Abstract
Patients with diabetes have a high risk of failure of H. pylori eradication therapy. The present study aims to evaluate the efficacy and safety of vonoprazan–amoxicillin (VA) dual therapy for the treatment of H. pylori infection in patients with type-2 diabetes mellitus (T2DM), and determine the influence of H. pylori eradication on the glycated hemoglobin A1C (A1C) level. The present prospective, single-center, single-arm, clinical trial enrolled 75 T2DM patients diagnosed with H. pylori infection. The patients were treated with the VA dual therapy regimen, which comprised of vonoprazan (20 mg, twice daily) and amoxicillin (750 mg, thrice daily), for 14 days (14-day VA dual therapy). The eradication rate in the intention-to-treat analysis and per-protocol analysis was 84.00% (63/75) and 87.14% (61/70), respectively. The multivariate analysis revealed that the independent risk factors for H. pylori eradication failure were smoking (OR: 4.59, 95% CI: 1.20–17.58, p = 0.026) and elevated A1C level (OR: 1.65, 95% CI: 1.01–2.68, p = 0.044). Patients in the successful eradication group presented with a significant decrease in the A1C level at 3 months, post-treatment, when compared to the pre-eradication level (7.70 ± 1.05% vs. 7.23 ± 1.00%, p = 0.006). VA dual therapy is a safe and effective regimen for patients with T2DM.
Similar content being viewed by others
Introduction
Helicobacter pylori (H. pylori) is a highly prevalent chronic infection classified by the World Health Organization as a Group 1 carcinogen1,2,3. The role of H. pylori in the causation of gastritis, gastroduodenal ulcers, and gastric cancer has been well-documented. In addition, H. pylori infection is associated to other systemic diseases, such as diabetes. For example, it was identified that patients with type-2 diabetes mellitus (T2DM) have a higher prevalence of H. pylori infection4,5. In addition, H. pylori infection has been identified as a risk factor for the development of nephropathy and cardiovascular complications in patients with diabetes6,7,8. Glycated hemoglobin A1C (A1C) is a serological marker of glycemic control, which is closely associated to outcomes in diabetic patients. In some studies, it was found that H. pylori eradication in T2DM patients can improve insulin resistance, and reduce A1C levels9. However, a research revealed that H. pylori eradication in patients with T2DM has no role in glycemic control10. At present, there is a lack of consensus on the effect of H. pylori eradication on A1C levels, and this relationship needs to be further explored.
The Maastricht VI/Florence Consensus reports, and the Sixth Chinese Consensus on H. pylori infection recommends the 14-day bismuth quadruple therapy as a first-line treatment in areas with a high prevalence of clarithromycin resistance (> 15%)11,12. However, these quadruple therapy regimens have some disadvantages, including high cost, numerous side effects, and poor treatment compliance due to the use of numerous drugs. In addition, previous studies reported that bismuth-containing quadruple therapies achieved a low H. pylori eradication rate in T2DM patients (38.20–81.00%)13,14,15. Diabetes patients are often prescribed multiple drugs to achieve glycemic control, and treat diabetes complications. The bismuth quadruple therapy regimen contains bismuth, a proton pump inhibitor (PPI), and two antibiotics. The intake of various drugs may lead to adverse reactions, reduce compliance, and may lead to confusion on the dose and timing of various drugs, resulting in poor compliance to the prescribed treatment. All these factors are liable to impact the efficacy of H. pylori eradication. According to a meta-analysis, T2DM patients have a higher risk of H. pylori eradication failure, when compared to non-diabetic patients (odds ratio [OR]: 2.59, 95% confidence interval [CI]: 1.82–3.70)9. Therefore, developing a safe and effective H. pylori eradication regimen for diabetic patients is of great clinical significance.
In recent clinical studies, the eradication rate for vonoprazan (VPZ)-amoxicillin (3 g/d) dual therapy (VA dual therapy), as the first-line treatment, was approximately 89.50–94.60%16,17,18, showing acceptable efficacy and safety. In our previous study, the VPZ-based regimen (amoxicillin 750 mg, three times daily) was identified as a safe and effective rescue therapy (eradication rate: 92.60%)19. Thus, VA dual therapy is an extremely promising therapeutic strategy for H. pylori eradication, due to simplicity, good efficacy, fewer side effects, and reduced amount of medication used. Nevertheless, all VA dual therapies have been conducted in the general population, and no prospective trials have evaluated the efficacy of the VA dual regimen in diabetic patients.
