Abstract
Tuta absoluta is one of the most destructive pests of tomatoes. Chemical insecticides used to control this leafminer harm all organisms, increasing the risk to public health and the environment. Developing natural alternatives, such as bioinsecticides formulated from essential plant oils, is a key strategy to address this problem. These volatile compounds, derived from the secondary metabolic pathways of plants, exhibit targeted activity against specific pest species. Their use is consistent with an environmentally responsible framework that reduces adverse impacts on ecosystems, protects non-target organisms, safeguards human health, and enhances the efficacy of integrated crop management systems. This study aims to determine the chemical composition of the essential oils (EOs) of from round leaf mint (Mentha rotundifolia) and crown chrysanthemum (Chrysanthemum coronarium) and to evaluate their toxicity to T. absoluta larvae in-vitro. The chemical composition of EOs obtained by steam distillation from the leaves of the plants was analyzed by gas chromatography coupled with mass spectrometry (GC-MS). Indeed, 77 volatile compounds representing 98.19% of the total oil of M. rotundifolia, including cyclobutane acetonitrile, 1-methyl-2-(1-methyl ethenyl)-, terpinene-4-ol, p-menthane, germacrene D, caryophyllene and myrcene, were the main compounds. However, farnesene, myrcene, eugenol, germacrene D, phytol, and pinene were significant components among 69 compounds representing 95.39% of the total oil of C. coronarium. Results showed that the EOs were toxic to the different larval stages. According to the Finney method, concentrations 2.88 and 1.07% are the LC50 of M. rotundifolia and C. coronarium oils, which induce 50% mortality of T. absoluta within 7 days of exposure. Statistical analysis of in-vitro tests showed that both EOs had a similar level of insecticidal efficacy by contact. The overall results showed that the oils used have been shown to have an important insecticidal effect and can be used as a source of biological and natural treatment against tomato leafminer (TLM).
Similar content being viewed by others
Introduction
The TLM, T. absolutais a highly destructive insect pest that attacks tomato plants1,2,3, causing yield losses of up to 100% if uncontrolled4. Chemical insecticides are the most common control measure farmers use to combat infestations5,6,7.
However, this control method can be expensive and sometimes ineffective due to reported resistance, potential adverse effects on the environment and human health, and secondary effects on natural enemies8,9,10. To mitigate these issues, natural alternatives such as biopesticides should be implemented6,11,12,13.
Entomopathogen and botanical-based biopesticides are important pest management options for farmers to control T. absolutapopulations, particularly in the agrobiological system14,15. Using active compounds of plant origin, such as plant extracts and essential oils, is more environmentally friendly than synthetic insecticides. Medicinal plants are a viable option for sustainable control of T. absoluta. Indeed, EOs are widely available, easy to apply, and inexpensive6. Furthermore, EOs and their bioactive compounds, such as terpenoids, sesquiterpenes, terpenes, phenylpropanoids, oxygenated terpenes, ketones, esters, and alcohols, are recognized as sustainable means for managing plant pests16.
EOs and their main components exhibit potent insecticidal activity against various agricultural pests, acting as contact insecticides, fumigants, antifeedants, and repellents17,18. T. absoluta integrated control programs may reduce the cost of controlling and the environmental impact of using broad-spectrum insecticides.
In different regions of the world, various medicinal plants belonging to different families have been used as botanical pesticides due to their effectiveness in controlling pests. These include Phoenician juniper (Juniperus phoenicea), marjoram (Origanum majorana), Greek oregano (Origanum vulgare ssp.), bay laurel (Laurus nobilis), leucate port (Echinophora spinosa), and lemon (Citrus limon)19. Peppermint (Mentha piperita), long-leaved mint (Mentha longifolia), sage (Salvia officinalis), and rosemary (Salvia rosmarinus) were studied by10. Basil (Ocimum basilicum), cultivated black cumin (Nigella sativa), and lavender (Lavandula angustifolia) were investigated by20.
Algeria is known for its diverse flora, resulting from the varied climate and soil types. The flora includes many species classified by their degree of rarity, including 289 fairly rare species, 647 rare species, 640 very rare species, 35 extremely rare species, and 168 endemic species21. Aromatic plants are widely distributed in Mostaganem, boasting a diverse flora that includes many species known for their medicinal and biological properties. This research focuses on M. rotundifolia and C. coronarium from the Lamiaceae and Asteraceae families, respectively. This study aimed to characterize the chemical composition of EOs using GC-MS and to evaluate their insecticidal potential. This study also represents the first description of the insecticidal properties of essential oils from M. rotundifolia and C. coronarium against T. absoluta larvae. These plants were selected based on their bioactive properties as demonstrated in previous studies on various organisms, making them promising candidates for biological control. In addition, their wide geographical availability, market presence and ease of cultivation increase their attractiveness to farmers seeking to reduce their reliance on chemical insecticides.
A perennial herb found in semi-arid and humid bioclimates in the Mediterranean basin in America and western Asia, M. rotundifolia22,23. This fragrant herb has long been utilized medicinally in North Africa as a tonic, stimulant, stomachic, carminative, and analgesic24,25. It has also been discovered to have biological properties, such as antioxidant25,26, insecticidal27, antibacterial28, and antifungal properties29.
