Abstract
Obesity is related to liver fibrosis, a condition marked by the collection of scar tissue in the liver due to the development of a profibrotic environment, which includes increased hepatocellular death and elevated reactive oxygen species production. The aim of study is to evaluate the effect of bariatric surgery on the association between liver fibrosis indices and obesity. This is a retrospective cohort, evaluating 1205 individuals diagnosed with type 2 diabetes (T2D) and living with obesity, who experienced bariatric surgery. These patients living with T2D and obesity were monitored after bariatric surgery for two years. The trajectory of biochemical markers and liver fibrosis indices were evaluated at five visits. These liver indices were Fibrosis-4 (FIB-4) index, aspartate aminotransferase (AST) to platelet ratio index, and non-alcoholic fatty liver disease (NAFLD) fibrosis score. FIB-4 index demonstrated notable trends based on its values. It showed an initial increase observed at the three-months visit, followed by a decline up to one year with a slight increase at the last follow-up (P-trend < 0.001). It should be mentioned that, mean FIB-4 in patients with FIB-4 ≥ 1.3 (pre-operation) did not exceed the value of 2.00, which is lower than the cut-off value of high risk for liver cirrhosis (FIB-4 ≥ 2.67). In addition, the NAFLD fibrosis score (NFS) demonstrated a substantial decline from − 0.32 ± 1.32 pre-operation to -0.86 ± 1.15 at the two-year mark (P-trend < 0.001). Finally, the AST to platelet ratio index (APRI) decreased from 0.27 ± 0.20 pre-operation to 0.23 ± 0.12 at the 12-month follow-up. Bariatric surgery significantly improves NFS and cause alterations in APRI and Fib-4 index levels without increasing the risk of liver cirrhosis development among patients with T2D and obesity.
Similar content being viewed by others
Background
Obesity, an important public health burden, has been increasingly associated with liver fibrosis, a state defined by excess collection of extracellular matrix proteins in the liver. This relationship is largely mediated by Metabolic dysfunction-associated fatty liver disease (MAFLD), which encloses a group of liver disorders from simple steatosis to metabolic dysfunction-associated steatohepatitis (MASH), the latest often progressing to liver fibrosis1. Recent studies highlight the prevalence of liver fibrosis among individuals living with obesity, emphasizing the importance of early detection and intervention in this high-risk population to prevent severe liver-related complications2. Additionally, obesity is a major driver of hepatic fat accumulation through mechanisms including adipose tissue dysfunction, altered lipid metabolism, and adipokine dysregulation.
Obesity leads to increased free fatty acid influx into the liver, resulting in hepatic steatosis due to enhanced triglyceride synthesis and diminished fatty acid oxidation. This accumulation triggers inflammatory pathways, promoting MASH defined by lobular inflammation and hepatocyte ballooning. Insulin resistance exacerbates these conditions, facilitating further liver damage and fibrosis progression, ultimately increasing cardiovascular diseases and liver-related morbidities2. Addressing obesity through lifestyle modifications, including physical activity and weight loss, is critical in the management of MAFLD and its related metabolic complications3.
MAFLD is a newly proposed term that includes a range of liver conditions characterized by hepatic steatosis in addition to metabolic dysfunction. The term was introduced to better reflect the multifactorial nature of the disease and its relationship with metabolic abnormalities besides fatty liver. MAFLD includes a wide range of liver conditions, from simple steatosis to MASH and liver failure, and is closely related to metabolic syndrome components such as insulin resistance, obesity, hypertension, and dyslipidemia. Diagnosis of MAFLD requires the presence of hepatic steatosis as well as either one of type 2 diabetes (T2D), metabolic dysregulation, or overweight/obesity4.
The worldwide prevalence of MAFLD is nearly 33%, with the greatest rates reported in the South America at 44% and lowest rates reported in Sub-Saharan Africa at 14%. Meanwhile, young people showed lower rates between 8 and 16%1.
The risk of MAFLD and MASH diagnosis increases with increasing body mass index (BMI). For instance, the risk of developing MAFLD will increase 5.5-times with a single unit increase in BMI. In a study conducted on 4,242 Iranians, individuals diagnosed with MAFLD had mean BMI of 32.67, which was 8.27 unit higher in individuals without MAFLD5. T2D is a substantial risk factor for MAFLD, accelerating hepatic disease progression6. However, as demonstrated previously, a substantial percentage of patients with MAFLD have normal weight, underscoring the importance of metabolic health, regardless of BMI. Multiple factors including poor dietary habits, sedentary lifestyles, and genetic predisposition play important role in MAFLD progression in normal-weight people. Therefore, lifestyle adjustments including dietary changes and physical activity should be considered to improve metabolic health and achieve an efficient metabolic adaptation7,8.