The present study aimed to assess the effectiveness and safety of VA dual therapy in T2DM patients, and determine the potential factors that influence the eradication rates. Furthermore, subgroup analyses were conducted to determine the impact of H. pylori eradication on A1C levels.
Methods
Study design
The present prospective, single-center, single-arm clinical trial was conducted in Changzhou No. 2 People’s Hospital, between April 2023 and December 2023. The ethics clearance for the present pilot study was obtained from Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University (No. [2022] YLJSA056). The present study adhered to the principles enshrined in the Declaration of Helsinki, and was registered in the Chinese Clinical Trial Registry (No. ChiCTR2300070231) in 06/04/2023.
Study population
T2DM patients diagnosed with H. pylori infection were eligible for inclusion in the present trial. The diagnosis of H. pylori infection was confirmed by rapid urease test or 13C-urea breath test (UBT). The exclusion criteria were, as follows: (1) patients who underwent prior H. pylori eradication therapy; (2) patients allergic to amoxicillin or VPZ; (3) patients who were treated with PPI, VPZ, bismuth, or antibiotics within four weeks preceding the study; (4) patients with acute upper gastrointestinal bleeding and history of gastric surgery; (5) pregnant or lactating women; (6) patients who did not provide an informed consent; (7) patients who changed their hypoglycemic regimen during the treatment and follow-up period; (8) patients with severe comorbid conditions, such as hepatic or renal dysfunction.
A written informed consent was obtained from all patients before enrollment. Data on the following demographic and clinical characteristics were obtained by face-to-face interviews, and electronic medical records: age, gender, height, weight, body mass index (BMI), body surface area (BSA), history of smoking and alcohol consumption, duration of diabetes, glycemic control regimen, A1C level, gastroscopy findings, and comorbidities.
Management of diabetes mellitus
The diagnosis of T2DM was based on the guidelines for the prevention and treatment of T2DM in China (2020 Edition). For newly diagnosed diabetes patients, lifestyle management (medical nutrition therapy and exercise therapy) was the primary treatment measure to achieve glycemic control. Patients who received any of the following oral hypoglycemic drugs (alone or in combination) were assigned to the oral hypoglycemic drug group: metformin, insulin secretomotor, α-glucosidase inhibitor, thiazolidinedione (TZD), dipeptidyl peptidase IV inhibitor (DPP-4i), sodium-glucose co-transporter 2 inhibitor (SGLT2i), or glucagon-like peptide-1 receptor agonist (GLP-1RA). Patients who received insulin injection in divided doses, or as a continuous subcutaneous insulin infusion were assigned to the insulin group. Patients who received a combination of oral hypoglycemic drugs and insulin were assigned to the combined group. The aforementioned treatment plans were individualized based on the condition of the patient. Blood glucose levels were monitored regularly during the treatment period to prevent hypoglycemia.
Intervention and follow-up
All subjects were treated with the VA dual regimen, which contained VPZ (20 mg, twice daily; Takeda Pharmaceutical, Tokyo, Japan) and amoxicillin (750 mg, thrice daily; Federal Pharmaceutical, Hong Kong, China), for 14 days (14-day VA dual therapy). VPZ was taken before meals, while amoxicillin was taken after meals. In case of any discomfort during treatment, these patients were instructed to immediately contact the investigators for further assistance. At least four weeks after treatment, the H. pylori status was confirmed using the 13C-UBT. An δ value that exceeded the baseline value of 4 was considered indicative of positive H. pylori status, while an δ value below the baseline value of 4 was considered indicative of negative H. pylori status. The A1C levels were again measured at approximately three months, post-treatment.
All patients were required to respond to a questionnaire within three days after completion of the eradication therapy. Adverse events (AEs) were assessed based on the questionnaire responses. Patient compliance was assessed based on the medication diaries maintained by the patients. The severity of AEs was classified, as follows: none, mild (causing discomfort, but not interfering with daily life), moderate (causing discomfort, and interfering with daily life), and severe (causing discomfort, necessitating treatment cessation). The intake of at least 80% of the prescribed medicine was defined as good compliance.
Outcomes
The primary outcome measure for the present study was the H. pylori eradication rate. All patients were included in the intention-to-treat (ITT) analysis. Patients who did not undergo the 13C-UBT during the follow-up period were classified as cases of treatment failure in the ITT analysis. Patients who were lost to follow-up, or patients with poor treatment compliance were excluded from the per-protocol (PP) analysis. The secondary endpoints were, as follows: rate of AEs, patient compliance, and factors associated to eradication. Lastly, the effect of H. pylori eradication on A1C levels was examined.