The C. couronariumis an annual plant found in the Mediterranean area, China, Japan, and the Philippines30,31. It has various biological properties, such as antibacterial, antioxidant, insecticidal, nematicidal, and cytotoxic properties, and is edible and used as a vegetable32,33,34,35.
Materials and methods
Plant materials
Both species were identified by a botanist at the University of Abdelhamid Ibn Badis of Mostaganem. They have been deposited at the herbarium of the plant ecology laboratory of the University Ahmed Ben Bella 1 of Oran (Algeria) and are registered under the following references: M. rotundifolia: H. O. ES. 2283; C. coronarium: H. O. ES. 2878 (Table 1).
EOs extraction
The plant material was dried and stored in a dry, well-ventilated area at room temperature until further use. EOs were extracted through steam distillation, a widely recognized method for isolating volatile oils from aromatic plants. This technique is highly efficient for extracting volatile and heat-sensitive compounds while maintaining their chemical integrity and bioactivity36,37. Moreover, steam distillation is particularly suitable for the plants examined in this study, as it produces high yields of pure essential oils devoid of solvent residues. This approach is environmentally friendly and well-suited for applications requiring high-quality extracts. Each plant sample was placed in the device with 2 L of distilled water for 2 h. After that, the essential oils were kept cold (4 °C) in an amber flask, awaiting additional examination. It was determined how much essential oil was extracted.
GC–MS analysis
The EOs analyses were performed simultaneously using GC–MS systems. The equipment used is very sensitive to identifying and quantifying volatile and semi-volatile compounds. The compounds were separated and identified in EOs using Clarus 690 SQ8T GC-MS with MultiPrep™ autosampler. The oven temperature was programmed to 50 °C for 10 min and raised to 280 °C at 10 °C/min. The temperature was kept constant at 200 °C for 10 min and then raised to 240 °C at a 1 °C/min rate. The injector temperature was set at 280 °C. A volume of 0.5 µl injected using micro syringes into a capillary column; the carrier gas is helium (1mL/min), an inert gas whose function is to push the volatile compounds in the sample toward the detector. Under the conditions of this work, the analysis is carried out in split mode, of which the ratio is 20:1. The split corresponds to a ratio between the part injected into the column and directed towards the outside of the device, intending to avoid saturation of the column.
Insect collection
Tomato leaves infested by T. absoluta were collected from the experimental farm at the University of Mostaganem on multiple occasions. The different larvae were then collected in the laboratory for in vitro tests.
Contact toxicity assay
Bioassays were conducted to evaluate the efficacy of five concentrations of essential oil (10, 20, 30, 40, and 50 µl/ml) under optimal conditions of 24 ± 3 °C and 51 ± 9% relative humidity. T. absolutalarvae, classified by their developmental stages (1st to 4th instars), were placed in Petri dishes containing fresh tomato leaves as a feeding substrate and a moistened absorbent paper layer to maintain adequate humidity. The essential oil was applied to the larvae via direct spraying. Positive controls were treated with 10% acetone, while negative controls received distilled water. Mortality was recorded at 24-hour intervals over 7 days. Corrected mortality rates were calculated using Abbott’s formula to assess the insecticidal effect accurately, accounting for natural mortality in the control groups38.
The efficacy of a toxic product is determined by its LC50 and LC90 values, which correspond to the concentrations of the toxic substance required to cause mortality in 50% and 90% of the exposed population, respectively. These values are obtained from the regression analysis of corrected mortality data. The calculation is typically performed using the Probit method, which transforms mortality percentages into a linear relationship to estimate lethal concentrations with precision and reliability39.
Statistical analyses
The results obtained were subjected to analysis of variance (ANOVA) to assess treatment effects, followed by a comparison of means using Tukey’s test to compare treatment means at P ≤ 0.05 using R 4.2.1 statistical software.
Results and discussion
EOs yields
The EOs yields obtained in the steam distillation were 0.70 and 0.20% for the M. rotundifolia and C. couronarium, respectively. The resulting vegetable extractions produced yellowish EOs with a strong and persistent odor.
A variation in EO yields was observed between species (Table 2). The quantity of M. rotundifoliaEO obtained in our study is higher than that (0.49%) obtained by25for the same species treated by hydro-distillation from Bejaia (Algeria). Higher values have been reported in various studies in other Algerian locations. The yield of mint leaves EO from Khemis Meliana is around 1.8%24. Meanwhile, samples from the Sétif region showed a rate of 1.27%29. Meanwhile, those from the El Tarf region yielded 1.65%26. M. rotundifoliacollected in Tunisia yielded 1.26%28. However, mint from Morocco was characterized by a yield of 1.17%40.
For C. couronarium, the leaves from the Mostaganem region showed a lower yield than in studies conducted in different parts of the world. In Tunisia, the study conducted by41 on C. couronariumoil noted a content of 0.31%. Nevertheless, the EO yields of this plant harvested from two regions in Cyprus, Değirmenlik and Salamis, were around 0.44 and 0.40%, respectively42. Furthermore, the study by43 obtained the following yields from samples from Greece: 0.31% (Porto Rafti) and 0.39% (Diminio).