Given the rising prevalence of MAFLD in populations with common metabolic diseases such as obesity and T2D, there is a pressing need for targeted screening and accurate diagnosis of liver diseases in these patient groups. Early prevention or treatment of MAFLD is crucial to mitigate both liver-specific and diabetes consequences and complications9.
Among non-invasive tools for assessing fibrosis severity, the most validated serum markers and scores include the aspartate aminotransferase (AST) to platelet ratio (APRI), the non-alcoholic fatty liver disease (NAFLD) fibrosis score (NFS), and the Fibrosis-4 (FIB-4) index10. The APRI is computed using the platelet count and AST level. The NFS is based on a combination of six parameters (BMI, age, AST/alanine aminotransferase (ALT) ratio, albumin, platelet count, and hyperglycemia), while FIB-4 is based on the combination of platelet count, age, ALT, and AST11.
Clinical studies have established that bariatric procedures exhibit superior effectiveness compared to lifestyle and medical interventions in facilitating weight reduction, enhancing serum lipid levels, optimizing blood glucose control, and reducing the need for medications used to control diabetes, hypertension, and dyslipidemia12,13,14. Enhancement in these conditions after bariatric surgery lead to reduced risk of cardiovascular diseases, as assessed by well-known tools such as the Framingham risk equation.
There is a lack of a well-developed study with an appropriate sample size and a significant follow-up duration regarding the performance of liver fibrosis indices after bariatric surgery among patients living with T2D in the current literature. In addition, there is a clinical problem regarding the development of liver failure in some individuals after bariatric surgery15,16, which was not evaluated before among patients with T2D. Accordingly, this study was aimed to assess the trends of three well-known liver fibrosis indices after bariatric surgery among individuals with T2D over a follow-up period of two years. Additionally, based on other studies, these indices have shown adequate performance for the diagnosis of liver fibrosis.
Methods
Study design and inclusion criteria
This study examined 1205 adults with obesity and T2D who have undergone three types of bariatric surgery including one anastomosis gastric bypass/mini gastric bypass (OAGB/MGB), sleeve gastrectomy (SG), and Roux-en-Y gastric bypass (RYGB) and were followed up for two years. This study is conducted retrospectively, with all patients being operated by only six surgeons. All participants were recruited consecutively to the study at the Surgical Department of Hazrat-e Rasool Hospital, a University Hospital located in Tehran, Iran. The criteria for diagnosing T2D were based upon the American Diabetes Association (ADA)17. Relevant demographic and metabolic information were collected from the National Iranian Obesity Surgery Database, with institutional review board and ethics approval obtained prior to the study. Patients provided informed consent at their first preoperative visit.
Data collection
The retrieved information included demographic information (sex, age, alcohol use, smoking, and addiction), comorbidities (dyslipidemia, hypertension (HTN), CVD, cerebrovascular accidents (CVA), deep vein thrombosis (DVT), heartburn, and pulmonary embolism (PE)), and anthropometric measures (BMI, height, and weight) which were obtained by an expert. Body weight was measured using a Seca scale while patients wore light clothing and no shoes (Seca 700, Hamburg, Germany). In addition, height was measured using a Seca stadiometer without shoes to the nearest of 0.5 cm (Seca 700, Hamburg, Germany). Additionally, total weight loss (TWL%) were computed using specific formulas as follows:
TWL % = [(initial weight) – (postoperative weight)] / [(initial weight)] * 100.
Blood samples were collected from individuals who were fast for 12 h. Afterward, samples were kept at − 70 °C until analysis after being centrifuged.
The hexokinase enzyme technique was utilized to measure fasting blood glucose (FBG), while standard laboratory enzymatic methods were utilized to measure hemoglobin A1c (HbA1c), total cholesterol (TC), triglyceride, and high-density lipoprotein-cholesterol (HDL-C). The Friedewald formula was used to estimate plasma low density lipoprotein-cholesterol (LDL-C) levels for triglyceride levels below 400 mg/dL, while the enzymatic method was used for triglyceride levels above 400 mg/dL. Serum liver enzymes, including AST and ALT, were evaluated using the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) method (AST intra-assay CV = 2.5%, ALT intra-assay CV = 3.7%). The participants’ serum creatinine values were also assessed using the Jaffe technique (Pars Azmun, Karaj, Iran).