Sample-size calculation and statistical analysis
In the present study, the H. pylori eradication rate for T2DM patients treated with the VA dual regimen was taken as the main evaluation index to calculate the sample size. The calculation formula was, as follows (where: n refers to the sample size, P0 refers to the reference eradication rate, and P1 refers to the expected eradication rate):
According to our previous research19, the reference eradication rate was P0 = 93.0% (n = 68), with a 95% CI of 86.60–98.80%. Furthermore, the expected upper limit eradication rate was P1 = 99.2%, the p-value (α) accepted as significant was 0.1, and the power (1 − β) was 0.8. According to the formula, the calculated sample size was 66 cases. Considering the dropout situation during the follow-up period, a 10% increase in sample size was needed. That is, at least 73 patients would be required. Categorical variables were presented in frequency (proportion), and between-group differences were assessed for statistical significance using Pearson’s chi-square test or Fisher’s exact test. Continuous variables were tested for normal distribution. If the data conformed to the normal distribution, the data was expressed in mean ± standard deviation (SD), the differences between groups were determined by independent samples test, and the differences within groups were determined by paired samples t-test. If the data did not conform to the normal distribution, the data was expressed in M (P25, P75), between-group comparisons were conducted using the Mann–Whitney U-test, and within-group comparisons were conducted using the Wilcoxon signed-rank test. In order to assess the risk factors for H. pylori eradication failure, binary logistic regression analysis was used to estimate the ORs and 95% CIs. A p-value of < 0.05 were considered statistically significant. The statistical analysis was performed using the Statistical Package for the Social Sciences v26.0 (IBM, Armonk, NY, USA).
Results
Patient characteristics
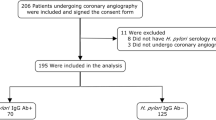
A total of 89 patients were screened for eligibility. Among these patients, 14 patients did not meet the inclusion criteria. Finally, 75 patients (44 male patients and 31 female patients) were enrolled. Among these patients, 58 patients underwent endoscopy, 39 patients consented to follow-up for the A1C level, and 37 patients underwent pre- and post-treatment liver and kidney function tests. Five patients were excluded from the PP analysis due to poor compliance (Fig. 1).
The mean age of these patients was 61.91 ± 9.37 years old. Forty patients (53.30%) had comorbid hypertension. Furthermore, smoking habits were reported by 21 patients (28.0%), and alcohol consumption was reported by 13 patients (17.3%). The median duration of T2DM was seven years. The mean A1C level at baseline was 7.79 ± 1.27%. Majority of the patients received oral hypoglycemic agents (n = 52, 69.3%). Among the 58 patients who underwent endoscopy, atrophic gastritis was diagnosed in 30 cases, peptic ulcer was diagnosed in 16 cases, and chronic non-atrophic gastritis was diagnosed in 12 cases (Table 1).
Eradication rate and factors that influence efficacy
The eradication rate in the ITT analysis and PP analysis was 84.0% (63/75) and 87.14% (61/70), respectively. Univariate and multivariate logistic regression were performed to identify the risk factors for H. pylori eradication (Table 2). The multivariate analysis revealed that the significant and independent risk factors for H. pylori eradication failure were smoking (OR: 4.59, 95% CI: 1.20–17.58, p = 0.026) and elevated A1C levels (OR: 1.65, 95% CI: 1.01–2.68, p = 0.044).
Adverse events, safety and compliance
The overall incidence of AEs in the present trial was 13.3% (10/75), and none of the patients developed any severe AEs. The main symptoms were, as follows: dry mouth (one case), nausea (two cases), rash (one case), abdominal pain (two cases), diarrhea (two cases), abdominal distension (one case), and fatigue (one case). Most of the AEs were mild and resolved, without additional intervention (Table 3). One patient experienced localized rash and pruritus on the upper limbs on day six, which subsequently improved with oral anti-allergic medication, and the treatment was not interrupted. Furthermore, one patient developed abdominal pain and one patient developed diarrhea on day two, and these patients refused to continue treatment. Their symptoms resolved after drug withdrawal. Moreover, 93.3% (70/75) of patients took at least 80% of all prescribed medications. None of the 37 patients who underwent liver and kidney function tests presented with any significant difference in pre- and post-treatment results (Table 4).