The difference in the number of EOs observed in M. rotundifolia and C. couronariummay be linked to several factors, including the plant family and biology, environmental conditions, climatic and edaphic factors related to geographical location, physiological variations, harvesting period44,45, plant organ, temperature, drying time and extraction method46,47,48.
Analysis of chemical composition
M. Rotundifolia EO
The GC-MS analysis of the dry leaves of M. rotundifolia EOs revealed the presence of 77 compounds, representing 98.19% of the total composition of the oil. The majority component was Cyclobutaneacetonitrile, 1-methyl-2-(1methylethenyl)- (45.60%), followed by terpinene-4-ol (6.36%), p-menthane (5.46%), germacrene D (4.49%), Caryophyllene (2.29%), and myrcene (3.04%) (Table 3).
Many studies have examined the chemical components of M. rotundifoliaEOs growing in different parts of the world, and distinct chemotypes have been identified24,49,50. M. rotundifoliaharvested in northeast Algeria was studied. Samples from Bejaia reported trans-piperine epoxide (30.2%), piperine oxide (8.7%), thymol (4.5%), germacrene D (3.5%), and terpinene-4-ol (2.7%) as the main ingredients25. In contrast, chemical analyses of the oil of the same species grown in Annaba revealed that the major compounds are carvacrol (60.54%), pulegone (5.95%), m-cymene (5.25%) and γ-terpinene (4.83%). However, the characterization of M. rotundifoliaoil from eastern Algeria (Batna) revealed a different composition with piperitenone oxide (35.49%) as the main component, followed by caryophyllene oxide (35.27%) and cis-cinerolone (10.95%)51. According to29, 3-cyclopenten-1-one and 2-hydroxy-3-(3-methyl-2-butenyl)- (89.09%) are the essential molecules of M. rotundifolia oil from Sétif.
In contrast28, analyzed the Tunisian species. They found that there was a difference in chemical composition between the Béja region (north-west Tunisia), where caryophyllene (26.67%) was considered the marginal substance followed by germacrene D (12.31%) and carveol (7.38%). Samples from Bizerte (north-east Tunisia) contained pulegone (32.9%), piperitenone oxide (17.28%), and 5-acetyl thiazole (11.26%). For those from Morocco, the most dominant component is 2 iso-propylidenecyclohexanone at 11.99%, followed by eucarvone at 11.42%, gamma-murolene at 8.61%, 2-Isopropyl-5-methyl-3-cyclohexen-1-one (6.83%) and p-menthan-1,2,3-triol (6.72%). In other reports, the main compound of the species studied is pulegone (85%)50.
C. Coronarium EOs
The chemical analysis of C. coronarium EOs allowed the identification of 69 compounds, representing approximately 95.39% of the total oil. The main substances were farnesene (10.19%), myrcene (10.12%), eugenol (7.97%), germacrene D (6.34%), phytol (4.88%), and pinene (3.66%) (Table 4).
In Morocco, researchers analyzed the aerial part of C. coronariumEOs obtained by hydrodistillation using GC/MS. The results indicated the presence of chrysanthenone (17.02%), camphor (15.38%), and pulegone (20.68%)52. Our oil’s chemical profile differs from those previously reported41. studied the Tunisian species and found that cis-chrysanthenyl acetate (21.82%), trans-chrysanthenyl acetate (12.78%), (E)-β-farnesene (8.97%), germacrene D (8.92%), and camphor (6.03%) are essential elements.
However, the main component of C. coronarium oil from Jordan was camphor (17.5%) followed by santolin triene (4.3%), neoiso-3-thujanol (5.6%), cis-chrysanthenyl acetate (10.8%), perilla aldehyde (11.7%), isoitalicene (4.7%) and phenylpropyl butanoate (4.9%)53. High concentrations of santolinatriene, yomogi alcohol, camphor, cis-chrysanthenyl acetate, and bornyl acetate were found in oils extracted from the aerial sections of C. couronariumplants in Cyprus42. On the other hand43, studied the chemical composition of flower heads from two locations in Greece. While samples from Diminio contained trans-chrysanthenyl acetate (13.2%), trans-chrysanthenyl isovalerate (10.2%), and cis-chrysanthenyl acetate (9.9%), those from Porto Rafti were rich in trans-chrysanthenyl acetate (7.8%), cis-chrysanthenyl acetate (9.1%) and camphor (15.7%). However, the species that are native to South Korea contain myrcene (31.9%), -bisabolol (16.5%), (E, E)-farnesene (11%), and (E)-farnesene (8.4%) as major constituents54.
The observed composition discrepancy between the plants’ essential oils and those documented by previous investigations is probably associated with intrinsic factors, such as species, chemotype/genotype, genetic structures, organogenesis, and development phases55. Geographical variation56, plant parameters (e.g., species, cultivated or wild plants), harvesting and post-harvest/predistillation parameters (e.g., harvesting season, biomass pre-treatment and storage), production conditions, storage conditions, and oil storage time EOs are additional factors that can affect the chemical composition of oils57.