The trend of all biochemical parameters in postoperative follow-ups were compared to preoperative levels. Additionally, APRI, NFS, and Fib-4 were evaluated throughout the two-year follow-up. The relative formula of each index is mentioned below:
FIB-4: Age (years) * AST(U/L) / [PLT (109/L) * ALT(U/L) * 1/2]
NFS: -1.675 + (0.037 * age (year)) + (0.094 * BMI (kg/m2)) + (1.13 * IFG/diabetes (yes = 1, no = 0)) + (0.99 * AST/ALT ratio) – (0.013 * PLT (*109/L) – (0.66 × albumin (g/dL)).
APRI: [(AST/upper limit of the normal AST range) * 100] / PLT (*109/L).
Afterward, the data regarding FIB-4 index were divided to low risk and indeterminate risk for liver fibrosis according to the index cut-off of 1.3, and were analyzed separately to evaluate its trend in these two population.
The present study was performed according to the principles of the Declaration of Helsinki and was accepted by the ethical committee of the Tehran University of Medical Sciences (TUMS) with the registered number of IR.TUMS.IKHC.REC.1401.162. Informed consent for publication and participation was acquired from all patients involved in the study.
Statistical analysis
For the statistical analysis, the IBM Statistical Package for the Social Sciences (SPSS) version 24 was utilized. The Kolmogorov-Smirnov test was used to evaluate the normality of the data. To investigate the differences in categorical and continuous variables among participants based on type of surgery in each time point, Chi square test and One-way analysis of variance (ANOVA) were used, respectively. The results were indicated as mean ± standard deviation (SD) for continuous and proportions for categorical variables.
Furthermore, the One-way repeated measure ANOVA was used to analyze the differences in liver fibrosis indices at various time points. These time points included baseline and three-months, six-months, 12 months, and 24 months after surgery (Fig. 1). The statistical significance is indicated by a P-value < 0.05. In addition, each index was adjusted for specific variables to omit the possible con-founding effects on the trend of these indices.
Trend of liver fibrosis indices in a two-year follow-up. Data are presented as mean ± SD in each follow-up visit. (a) Trend of FIB-4 for lower than cutoff (1.3) values in two years of follow-up; (b) Trend of FIB-4 for higher than cutoff (1.3) values in two years of follow-up; (c) Trend of NFS in two years of follow-up; (d) Trend of APRI in two years of follow-up. FIB-4 Fibrosis-4, NFS NAFLD Fibrosis Score, APRI aspartate aminotransferase to platelet ratio index to platelet ratio.
Results
One-thousand two hundred and five individuals with the mean age (SD) of 45.37 (10.70) years, 79.7% women, from the national obesity surgery database with T2D have undergone three types of bariatric surgery. In the current study, 677 (56.18%) OAGB/MGB, 332 (27.55%) RYGB, and 196 (16.26%) SG were performed. Table 1 demonstrates the preoperative demographics of all patients based on the type of surgery. At six-month follow-up, one-year follow-up, and two-year follow-up, 947 (78.58%), 588 (48.79%), and 263 (21.82%) patients participated in the study, respectively. The frequency of dyslipidemia, HTN, CVD, CVA, and hypothyroidism were 43.5%, 38.1%, 6.7%, 0.3%, and 26.1%, respectively. A significant reduction of BMI in consecutive follow-ups was observed regardless of type of surgery. The preoperative mean of BMI was 44.75 (6.89) which was significantly reduced to 36.19 (± 9.01), 32.55 (± 6.93), 30.14 (± 5.19), and 30.79 (± 12.37) in three-month, six-month, one-year, and two-year follow-ups, respectively.
Anthropometric indices and biochemical parameters
In this study, the impact of these types of surgery on various measurements and outcomes in participants undergoing bariatric surgery were analyzed. The peak TWL% was observed at the one-year visit with the value of 32.86 (7.45) and remained stable until the last follow-up visit. The mean (SD) values of BMI, weight and TWL% are indicated in Table 2.
Serum levels of AST and ALT declined significantly at first visit after the surgery, and remained unchanged in all follow-up visits. Although all the changes were in normal range, SG showed better reduction in liver transaminases blood levels with statistically significant difference (P-value < 0.05) with other techniques in some follow-up visits (Supplementary Materials).
