked eyes and fluorescent one in iBPS patient (case 4), respectively. (eBPS, extralobar bronchopulmonary sequestration; iBPS, intralobar bronchopulmonary sequestration; CPAM, congenital pulmonary airway malformation; NIR, near infrared; N, normal; L, lesion)
(A) and (B) manifested the existing demarcation (yellow arrows) and fluorescent one (red arrows) in a CPAM patient (case 7), respectively; (C) presented there is no significant borderline using the existing method; (D) displayed distinct fluorescence demarcation delineated by NIR fluorescence imaging in a CPAM patient (case 9).
Standard fluorescence imaging (left upper) and white light (right) modes were compared at the same screen, which demonstrated as the normal lung reexpanded, the fluorescent green would become lighter and lighter and finally disappeared. The yellow arrow indicated the collapse lung and the blue arrow reinflated lung.
As is well known to us, near infrared imaging with ICG delivery of intravenous injection has been widely used in adult cancer surgery3,4,5,6 in recent decades and has achieved good results in intraoperative navigation. More recently, this technique has been successfully used in the pediatric population7,8,9. Regardless of how the diseases or population varies, the basic rules behind the differences in ICG distribution between the target lesion (tumor or organ) and adjacent tissue following intravenous delivery. In contrast to adult tumors, congenital lung malformations in children are benign, and it may not be appropriate to copy the same style of ICG delivery as adult segmentectomy. Theoretically, ICG fluorescence imaging can also be used for intraoperative navigation in CLM as long as certain differences can be found. CLM lesions present abnormalities in the lungs and airways, which may differ in ventilation from normal lung tissue. In addition, some authors have mentioned that ICG administration by nebulization inhalation is feasible to delineate a lung tumor margin in animal experiments and in the adult population10.
Based on our experience, CPAM and BPS are regarded as foregut duplication malformation. In addition, the lesion boundaries, including the external and internal boundaries, were identifiable. The existing method for identifying an external demarcation depends on the presence of normal lobuli on the surface of the lung. The pulmonary vein plane is defined as the internal demarcation under the condition that the lesion is close to the pulmonary hilum. Based on the above, we consider that TALR is the best policy for CLM patients, the core of which is the identification of internal and external boundaries. However, the existing external demarcation is not always complete and the lesion is not always located close to the pulmonary hilum. To identify a more effective method for identifying the lesion demarcation, fluorescence imaging with ICG was introduced.
All 12 cases of CLM were successfully treated with atomized ICG inhalation, and intraoperative NIR fluorescence imaging indicated that the normal lung was dyed as fluorescence green and the lesion was not, thus delineating the external demarcation of the lesion. Establishment of the fluorescent demarcation of the lesion demonstrated that the CLM lesion had little or no ventilation. The fluorescent demarcation was highly consistent with the existing one (cases 6 and 9 excluded) and the pulmonary vein plane (case 2 excluded). Thus, we were able to perform TALR using NIR fluorescence imaging. The demarcation of the lesion is stereoscopic, and sometimes part of the demarcation is difficult to identify with the naked eye. Without a fluorescent demarcation, cases 6 and 9 were not suitable for TALR because of the lack of a complete demarcation. To conduct TALR procedure, identification of the lesion demarcation was crucial. The surgeon would mobilize the internal lesion boundary along the pulmonary vein plane. Using the external lesion borderline combined with the internal one, the surgeon could remove the entire lesion while preserve all the normal parenchyma. Incomplete lesion demarcation means that there is no chance for undergoing TALR. However, under the guidance of fluorescence imaging the incomplete lesion demarcation became complete in another form, thus creating an opportunity for undergoing TALR procedure.
In case 2, the lesion was not close to the pulmonary vein, and the pulmonary-vein plane failed to function as the internal demarcation. Under these circumstances, fluorescence imaging also plays a role in identifying the internal boundaries owing to the three-dimensional sense of fluorescence imaging. For example, stereoscopic uniform fluorescence areas encountered during parenchyma-dividing suggested that the operative area may have been close to or already exceeded the internal demarcation of the lung lesion. In other words, NIR fluorescence imaging can be used as a real-time navigation tool that assists surgeons in making precise judgments on lesion demarcation. Based on fluorescence imaging techniques, the indication for TALR in CLM may be widened to some degree. It is difficult for novice doctors to quickly recognize the external boundaries of the lesion. With the help of fluorescent borders, novice doctors are more confident and willing to attempt TALR. Owing to better identification of the lesion demarcation, based on our experiences, the operative time may be shortened to a certain extent via fluorescence imaging in performing TALR procedure. In other words, the learning curve for TALR procedure may be more easily overcome to some degree, thus facilitating the promotion of TALR procedure.