Insecticidal activity
Contact toxicity of M. Rotundifolia EO
The toxicity of M. rotundifolia oil to T. absoluta larvae was demonstrated. Mortality rates ranging from 7.01 to 38.58% were observed 24 h after application. After 4 days, the various concentrations of peppermint oil resulted in mortality rates ranging from 46.15 to 84.8%. On the seventh day of the experiment, peppermint oil achieved 86.20% mortality at the highest concentration (50 µl/ml). Meanwhile, 65.51%, 66.65%, 82.18%, and 83.32% mortality rates were observed with 10, 20, 30, and 40 µl/ml concentrations, respectively (Fig. 01).
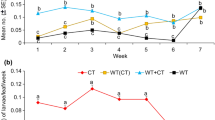
The mortality rate of T. absoluta caterpillars varied highly significantly (P < 0.001) among different concentrations of M. rotundifolia oil and control groups (positive and negative) (Fig. 02).
Contact toxicity of C. Coronarium EO
The toxicity of C. couronarium EO against T. absoluta caterpillars was remarkable. After 24 h of oil application, larval mortalities ranged from 5.35 to 19.64. However, mortality rates of 46.5% and 65.57% were caused by the lowest (10 µl/ml) and highest concentration (50 µl/ml), respectively, four (04) days later.
On the seventh day of the test, the highest concentration (50%) caused 82.91% mortality, while 10 and 20 µl/ml concentrations caused 61.29% mortality (Fig. 03).
The variance analysis conducted on the larvae’s mortality, based on the concentrations of the EO of the C. couronarium and the controls, yielded highly significant results (P < 0.001). The highest mortality was observed in the highest concentration for all developmental stages of T. absoluta. Additionally, the results revealed that the 1st and 2nd larval instars were more sensitive than the 3rd and 4th instars (Fig. 04).
The analysis of variance for the insecticidal effect EOs on T. absoluta larvae did not show any significant difference (P > 0.05) for both the concentration and oil factors. The larvae were sensitive to the toxicity of the essential oils, even at the lowest concentration (10 µl/ml) (Fig. 05).
LC50 and LC90 of the EOs tested on T. absoluta larvae
After seven days of exposure to M. rotundifolia EO to T. absoluta larvae, the calculated LC50 was 2.88 µl/ml, and the LC90 obtained was equal to 54.95 µl/ml. In contrast, the LC50 and LC90 values obtained for C. couronarium oil are 1.07 µl/ml and 17.37 µl/ml, respectively.
One day after spraying, significant mortality of TLM caterpillars was observed. However, live individuals moved more slowly before stopping feeding. The caterpillars then became discoloured, desiccated, and died a few days later. The insecticidal effect of EOs against T. absolutalarvae could be explained by the high content of the major volatile components of the plant’s essential oil. It may also be due to certain minority constituents or to a synergistic effect of several constituents58. reports that monoterpenes, sesquiterpenes, and phenylpropanoids are among the main constituents of several botanical EOs that are important for their effects on insect pests. Indeed, several studies have explored the effectiveness of the EOs from certain species of Menthaas insecticides. The results obtained in our research are consistent with a previous study that highlighted the high toxicity of the EOs of this genus against various insect species40. In contrast, the toxicity of M. rotundifoliaEOs at different concentrations was tested against adult wheat weevils and red flour beetles. It was found that the mortality rate was influenced by several factors, including insect species and exposure duration51. However, the in-vitro test showed that the essential oil had insecticidal activity against fourth-instar Culex pipiens larvae.
On the other hand, various research studies have also demonstrated the insecticidal activity of the EOs of C. couronarium. The application of C. coronarium oil to the nymphs of the stored product pest Tribolium confusumcaused significant mortality, which reached 67% after 7 days of treatment35. Also, the study conducted by42 showed the insecticidal effect of this oil on individuals of Sitophilus granarius.
As with other insect pests, the most developed botanical insecticides against T. absolutahave been formulated with EOs59. Several studies investigating essential oils’ lethal and/or sublethal effects (e.g., on behaviour) have demonstrated insecticidal efficacy against TLM. The impact of ajwain essential oils (Trachyspermum ammi) on T. absolutalarvae in their fourth instar was studied. The study determined each compound’s effectiveness and whether it was antagonistic or synergistic. The findings indicated that thymol and ajwain EOs had the maximum toxicity against larvae60.
61 investigated the biocidal activity of thyme (Thymus vulgaris) and citronella (Cymbopogon citratus) EOs against the pest T. absoluta. The results showed that both oils had similar extermination and insecticidal efficacy by direct contact and fumigation. Both prolonged the life cycle of the pest. Furthermore, Syzygium aromaticumbud essential oil showed high insecticidal toxicity against TLM larvae under laboratory conditions62. On the other hand63, showed good insecticidal activity of citrus EOs with contact mortality on T. absoluta eggs and larvae.