In Table 2, an overview of the trends of biochemical measurements and anthropometric indices is provided at different follow-up time points. Based on paired sample t-test comparisons, it is noted that HDL levels decreased significantly in the first three-month visit after the surgery compared to the pre-operative stage from 45.70 (± 12.76) to 42.31 (± 10.96) (P-value < 0.001). However, the overall HDL levels from the pre-operation stage (45.70 ± 12.76) to two years after the surgery (51.93 ± 12.03) was increased (P-value < 0.001), and no difference in HDL levels was observed in the last follow-up, based on type of surgery (P-value = 0.464). TC levels exhibited a decline after the surgery in the first visit, and this reduction was maintained throughout the follow-up period from 187.33 (± 43.69) to 176.76 (± 34.09) (P-value = 0.001). However, OAGB/MGB and RYGB outperformed SG in reducing TC levels after the surgery until one-year follow-up visit (P-value = 0.002 and P-value = 0.003, respectively). Additionally, LDL levels were significantly reduced in the first visit after the surgery, with a sustained decrease until the one-year follow-up visit from 106.67 (± 35.45) to 99.16 (± 28.81) (P-value < 0.001). In comparison of types of surgery, OAGB/MGB and RYGB had better effect on reducing LDL-C levels compared to SG, specially, in 6-months and one-year follow-up visits (P-value < 0.001 and P-value < 0.001, respectively).
Impact of bariatric surgery on liver fibrosis indices
The values regarding liver fibrosis indices in all follow-up visits are illustrated in Table 3. Firstly, FIB-4 index was divided into two populations, the low-risk and the indeterminate risk, for liver fibrosis with the cut-off of 1.3. The trend of its levels in the low risk population (FIB-4 < 1.3) (based on One-way repeated measure ANOVA) showed an initial increase in the first postoperative visit, three months after the surgery. However, its levels showed a decline from the second postoperative visit to one-year follow-up visit which experienced an elevation in the last visit with a peak value of 0.92 (± 0.36). (Fig. 1a) (P-trend < 0.001). It is worth mentioning that the cut off value of 1.3 for FIB-4 is adjusted for individuals with less than 65 years of age; however, according to the mean (SD) of age in our population, this cut-off is almost applicable to all of the patients.
In addition, in the population with an indeterminate risk of liver fibrosis (FIB-4 ≥ 1.3), FIB-4 levels showed an initial increase toward the peak value of 1.88 (± 1.06) in the first post-operation visit, followed by a steady decline to the one-year visit with a subsequent increase in the last appointment with a value of 1.70 (± 0.99) (Fig. 1b) (P-trend < 0.001). In addition, the trends of FIB-4 index was adjusted for waist circumference, HbA1C, sex, and 2-years-TWL% and remained significant afterward, suggesting a substantial association with bariatric surgery (P-trend < 0.001).
Secondly, NFS exhibited a decreasing pattern from the pre-operative visit through the one-year post-operative follow-up with the lowest value of -0.88 (± 1.10), followed by a subsequent elevation at the two-year follow-up with the value of -0.69 (1.07) (P-trend < 0.001) (Fig. 1c). Moreover, since NFS has BMI and diabetes in its formula, the analysis was only adjusted for waist circumference and sex. The trend of NFS remained significant (P-trend < 0.001), indicating a robust association with bariatric surgery.
Finally, the levels of APRI showed a slight increase in the first follow-up period to reach the peak value of 0.28 (± 0.20). This trend was continued by a decreasing pattern until the six-months visit with a final increase up to the end of follow-up period with a value of 0.25 (± 0.14) (P-trend < 0.05). However, in the last visit, two-years after the surgery, its levels returned to the preoperative stage (P-value = 0.76) (Fig. 1d). Additionally, the analysis was adjusted for waist circumference, HbA1C, sex, and 2-years-TWL% and remained significant afterward, demonstrating a significant association with bariatric surgery (P-trend < 0.001).
Finally, no significant difference on the levels FIB-4, NFS, and APRI was observed comparing different surgical procedures in the last follow-up visit (P-values: 0.663, 0.214, and 0.472, respectively).