Effect of H. pylori eradication on the A1C level
After the three-month follow-up period, a significant decrease in A1C levels was observed (7.70 ± 1.05 vs. 7.23 ± 1.00, p = 0.006). The subgroup analysis revealed a significant decrease in A1C levels in the successful eradication group, when compared to the pre-treatment level (7.63 ± 1.11 vs. 7.23 ± 0.98, p = 0.026). The failed eradication group presented with no significant differences between the pre-treatment A1C levels and A1C level at three months after completion of treatment (8.06 ± 0.61 vs. 7.23 ± 1.16, p = 0.117).
Discussion
To the best of our knowledge, the present study was the first prospective study to investigate the efficacy and safety of the 14-day VA dual therapy for eradicating H. pylori in T2DM patients. In the present study, the 14-d VA regimen achieved a high eradication rate (87.14% in the PP analysis and 84.0% in the ITT analysis). In recent studies, the efficacy of triple or quadruple regimens based on PPIs was significantly lower in diabetic patients, when compared to their non-diabetic counterparts15,20,21. For instance, Vafaeimanesh et al.15 reported a low 63% eradication rate for diabetic patients who received the 14-day quadruple therapy regimen (20 mg of omeprazole, 500 mg of metronidazole, 1 g of amoxicillin, and 240 mg of bismuth citrate, twice daily). Similarly, Sargyn et al. reported a 50% eradication rate for diabetes patients who received the standard 10-day triple therapy regimen (500 mg of clarithromycin, 20 mg of omeprazole, 1 g of amoxicillin, twice a day)20. Several factors may have contributed to the low eradication rate in diabetic patients. A key factor that contributed to the low eradication rate was the increase in prevalence of antibiotic resistance in diabetic patients22. Diabetes patients typically have compromised immune responses, rendering them more susceptible to infections, resulting in frequent antibiotic usage, and the subsequent emergence of drug-resistant strains. Furthermore, diabetic microvascular disease compromises the gastric microvasculature, impairing blood circulation in the gastric mucosa. This may reduce the absorption of drugs through the gastric mucosa23. Moreover, the poor compliance of patients is a crucial factor that influences eradication. Most diabetic patients are prescribed multiple types of hypoglycemic drugs, which need to be adjusted based on glucose fluctuations. The concurrent use of multiple medications often leads to confusion on the dose and timing, affecting compliance. All these factors are liable to impact the efficacy of H. pylori eradication.
T2DM patients have significantly higher rates of clarithromycin resistance, when compared to non-diabetic patients13. Yao et al. reported a 50% prevalence rate of resistance for both clarithromycin and levofloxacin in diabetic patients, while the corresponding rate in non-diabetic patients was 17.10% and 22.90%, respectively. Clarithromycin resistance was identified as an independent risk factor for H. pylori eradication failure (OR: 25.472, p = 0.023)21. In contrast to the aforementioned antibiotics, amoxicillin has a consistent low primary and secondary resistance rate24. The bactericidal effect of amoxicillin is both pH- and time-dependent.
Traditional PPIs have a short half-life (0.5–2.0 h), and the metabolism is influenced by CYP2C19 gene polymorphism, resulting in a limited duration of acid inhibition. Compared to PPIs, VPZ (as the first potassium-competing acid blocker employed in clinical practice) causes a more rapid and sustained acid inhibition25. Therefore, VPZ-amoxicillin dual therapy is expected to be more effective in eradicating H. pylori in T2DM patients.
In the present study, the multivariate analysis revealed that elevated A1C level (OR: 1.65, 95% CI: 1.01–2.68, p = 0.044) is an independent risk factor for H. pylori eradication failure, suggesting that T2DM patients with poor glycemic control are more likely to fail in achieving eradication. In the study conducted by Nam et al.26 the A1C level of ≥ 7.5% was a risk factor for failure to achieve H. pylori eradication. This was likely attributable to the detrimental impact of hyperglycemia on innate immune responses to infection, immunoglobulin-mediated bacterial opsonization and complement fixation to bacteria, and phagocytosis27. In addition, hyperglycemia can impair endothelial function, leading to damage to the gastric mucosal microvasculature, and the subsequently reduced absorption of antibiotics23. Therefore, optimizing glycemic control is the key to achieve H. pylori eradication in T2DM patients.
In addition, smoking is a risk factor for failure of H. pylori eradication in T2DM patients. Smoking is known to decrease gastric blood flow and mucus secretion, which can reduce the delivery of antibiotics to the gastric mucosa28. Furthermore, compared to non-smokers, long-term heavy smokers exhibit elevated pentapeptide secretion, leading to increased gastric acid secretion29. Consequently, the effectiveness of amoxicillin, which is an acid-sensitive antibiotic, may be diminished in smokers.