The insecticidal activity of EOs is promising; however, their application in real-world scenarios presents challenges related to resistance development and environmental limitations. Repeated exposure to sub-lethal doses of EOs may lead to behavioral or physiological resistance in pest populations, akin to resistance seen with synthetic insecticides. This is particularly concerning if EOs act on specific molecular targets, as pests can evolve mechanisms to counteract these effects. Furthermore, the volatility and susceptibility of essential oils to environmental degradation, such as oxidation or UV light exposure, limit their efficacy in outdoor applications. Their short residual activity necessitates frequent reapplications, which can increase costs and labor demands. Additionally, EOs are often non-specific, potentially affecting beneficial insects like pollinators and predators, which could disrupt ecological balance. The economic feasibility of large-scale EO production and regulatory hurdles also pose significant barriers to widespread pest management adoption. These limitations highlight the need to integrate EOs into broader pest control strategies, combining them with other methods to mitigate resistance and optimize efficacy64,65.
Farmers are advised to use EOs products alone and with other biorational options, such as predatory arthropods and microbial pesticides66. However, several limitations exist, such as optimized and approved formulations, for practically incorporating essential oils into IPM programs67. In addition, the compatibility of EOs with effective biological control agents needs to be assessed on a case-by-case basis68,69. These reasons, as well as cost, efficacy, and reliability, may hinder growers’ use of this control option.
Conclusion
Plants synthesize several secondary metabolites. These molecules are of interest as safe environmental practices to mitigate severe damage caused by pests in agroecosystems. The bioactivity of the medicinal plants (EOs) evaluated against T. absoluta larvae is explained by the secondary metabolites produced by these plants, which have different modes of action. In this experiment, the essential oils and extracts affected the survival of TLM larvae. The results demonstrated a clear correlation between the concentrations and length of exposure and the mortality rate of T. absoluta caterpillars. The first and second larval instars were the most susceptible to all concentrations, according to the in-vitro test. Due to its insecticidal properties, this essential oil may be utilized as a substitute in integrated biological control systems to combat TLM while reducing the need for synthetic pesticides.
Future studies could focus on evaluating the synergy between these compounds and other integrated pest management practices for more effective control of the TLM. It would also be relevant to characterize the sublethal effects of essential oils on the growth, reproduction, and behavior of pests while investigating the genetic variability of T. absoluta responses across different geographical populations. Analyzing the bioavailability and persistence of bioactive compounds in various agricultural environments and examining the modes of action of EOs on the insect nervous system using transcriptomic and proteomic techniques are also promising avenues. Furthermore, the environmental impact of essential oils on non-target biodiversity and the development of nanotechnological formulations to improve their stability and controlled release in agricultural applications should be explored. Finally, longitudinal studies would allow the assessment of long-term effects on pest populations and the resilience of agricultural ecosystems.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Zhang, P., Min, L., Tang, J., Rafiq, M. K. & Sun, H. Sorption and degradation of imidacloprid and clothianidin in Chinese paddy soil and red soil amended with biochars. Biochar 2, 329–341 (2020).
Erasmus, R., van den Berg, J. & du Plessis, H. Susceptibility of Tuta absoluta (Lepidoptera: Gelechiidae) pupae to soil applied entomopathogenic fungal biopesticides. Insects 12, 515 (2021).
Bakheit, E. H. et al. Prospects of resistance of some Sudanese tomato accessions to tomato fruit borer (Tuta absoluta)(Meyrick, 1917)(Lepidoptera: Gelechiidae) and its mechanism. (2022).
Biondi, A., Guedes, R. N. C., Wan, F-H. & Desneux, N. Ecology, worldwide spread, and management of the invasive south American tomato pinworm, Tuta absoluta: past, present, and future. Annu. Rev. Entomol. 63, 239–258 (2018).
Srinivasan, K. Fenugreek (Trigonella foenum-graecum L.) seeds used as functional food supplements to derive diverse health benefits. In: Nonvitamin and Nonmineral Nutritional Supplements. Elsevier; 217–221. (2019).
Tarusikirwa, V. L., Machekano, H., Mutamiswa, R., Chidawanyika, F. & Nyamukondiwa, C. Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on the Offensive in Afprospectsspects for Integrated Management Initiatives. Insects 11, 764 (2020).
Langa, T. P. et al. Basis and monitoring of methoxyfenozide resistance in the South American tomato pinworm Tuta absoluta. J Pest Sci 2022;95:351–64. (2004).
Ali, W., Ahmad, R., Nor, Z. M. & Hassan, A. Spatial distribution, enzymatic activity, and insecticide resistance status of Aedes aegypti and Aedes albopictus from dengue hotspot areas in Kuala Lumpur and Selangor, Malaysia. Serangga 25, 65–92 (2020).
Krache, F. et al. Effect of botanical extract of garlic (Allium sativum L.) on larvae of tomato leaf miner Tuta absoluta (Meyrick)(Lepidoptera: Gelechiidae). Pap Life Sci. Mar. Environ. Res. July, 6–9 (2022).