Discussion
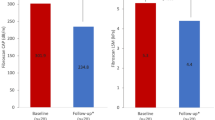
This study classified patients to two groups: low risk and intermediate risk for development of liver fibrosis with a well-known cutoff value of 1.3 for FIB-418. In the low risk group, FIB-4 levels demonstrated an overall increasing trend, while in the intermediate risk group, FIB-4 levels demonstrated an overall decreasing trend over the course of two years following surgery. However, the risk of progression to liver fibrosis did not increase in either group, indicating that the probability of developing liver fibrosis remained unchanged over two years after the surgery compared to pre-operation levels. In addition, APRI values two years after the surgery returned to the pre-operative levels, indicating a return to pre-operative stage of liver fibrosis. Finally, NFS showed a consistent decrease until the one-year visit, followed by an increase until the last follow-up visit; however, its values never surpassed the normal range over two-years of follow-up. Figure 2 demonstrates a visual summary of the mentioned findings with a focus on the trend of live fibrosis indices.
Visual summary of study’s findings. APRI: aspartate aminotransferase to platelet ratio index to platelet ratio; FIB-4: Fibrosis-4; MAFLD: metabolic dysfunction-associated steatotic liver disease; NFS: NAFLD Fibrosis Score; OAGB/MGB: one-anastomosis gastric bypass/mini gastric bypass; RYGB: Roux-en-Y gastric bypass; SG: sleeve gastrectomy; T2D: type 2 diabetes; TWL%: total weight loss percentage.
Recent research has illustrated the usefulness of FIB-4 index for the evaluation of liver fibrosis after bariatric surgery. In a cohort study with a follow-up of 5-years, FIB-4 index demonstrated appropriate performance for predicting liver fibrosis assessed by liver biopsy, in baseline, 12-months and 60-months19. In a prospective cohort, Jagtap et al. reported that APRI levels showed a reduction from pre-operative stage (1.191 (0.37)) to 6-months (0.952 (0.26)) and 12-months (0.785 (0.25)) after the surgery (P-value = 0.001)20. In contrast, the present study demonstrated an initial increase in APRI levels in the first three months with a continuous decrescendo pattern, which highlights the importance of shorter intervals for follow-up visits near the surgery. In the Japtap et al. study, NFS also showed a decrescendo trend from pre-operative stage (0.228 (1.00)) until 12-months (-0.552 (1.08)) after the surgery (P-value = 0.001), which aligns with the present study’s results (Jagtap et al., 2021). Moreover, in a cohort study performed by Kreve et al. NFS levels showed an initial reduction from pre-operative stage (1.137 ± 1.228) until 1-year post-surgery (0.269 ± 0.996) with subsequent increase until the last follow-up visit, 5 years after the surgery (0.476 ± 1.043) (P-value < 0.05)21.
In recent studies, cirrhosis is considered a contraindication for bariatric surgery, which underscores the impact of bariatric surgery on liver health. Investigators have suggested that MAFLD should also be a comorbidity for bariatric surgery candidates to prevent the progression of steatosis to cirrhosis and end-stage liver disease22. In the present study, an initial increase in FIB-4 and APRI values was observed after the surgery. Regarding these changes, it should be stated that rapid weight loss leads to an influx of free fatty acids into the liver, aggravating inflammation and hepatic steatosis, which are precursors of fibrosis, and can deteriorate liver health23,24. However, as demonstrated by liver fibrosis indices in the current study, weight loss after bariatric surgery is beneficial for liver health in the long-term.
Moreover, this study’s findings regarding the enhancement of liver health are in line with those of the BRAVES randomized trial, which evaluated patients for one year after the bariatric surgery for liver fibrotic changes25. It was demonstrated that 37 to 39% o patients in the surgery groups and 23% of patients in the lifestyle modification group experienced improvement of at least one stage of liver fibrosis with no worsening of MASH, as was also supported by significant changes of NAFLD activity score and major reductions of AST and ALT values in surgery groups after one year. Additionally, individuals in the surgery groups experienced 3.60 to 3.67 times higher MASH resolution compared to the lifestyle modification group. These findings emphasize on the potency and safety of bariatric surgery on the liver health improvement compared to the lifestyle modification.
Recent data also suggest effects of bariatric surgery on glucagon-like peptide-1 (GLP-1) and other gut hormones, as well as various lipid indices and inflammatory abnormalities relevant to the pathophysiology of fatty liver disease26. In the present study, significant reductions in liver enzymes were noted at all time points (Table 2). These findings align with Elhelw et al.‘s research, where patients with ALT levels over 40 U/L experienced significant decreases in ALT levels at 4 months following bariatric surgery, with these reductions being sustained throughout a 60-month follow-up period27. Similarly, Hassan-Zadeh et al. which studied 151 patients from general population, reported considerable decline in serum AST and ALT at the 3- and 6-month follow-up28.