There were no remnant lesions or air leaks, thus demonstrating the effectiveness of TALR under the guidance of NIR fluorescence imaging. There were no major complications, except subcutaneous emphysema (n = 9) and absorptive fever (n = 8), both of which spontaneously resolved within several days. Regarding the allergic reaction rate of ICG, Speich et al.11 reported the rate of intravenous injection was 0.003% with doses below 0.5 mg/kg. Pischik VG used an intravenous dose of 0.15 mg/kg with intravenous method in 75 cases, and no adverse reactions were observed3. The dose of ICG (0.25 mg/kg) used by inhalation is below 0.5 mg/kg and the drug does not directly contact the blood flow compared to intravenous injection, so we inferred that the allergic reaction rate by inhalation would be much lower than 0.003%. Although no intraoperative ICG-related allergic reactions occurred in this study, it is inadequate to conclude that ICG inhalation is 100% safe owing to the small sample size. Herein, precautions were warranted before ICG delivery to keep children (especially for those with airway hyperresponsiveness or hypersensitivity). Exclusive caregivers were responsive for monitoring the vital signs and symptoms of the patients. Anaphylactic drugs (Norepinephrine and methylprednisolone) were well prepared in case that the patient displayed allergic reactions such as rash, wheal or even shock. Drug dosage was referenced from the literature10 in which the authors recommended 0.25 mg/kg of ICG was an optimal dose during their animal experiments and clinical trial concerning adult with lung cancer. As for the timing administration, based on our clinical experiences, the normal lung would display obvious fluorescence green immediately under the NIR thoracoscope at the end of ICG inhalation (5–8 min duration). Moreover, the fluorescence imaging time could last a long time (3.5–4 h). One to two hours was considered an optimal interval between inhalation and surgery due to one hour for transferring the patients from the wards to the operative room and getting ready for anesthesia.
We hypothesized that ICG inhalation would be feasible for the treatment of children with CLM. In case 1, we directly administered by nebulization inhalation to the infant in the operating room for a longer imaging time, which was proven to be time-consuming and cumbersome. Subsequently, the patients were administered atomized ICG inhalation in pediatric wards 1.5–2 h in advance. It can be seen from the results that the fluorescence imaging of normal lung tissue was very obvious at this dose. However, whether this dose is the minimum dose required for clear visualization needs to be confirmed in further clinical studies. Because ICG nebulization inhalation was completed 1.5–2 h earlier in the pediatric wards and the median operation time was 130 min, we speculated that if ICG administration was completed in the operating room, the fluorescence imaging time could be extended to 3.5–4 h. Significant fluorescence imaging was still observed on the normal lung surface at the end of the operation, indicating that the imaging effect was persistent and stable. It has been reported that lung tissue has a high affinity for ICG, and the fluorescence imaging intensity in animal experiments is maintained at a high level 24 h after inhalation10. It is worth mentioning that fluorescence imaging should be established based on single-lung ventilation (creating lung consolidation or collapse of the lung), and once the collapsed lung was reinflated to a normal state, the fluorescence imaging soon became less obvious and finally disappeared owing to the prompt declination of the concentration of ICG dwelling at the alveolar wall (see Fig. 5).
The fluorescence demarcation is not a perfect one and has some overflow phenomena. This may be related to the presence of a small airway path between the lesion margin and normal lung. Comparing our study with the fluorescence boundaries of lung tumors in animal experiments10, it can be seen that there is no ventilation in lung tumors, and indocyanine green molecules cannot directly ingested by tumor cells; therefore, the tumor boundaries outlined by fluorescence imaging are clearer. Therefore, the fluorescent demarcation of the CLM should be defined. In this study, the fluorescent demarcation was defined as the edge of a normal lung that uniformly displayed fluorescent green around the lesion.
There were only 12 cases of CLM in the study, but it incorporated two types of bronchopulmonary sequestration (intralobar and extralobar types) and three types of congenital pulmonary airway malformations (type 1, 2, and hybrid according to Stocker’s classification), which were deemed representative of CLM.
Limitations
This study has several limitations. First, this was a retrospective pilot study without control. Second, the sample size of this study was small. Therefore, prospective studies with larger sample sizes on the safety and minimum effective dose for fluorescence imaging should be conducted. In this study, ICG administration by nebulized inhalation was applied to infants with CLM for TALR, and promising outcomes were obtained, which are of great significance. Additionally, the application of fluorescence imaging is not limited to tumor navigation, but extends to other areas such as congenital structural malformations, as long as the differences between the target tissue and its surrounding tissue are ascertained and a reasonable ICG delivery method is selected. With further research, we are willing to foresee that fluorescence imaging technology will play a better role in treating CLM in the near future.