Sayed, S. et al. Toxicity, deterrent and repellent activities of four essential oils on Aphis punicae (Hemiptera: Aphididae). Plants 11, 463 (2022).
Titouhi, F. et al. Protective effects of three Artemisia essential oils against Callosobruchus maculatus and Bruchus rufimanus (Coleoptera: Chrysomelidae) and the extended side-effects on their natural enemies. J. Stored Prod. Res. 72, 11–20 (2017).
Banaras, S., Javaid, A. & Khan, I. H. Bioassays guided fractionation of Ageratum conyzoides extract for the identification of natural antifungal compounds against Macrophomina Phaseolina. Int. J. Agric. Biol. 25, 761–767 (2021).
Iqbal, J. et al. Acidified Biochar confers improvement in Quality and Yield attributes of Sufaid Chaunsa Mango in saline soil. Horticulturae 7, 418 (2021).
Amizadeh, M., Hejazi, M. J., Niknam, G. & Arzanlou, M. Compatibility and interaction between Bacillus thuringiensis and certain insecticides: perspective in management of Tuta absoluta (Lepidoptera: Gelechiidae). Biocontrol Sci. Technol. 25, 671–684 (2015).
Abd El-Ghany, N. M., Abdel-Razek, A. S., Djelouah, K. & Moussa, A. Efficacy of bio-rational insecticides against Tuta absoluta (Meyrick)(Lepidoptera: Gelechiidae) on tomatoes. Biosci. Res. 15, 28–40 (2018).
El Kasimi, R., Douiri, F., Haddi, K. & Boughdad, A. Bioactivity of essential oil from Citrus aurantium Peel against the pulse Beetle Callosbruchus Maculatus F. on Chickpea. Agriculture 13, 232 (2023).
Wanzala, W., Hassanali, A., Mukabana, W. R. & Takken, W. Essential oils of indigenous plants protect livestock from infestations of Rhipicephalus appendiculatus and other tick species in herds grazing in natural pastures in western Kenya. J. Pest Sci. 91, 395–404 (2018).
Arena, J. S., Merlo, C., Defagó, M. T. & Zygadlo, J. A. Insecticidal and antibacterial effects of some essential oils against the poultry pest Alphitobius diaperinus and its associated microorganisms. J. Pest Sci. 93, 403–414 (2020).
Papanikolaou, N. E. et al. Essential oil coating: Mediterranean Culinary plants as grain protectants against larvae and adults of Tribolium castaneum and Trogoderma granarium. Insects 13, 165 (2022).
Al-Harbi, N. A. et al. Evaluation of insecticidal effects of plants essential oils extracted from basil, black seeds and lavender against Sitophilus oryzae. Plants 10, 829 (2021).
Sahi, L. La Dynamique Des plantes aromatiques et médicinales en Algérie [Troisième Partie]. Le marché Des plantes Aromat médicinales Anal Des Tend Du marché Mond des Strat économiques en albanie en Algérie. ;73:101–140. (2016).
Quézel, P. & Santa, S. Nouvelle flore de l’Algérie et des régions désertiques méridionales. (1962).
Derwich, E., Benziane, Z., Taouil, R., Senhaji, O. & Touzani, M. Comparative essential oil composition of leaves of Mentha rotundifolia and Mentha pulegium a traditional herbal medicine in Morocco. (2010).
Brada, M., Bezzina, M., Marlier, M., Carlier, A. & Lognay, G. Variabilité de la composition chimique des huiles essentielles de Mentha rotundifolia du Nord de l’Algérie. Biotechnol. Agron. société Environ. ;11. (2007).
Brahmi, F. et al. Chemical composition and in vitro antimicrobial, insecticidal and antioxidant activities of the essential oils of Mentha pulegium L. and Mentha rotundifolia (L.) huds growing in Algeria. Ind. Crops Prod. 88, 96–105 (2016).
Benabdallah, A. et al. Chemical composition, antioxidant activity and acetylcholinesterase inhibitory of wild Mentha species from northeastern Algeria. South. Afr. J. Bot. 116, 131–139 (2018).
Aouadi, G. et al. Chemical investigations on Algerian Mentha rotundifolia and Myrtus communis essential oils and assessment of their insecticidal and antifungal activities. Int. J. Agric. Biol. 26, 667–680 (2021).
Riahi, L. et al. Phytochemistry, antioxidant and antimicrobial activities of the essential oils of Mentha rotundifolia L. in Tunisia. Ind. Crops Prod. 49, 883–889 (2013).
Leblalta, A., Harzallah, D., Demirtas, I. & Gül, F. Insecticidal activity of Mentha rotundifolia essential oil against Rhopalosiphum padi and Sitobion avenae (Hemiptera: Aphididae), cereal aphids in Setif, Algeria. Arch. Phytopathol. Plant. Prot. 53, 793–805 (2020).
Couplan, F. The Vegetable Treat: Edible Wild Plants (Vol. 1). (2009).
Kim, J-H., Kang, W-S. & Yun, S-C. Development of a model to predict the primary infection date of bacterial spot (Xanthomonas campestris Pv. Vesicatoria) on Hot Pepper. Plant. Pathol. J. 30, 125–135 (2014).