In the current study, significant improvements in dyslipidemia following bariatric surgery were also observed (Table 2). These findings are consistent with a systematic review and meta-analysis involving 279 patients from randomized controlled trials and 1477 patients from observational studies, which reported remission rates of 76% and 68%, respectively29. Another analysis supported these results, showing that RYGB surgery led to a substantial reduction in triglyceride levels after 3 months post-surgery, which was sustained throughout follow-up. Additionally, there was a notable increase in HDL-C levels after 12 months post-surgery, with no significant changes at 3- and 6-months intervals. Furthermore, TC and LDL-C levels decreased after 1-month post-surgery, and these changes were maintained in the long-term30.
However, in the present study, there was an initial decrease regarding HDL-C levels observed in the 3-months visit, which could be linked to physiological changes. During the active weight loss phase after the bariatric surgery, energy metabolism comes from fat stores, which may cause imbalance in lipid transport and metabolism. This chain of events may cause the buildup of unstable TG-enriched HDL-C particles, which will be hydrolyzed by hepatic lipase, lowering HDL-C blood levels. In addition, because of the redistribution of body fat, especially visceral adipose tissue, inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) secret, which may cause displacement of apolipoprotein A-I (APO-I) from HDL-C particles, leading to degradation of HDL-C immediately after the surgery (Thakkar et al., 2021). It should be mentioned that HDL-C levels improved significantly from the 3-months visit until two years after the surgery.
Strength and limitations
The relatively large sample size of the study with two-years follow-up on a national scale, is one of the major strengths of the current study increasing the reliability and generalizability of the findings. In addition, this is the first study to date, focusing on patients with T2D, comparing the three noninvasive indices of liver fibrosis among patients undergoing three types of bariatric surgeries. However, it is essential to indicate some limitations of the current study. Lack of randomization in patient selection for each type of surgery could be source of biases. Additionally, data regarding the duration of diabetes and use of insulin was not available, which could deliver insightful information regarding the control over blood glucose. In addition, this study could benefit from a standardized method for diagnosing liver fibrosis, such as liver biopsy to strengthen the results of the study. The low rates of follow-up participation are also a limitation of the present study, which was most likely due to the observation that some patients had achieved their goals in terms of weight reduction, blood glucose control, and improvement in lipid profiles and did not find it necessary to continue follow-up. Finally, it would be beneficial to evaluate the effect of life style modification and diet which have substantial role in the process.
Conclusion
This study demonstrated that bariatric surgery significantly improves NFS and cause alterations in FIB-4 and APRI levels without increasing the risk of liver cirrhosis development, regardless of type of surgery. Based on FIB-4 values, patients with T2D, obesity, and low to indeterminate risk of liver fibrosis undergoing bariatric surgery, show no significance increase in the probability of liver failure. Additionally, bariatric surgery reduces liver enzymes, and enhances lipid and glycemic profiles in individuals with obesity and T2D. These findings underscore the potential and safety of bariatric surgery as an efficient treatment strategy for morbid obesity in individuals with T2D. We recommend further studies to involve histological confirmation for better validation of these findings.
Data availability
This study’s data is available upon request to the corresponding author through e-mail.
Abbreviations
- ADA:
-
American Diabetes Association
- ALT:
-
Alanine aminotransferase
- APO-I:
-
Apolipoprotein A-I
- APRI:
-
Aspartate aminotransferase to platelet ratio
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- CKD:
-
Chronic kidney disease
- CVA:
-
Cerebrovascular accident
- CVD:
-
Cardiovascular disease
- DVT:
-
Deep vein thrombosis
- FBG:
-
Fasting blood glucose
- FIB-4:
-
Fibrosis-4 index
- GLP-1:
-
Glucagon-like peptide-1
- HbA1c:
-
Hemoglobin A1C
- HDL-C:
-
High density lipoprotein-cholesterol
- HTN:
-
Hypertension
- IL-6:
-
Interleukin-6
- LDL-C:
-
low density lipoprotein-cholesterol
- LSG:
-
Laparoscopic sleeve gastrectomy
- MASH:
-
Metabolic dysfunction-associated steatohepatitis
- MAFLD:
-
Metabolic dysfunction-associated fatty liver disease
- NFS:
-
NAFLD fibrosis score
- OAGB/MGB:
-
One anastomosis gastric bypass/mini gastric bypass
- OSA:
-
Obstructive sleep apnea
- PE:
-
Pulmonary embolism
- RYGB:
-
Roux-en-Y gastric bypass
- SD:
-
Standard deviation
- SG:
-
Sleeve gastrectomy
- T2D:
-
Type 2 diabetes mellitus
- TC:
-
Total cholesterol
- TNF-α:
-
Tumor necrosis factor-alpha
- TWL%:
-
Total weight loss
References
Fouad, Y., Alboraie, M. & Shiha, G. Epidemiology and diagnosis of metabolic dysfunction-associated fatty liver disease. Hepatol. Int. 1–7. (2024).