Conclusion
ICG inhalation is a novel method of drug delivery for CLM patients. Despite its pilot nature, ICG administration by nebulized inhalation was the first successful application in infants with CLM. Additionally, using near-infrared fluorescence imaging, the lesion demarcation (especially external borderline) of CLM can be clearly delineated, thus facilitating the completion of more precise TALR via lesion demarcation navigation to a certain extent. Conversely, the establishment of a fluorescent demarcation proves that the diseased lung has little or no ventilation.
Data availability
Data is provided within the manuscript or supplementary information files.
References
1 Yuan, M. et al. A novel surgical method for congenital lung malformation: A pilot study. Seminar in Thoracic and Cardiovascular Surgery, publication online. (2022). https://doi.org/10.1053/j.semtcvs.2022.06.017
2 Matthew, J. P. & Benedict, D. T. D. Commentary the road less traveled. In Seminars in thoracic and cardiovascular surgery. https://doi.org/10.1053/j.semtcvs.2022.07.007
4 Pischik, V. G. & Kovalenko, A. The role of indocyanine green fluorescence for intersegmental plane identification during video-assisted thoracoscopic surgery segmentectomies. J. Thorac. Dis. 10, S3704. https://doi.org/10.21037/jtd.2018.04.84 (2018).
5 Predina, J. D. et al. Near-infrared intraoperative imaging for minimally invasive pulmonary metastasectomy for sarcomas. J. Thorac. Cardiovasc. Surg. 157, 2061–2069. https://doi.org/10.1016/j.jtcvs.2018.10.169 (2019).
6 Lake, C. M. et al. Indocyanine green is a sensitive adjunct in the identification and surgical management of local and metastatic hepatoblastoma. Cancer Med. 104, 322–4343. https://doi.org/10.1002/cam4.3982 (2021).
7 Jeremiasse, B. et al. Systematic review and meta-analysis concerning near-infrared imaging with fluorescent agents to identify the sentinel lymph node in oncology patients. Eur. J. Surg. Oncol. 46, 2011–2022. https://doi.org/10.1016/j.ejso.2020.07.012 (2020).
Esposito, C. et al. Efficacy of indocyanine green (ICG) fluorescent cholangiography to improve intraoperative visualization during laparoscopic cholecystectomy in pediatric patients: A comparative study between ICG-guided fluorescence and standard technique. Surg. Endosc 4369–4375 https://doi.org/10.1007/s00464-021-08784-5 (2022).
9 Masuya, R. et al. Using indocyanine green fluorescence in laparoscopic surgery to identify and preserve rare branching of the right hepatic artery in pediatric congenital biliary dilatation. Surg. Today 52, 1–4. https://doi.org/10.1007/s00595-022-02516-5 (2022).
10 Keren, S. et al. Indocyanine green assisted removal of orbital lacrimal duct cysts in children. J. Ophthalmol. 130215 https://doi.org/10.1155/2015/130215 (2015).
Quan, Y. H. et al. Evaluation of intraoperative near-infrared fluorescence visualization of the lung tumor margin with indocyanine green inhalation. JAMA Surg. 155, 732–740 https://doi.org/10.1001/jamasurg.2020.1314 (2020).
Speich, R. et al. Anaphylactoid reactions after indocyanine-green administration. Ann. Intern. Med. 109, 345–346 https://doi.org/10.1059/0003-4819-109-4-345_2 (1988).
Acknowledgements
We would like to thank the Optomedic Technique Incorporation from Guangdong, China, for their support in this study. We would also like to express our gratitude to Doctor Wei Yang from the Department of Oncological Surgery in Peking Children’s Hospital for his constructive suggestions regarding this study.
Funding
Chang Xu was granted by the National Natural Science Foundation of China (NO.31201095). and Natural Science Foundation of Sichuan Province (No.2022NSFSC0354).
Author information
Authors and Affiliations
Contributions
TH: conceptualization, data collection and investigation, writing original draft, writing – review & editing; GC: methodology, conceptualization, formal analysis; KC&DL: software, formal analysis and draft revision; MY: contributed to the methodology, writing and data curation; XS: writing, review and editing, supervision; GY: Resources, investigation, data curation; CX: validation, data curation, project administration, Funding acquisition. TH and XS contributed equally to this manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
IRB approval was waived because this was a retrospective study.
Informed consent
was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, T., Sun, X., Che, G. et al. Fluorescence imaging-assisted thoracoscopic anatomical lesion resection in treating congenital lung malformation. Sci Rep 15, 755 (2025). https://doi.org/10.1038/s41598-025-85404-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85404-5