Dokuparthi, S. K. & Manikanta, P. Phytochemical and pharmacological studies on Chrysanthemum coronarium L.: a review. J. Drug Discov Ther. 3, 11–16 (2015).
Pukalskas, A., Venskutonis, P. R., Dijkgraaf, I. & van Beek, T. A. Isolation, identification and activity of natural antioxidants from costmary (Chrysanthemum balsamita) cultivated in Lithuania. Food Chem. 122, 804–811 (2010).
Haouas, D., Guido, F., Monia, B. H. K. & Habib, B. H. M. Identification of an insecticidal polyacetylene derivative from Chrysanthemum macrotum leaves. Ind. Crops Prod. 34, 1128–1134 (2011).
Haouas, D., Cioni, P. L., Ben Halima-Kamel, M., Flamini, G. & Ben Hamouda, M. H. Chemical composition and bioactivities of three Chrysanthemum essential oils against Tribolium confusum (du Val) (Coleoptera: Tenebrionidae). J Pest Sci 2012;85:367–79. (2004).
Djilali, B. et al. Kinetic of batch production of lactic acid from carob pods syrup. Bull. Pharm. Res. 7, 1–6 (2017).
Danilchuk, Y. V. Selective crystallization of maltose by isopropanol and acetone from glucose-maltose syrups. Banat J. Biotechnol. 7, 120 (2016).
Abbott, W. S. A method of computing the effectiveness of an insecticide. J. econ. Entomol. 18, 265–267 (1925).
Finney, M. A. & Ogden, U. T. FARSITE: Fire area simulator—model development and evaluation. Ogden UT: US Department of Agriculture; (1998).
Kasrati, A. et al. Chemical characterization and Insecticidal properties of essential oils from different wild populations of Mentha suaveolens subsp. timija (Briq.) Harley from Morocco. Chem. Biodivers. 12, 823–831 (2015).
Hosni, K., Hassen, I., Sebei, H. & Casabianca, H. Secondary metabolites from Chrysanthemum coronarium (Garland) flowerheads: Chemical composition and biological activities. Ind. Crops Prod. 44, 263–271 (2013).
Polatoğlu, K., Karakoç, Ö. C., Demirci, B. & Başer, K. H. C. Chemical composition and insecticidal activity of edible garland (Chrysanthemum coronarium L.) essential oil against the granary pest Sitophilus granarius L. (Coleoptera). J. Essent. Oil Res. 30, 120–130 (2018).
Basta, A., Pavlović, M., Couladis, M. & Tzakou, O. Essential oil composition of the flowerheads of Chrysanthemum coronarium L. from Greece. Flavour. Fragr. J. 22, 197–200 (2007).
Kumar Sharma, P., Singh, V. & Ali, M. Chemical composition and antimicrobial activity of Fresh Rhizome essential oil of Zingiber Officinale Roscoe. Pharmacogn J. 8, 185–190 (2016).
Mehalaine, S. & Chenchouni, H. Quantifying how climatic factors influence essential oil yield in wild-growing plants. Arab. J. Geosci. 14, 1257 (2021).
Haddouchi, F. et al. Chemical composition and antimicrobial activity of the essential oils from four Ruta species growing in Algeria. Food Chem. 141, 253–258 (2013).
Bouyahya, A. et al. Résistance aux antibiotiques et mécanismes d’action des huiles essentielles contre les bactéries. Phytothérapie 16, 173–183 (2017).
Madhumita, M., Guha, P. & Nag, A. Extraction of betel leaves (Piper betle L.) essential oil and its bio-actives identification: process optimization, GC-MS analysis and anti-microbial activity. Ind. Crops Prod. 138, 111578 (2019).
Lorenzo, D. et al. Essential oils of Mentha pulegium and Mentha rotundifolia from Uruguay. Brazilian Arch. Biol. Technol. 45, 519–524 (2002).
El Arch, M. et al. Composition chimique et activités antimicrobienne et insecticide de l’huile essentielle de Mentha rotundifolia du Maroc. Acta Bot. Gall. 150, 267–274 (2003).
Yakhlef, G., Hambaba, L., Pinto, D. C. G. A. & Silva, A. M. S. Chemical composition and insecticidal, repellent and antifungal activities of essential oil of Mentha rotundifolia (L.) from Algeria. Ind. Crops Prod. 158, 112988 (2020).
Ainane, A. et al. Behaviour desorption study of the essential oil of Cedrus Atlantica in a porous clay versus insecticidal activity against Sitophilus granarius: explanation of the phenomenon by statistical studies. Int. J. Metrol. Qual. Eng. 12, 12 (2021).
Tawaha, K. & Hudaib, M. Volatile oil profiles of the aerial parts of Jordanian garland, Chrysanthemum coronarium. Pharm. Biol. 48, 1108–1114 (2010).
Zheng, C. H., Kim, T. H., Kim, K. H., Leem, Y. H. & Lee, H. J. Characterization of potent aroma compounds in Chrysanthemum coronarium L. (Garland) using aroma extract dilution analysis. Flavour. Fragr. J. 19, 401–405 (2004).