Yanai, H., Adachi, H., Hakoshima, M., Iida, S. & Katsuyama, H. Metabolic-dysfunction-Associated Steatotic Liver Disease—its Pathophysiology, Association with atherosclerosis and Cardiovascular Disease, and treatments. Int. J. Mol. Sci. 24, 15473 (2023).
Beygi, M., Ahi, S., Zolghadri, S. & Stanek, A. Management of metabolic-Associated fatty liver Disease/Metabolic Dysfunction-Associated Steatotic Liver Disease: from medication therapy to Nutritional interventions. Nutrients 16, 2220 (2024).
Eslam, M. et al. Yki-Järvinen, MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 158, 1999–2014 (2020).
Taheri, E. et al. Predictors of metabolic-associated fatty liver disease (MAFLD) in adults: a population-based study in northeastern Iran. Gastroenterol. Hepatol. Bed Bench 14, S102 (2021).
Binet, Q. et al. Non-invasive screening, staging and management of metabolic dysfunction-associated fatty liver disease (MAFLD) in type 2 diabetes mellitus patients: what do we know so far. Acta Gastroenterol. Belg. 85 (2022).
Eslam, M., Fan, J. G. & Mendez-Sanchez, N. Non-alcoholic fatty liver disease in non-obese individuals: the impact of metabolic health. Lancet Gastroenterol. Hepatol. 5, 713–715 (2020).
Eslam, M. et al. Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat. Rev. Gastroenterol. Hepatol. 19, 638–651 (2022).
Stefan, N. & Roden, M. Diabetes and fatty liver. Exp. Clin. Endocrinol. Diabetes 127, S93–S96 (2019).
Panel, C. P. G. et al. For the S. of the liver, EASL Clinical Practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis–2021 update. J. Hepatol. 75, 659–689 (2021).
Zhang, F. et al. Association of non-invasive markers with significant fibrosis in patients with nonalcoholic fatty liver disease: a cross-sectional study. Diabetes Metabolic Syndrome Obes. 2255–2268. (2023).
Fisher, D. P. et al. Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. JAMA 320, 1570–1582 (2018).
Kirwan, J. P. et al. Diabetes remission in the alliance of randomized trials of medicine versus metabolic surgery in type 2 diabetes (ARMMS-T2D). Diabetes Care 45, 1574–1583 (2022).
Salehi, S. S. et al. The effect of vitamin D deficiency state on oxidized low-density lipoprotein alteration in patients with type 2 diabetes. Funct. Foods Health Disease 11, 357–367 (2021).
Moolenaar, L. R. et al. Liver injury and acute liver failure after bariatric surgery: an overview of potential injury mechanisms. J. Clin. Gastroenterol. 56, 311–323 (2022).
Geerts, A. et al. The Multicenter Belgian Survey on Liver Transplantation for Hepatocellular Failure after Bariatric Surgerypp. 4395–4398 (Transplant Proc, Elsevier, 2010).
Care, D. 2. Classification and diagnosis of diabetes: standards of Care in. Diabetes Care 46, S19 (2023).
Bertot, L. C. et al. Diabetes impacts prediction of cirrhosis and prognosis by non-invasive fibrosis models in non‐alcoholic fatty liver disease. Liver Int. 38, 1793–1802 (2018).
Raverdy, V. et al. Performance of non-invasive tests for liver fibrosis resolution after bariatric surgery. Metabolism 153, 155790 (2024).
Jagtap, N. et al. Endoscopic sleeve gastroplasty—minimally invasive treatment for non-alcoholic fatty liver disease and obesity. Indian J. Gastroenterol. 40, 572–579 (2021).