Ghasemi Pirbalouti, A., Nourafcan, H. & Solyamani-Babadi, E. Variation in Chemical composition and antibacterial activity of essential oils from Bakhtiari Savory (Satureja Bachtiarica Bunge). J. Essent. Oil Bear. Plants. 20, 474–484 (2017).
Sanli, A., Karadogan, T., Tosun, B. & Erbas, S. Variation of Chemical composition of essential oils in wild populations of Ferulago cassia Boiss. From Turkey. J. Essent. Oil Bear. Plants. 23, 1386–1394 (2020).
De Groot, A. C. & Schmidt, E. Essential oils, part III: chemical composition. Dermatitis 27, 161–169 (2016).
Isman, M. B. Botanical insecticides in the twenty-First Century—Fulfilling their Promise? Annu. Rev. Entomol. 65, 233–249 (2020).
SoaresMA et al. Botanical insecticide and natural enemies: a potential combination for pest management against Tuta absoluta. J. Pest Sci. (2004). 92, 1433–1443 (2019).
Piri, A. et al. Toxicity and physiological effects of ajwain (Carum copticum, Apiaceae) essential oil and its major constituents against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Chemosphere 256, 127103 (2020).
Ngongang, M. D. T. et al. Chemical constituents of essential oils from Thymus vulgaris and Cymbopogon citratus and their insecticidal potential against the tomato borer, Tuta absoluta (Lepidoptera: Gelechiidae). Int. J. Trop. Insect Sci. 42, 31–43 (2022).
Benchouikh, A., Allam, T., Kribii, A. & Ounine, K. The study of the insecticide effect of the essential oil of Syzygium aromaticum L. against larvae of Tuta absoluta. Int. J. Innov. Sci. Res. 20, 188–194 (2016).
Campolo, O. et al. Citrus peel essential oil nanoformulations to control the tomato borer, Tuta absoluta: chemical properties and biological activity. Sci. Rep. 7, 13036 (2017).
Mihaylova-Garnizova, R., Davidova, S., Hodzhev, Y. & Satchanska, G. Antimicrobial peptides derived from Bacteria: classification, sources, and mechanism of action against Multidrug-resistant Bacteria. Int. J. Mol. Sci. 25 (19), 10788. https://doi.org/10.3390/ijms251910788 (2024).
Sergeiko, A. et al. Cyclobutane-containing alkaloids: origin, synthesis, and biological activities. Open Med. Chem. J. 2, 26 (2008).
Mansour, R. & Biondi, A. Releasing natural enemies and applying microbial and botanical pesticides for managing Tuta absoluta in the MENA region. Phytoparasitica 49, 179–194 (2021).
Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: a review. Ind. Crops Prod. 76, 174–187 (2015).
Soares, J. C., Santos, C. S., Carvalho, S. M. P., Pintado, M. M. & Vasconcelos, M. W. Preserving the nutritional quality of crop plants under a changing climate: importance and strategies. Plant. Soil. 443, 1–26 (2019).
Campolo, O., Giunti, G., Laigle, M., Michel, T. & Palmeri, V. Essential oil-based nano-emulsions: Effect of different surfactants, sonication and plant species on physicochemical characteristics. Ind. Crops Prod. 157, 112935 (2020).
Acknowledgements
A grant from the Ministry of High Education and Scientific Research of Algeria supported this work. The authors are thankful to Dr. Sekkal Fatima for identifying the plant studied and other people who contributed to the realization of this work. The authors extend their appreciation to the Researchers Supporting Project number (RSP2025R98), King Saud University, Riyadh, Saudi Arabia, for financial support.
Funding
The authors extend their appreciation to the Researchers Supporting Project number (RSP2025R98), King Saud University, Riyadh, Saudi Arabia, for financial support.
Author information
Authors and Affiliations
Contributions
K.F.; B.M.; B.F.; contributed to the conceptualization and design of the study, K.F.; B.M.; B.F.; data collection; K.F.; B.M.; B.F.; analysis, K.F.; B.M.; B.F.; interpretation. T.N.; M.A.; S.D.; S.A.A.; A.A.A.; M.J.A.; contributed to the statistical analysis; T.N.; M.A.; S.D.; S.A.A.; A.A.A.; M.J.A.; interpretation of the data. All authors have reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Not Applicable.
Ethics approval and consent to participate
We all declare that manuscript reporting studies do not involve any human participants, human data, or human tissue. So, it is not applicable. Study protocol must comply with relevant institutional, national, and international guidelines and legislation. Our experiment follows the with relevant institutional, national, and international guidelines and legislation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Farial, K., Malika, B., Fouzia, B. et al. Chemical composition and insecticidal activity of Mentha rotundifolia and Chrysanthemum coronarium essential oils against the tomato leaf miner, Tuta absoluta. Sci Rep 15, 1411 (2025). https://doi.org/10.1038/s41598-025-85606-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85606-x
Keywords
This article is cited by
-
Current advances and prospects in integrated pest management of Phthorimaea absoluta
Discover Agriculture (2026)