Kreve, F. et al. Trajectory of NAFLD characteristics after Roux-en-Y gastric bypass: a five-year historical cohort study. Sao Paulo Med. J. 140, 739–746 (2022).
Agarwal, L., Sahu, A. K., Baksi, A., Agarwal, A. & Aggarwal, S. Safety of metabolic and bariatric surgery in obese patients with liver cirrhosis: a systematic review and meta-analysis. Surg. Obes. Relat. Dis. 17, 525–537 (2021).
Huang, Y., Dong, S., Wang, C., Dong, Z. & Chen, W. Significant fibrosis assessed by liver biopsy among Chinese bariatric surgery patients: a prospective cross-sectional study. Front. Endocrinol. (Lausanne) 14, 1090598 (2023).
Moretto, M., Kupski, C., Da Silva, V. D., Padoin, A. V. & Mottin, C. C. Effect of bariatric surgery on liver fibrosis. Obes. Surg. 22, 1044–1049 (2012).
Verrastro, O. et al. Bariatric–metabolic surgery versus lifestyle intervention plus best medical care in non-alcoholic steatohepatitis (BRAVES): a multicentre, open-label, randomised trial. Lancet 401, 1786–1797 (2023).
Laursen, T. L. et al. Bariatric surgery in patients with non-alcoholic fatty liver disease - from pathophysiology to clinical effects. World J. Hepatol. 11, 138–149. https://doi.org/10.4254/wjh.v11.i2.138 (2019).
Elhelw, O. et al. The impact of bariatric surgery on liver enzymes in people with obesity: a 5-year observational study. Surgeon. https://doi.org/10.1016/j.surge.2023.07.006 (2023).
Zadeh, M. H. et al. Changes in serum albumin and liver enzymes following three different types of bariatric surgery: six-month follow-up. A retrospective cohort study. Sao Paulo Med. J. 139, 598–606. https://doi.org/10.1590/1516-3180.2021.00065.r1.1504221 (2021).
Chang, S. H. et al. The effectiveness and risks of bariatric surgery. JAMA Surg. 149, 275. https://doi.org/10.1001/jamasurg.2013.3654 (2014).
Carswell, K.A., Belgaumkar, A.P., Amiel, S.A. & Patel, A.G. A systematic review and Meta-analysis of the Effect of gastric bypass surgery on plasma lipid levels. Obes. Surg. 26, 843–855. https://doi.org/10.1007/s11695-015-1829-x (2016).
Author information
Authors and Affiliations
Contributions
A.S.: Conceptualization, Formal analysis, Methodology, Project administration, Writing - original draft; S. Rabizadeh: Conceptualization, Formal analysis, Methodology; S. K. R.: Validation, Methodology, Writing - review & editing; S. H.: Writing - review & editing, Investigation; S. A. A.: Writing - original draft, Investigation ; M. S.: Writing - original draft, S. A. N.: Formal analysis, N. A. S.: Data curation; A. Y.: Investigation, F. Mohammadi: Methodology, F. Moosaei: Writing - review & editing, E. S.: Visualization, S. Riazi: Data curation; F. S.: Data curation, M. N.: Supervision, Conceptualization; A. P.: Supervision, Data curation; A. E.: Supervision, Conceptualization.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The ethical committee of the Tehran University of Medical Sciences (TUMS) approved the study with the registered number of IR.TUMS.IKHC.REC.1401.162. Informed consent for participation and publication was obtained from all individual participants included in the study.
Artificial Intelligence (AI) statement
Generative AI and AI-assisted technologies were only used during the writing process to improve the readability and language of the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Seyedi, A., Rabizadeh, S., Reyhan, S.K. et al. Impact of bariatric surgery on liver fibrosis indices among type 2 diabetes patients in a national cohort. Sci Rep 15, 1235 (2025). https://doi.org/10.1038/s41598-025-85427-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85427-y
Keywords
This article is cited by
-
Recurrent Weight Gain after Metabolic Bariatric Surgery (MBS): Emerging Insights on Kidney Function
Obesity Surgery (2026)
-
Efficacy and Safety of Bariatric Surgery in Well-Compensated Liver Cirrhosis: A Systematic Review and a Single-Arm Meta-analysis
Obesity Surgery (2025)
-
Comment on “Efficacy and Safety of Bariatric Surgery in Well-Compensated Liver Cirrhosis: A Systematic Review and Single-Arm Meta-analysis”
Obesity Surgery (2025)