In the present study, the three-month follow-up assessment of A1C levels after eradication revealed a significant reduction in A1C levels in patients who achieved successful eradication. Although all included patients did not change their hypoglycemic regimen during the treatment and follow-up period, merely 39 patients consented to follow-up for the A1C level, and merely six patients failed to be eradicated. In addition, the confounding influence of factors that could affect A1C levels (e.g. diet, exercise, and drug compliance) were not analyzed in the study. Thus, the presented data was not adequate to comment on the impact of H. pylori eradication on the change in A1C level.
Some limitations of the present study should be acknowledged. First, the present study was a prospective single-center, single-arm study. Larger, multicenter, randomized, controlled trials that include non-diabetic control subjects are required to obtain more robust evidence. Second, no antibiotic susceptibility tests were performed for the present study. Therefore, the influence of different antibiotic resistance on the eradication rate of the 14-day VA dual therapy could not be evaluated. Third, the bilirubin levels increased at post-treatment, when compared to pre-treatment, although the difference was not statistically significant. When using dual therapy for H. pylori infection in diabetes patients, more attention should be given in monitoring the bilirubin level. Fourth, no amoxicillin absorption test was performed to support the VA dual therapy. Amoxicillin is mainly absorbed by the gastrointestinal tract. Compared to non-diabetic patients, it remains unclear whether there are differences in amoxicillin absorption in diabetic patients. Research would be conducted with this regard in the future. Finally, the confounding influence of factors that can affect the A1C levels (e.g. diet, exercise, and medications) was not analyzed in the study. Thus, future large-scale, multicenter prospective studies should account for these confounding factors in the statistical analysis to confirm the present results.
Conclusion
In conclusion, the present study revealed the efficacy and safety of the 14-day VA dual therapy for eradicating H. pylori in T2DM patients. T2DM patients should abstain from smoking, and achieve optimal glycemic control to increase the eradication rate of H. pylori.
Data availability
The data sets supporting the results of the current research are available from the corresponding authors upon request.
References
Guevara, B. & Cogdill, A. G. Helicobacter pylori: A review of current diagnostic and management strategies. Dig. Dis. Sci. 65, 1917–1931. https://doi.org/10.1007/s10620-020-06193-7 (2020).
Hooi, J. K. Y. et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 153, 420–429. https://doi.org/10.1053/j.gastro.2017.04.022 (2017).
Zeng, R., Gou, H., Lau, H. C. H. & Yu, J. Stomach microbiota in gastric cancer development and clinical implications. Gut https://doi.org/10.1136/gutjnl-2024-332815 (2024).
Han, X. et al. Helicobacter pylori infection is associated with type 2 diabetes among a middle- and old-age Chinese population. Diabetes Metab. Res. Rev. 32, 95–101. https://doi.org/10.1002/dmrr.2677 (2016).
Kayar, Y. et al. Relationship between Helicobacter pylori infections in diabetic patients and inflammations, metabolic syndrome, and complications. Int. J. Chronic Dis. 2015, 290128. https://doi.org/10.1155/2015/290128 (2015).
Shi, Y. et al. Helicobacter pylori infection is associated with occurrence of proteinuria in type 2 diabetes patients: A systemic review and meta-analysis. Chin. Med. J. (Engl) 131, 2734–2740. https://doi.org/10.4103/0366-6999.245269 (2018).
Wang, F., Fu, Y. & Lv, Z. Association of Helicobacter pylori infection with diabetic complications: A meta-analysis. Endocr. Res. 39, 7–12. https://doi.org/10.3109/07435800.2013.794426 (2014).
Yang, Y. F. et al. Relation of Helicobacter pylori infection to peripheral arterial stiffness and 10-year cardiovascular risk in subjects with diabetes mellitus. Diab. Vasc Dis. Res. 17, 1479164120953626. https://doi.org/10.1177/1479164120953626 (2020).
Song, X., Cai, C., Jin, Q., Chen, X. & Yu, C. The efficacy of Helicobacter pylori eradication in diabetics and its effect on glycemic control: A systematic review and meta-analysis. Helicobacter 26, e12781. https://doi.org/10.1111/hel.12781 (2021).
Vafaeimanesh, J. et al. Effect of Helicobacter pylori eradication on glycaemia control in patients with type 2 diabetes mellitus and comparison of two therapeutic regimens. Arab J. Gastroenterol. 14(2), 55–8. https://doi.org/10.1016/j.ajg.2013.03.002 (2013).
Malfertheiner, P. et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut https://doi.org/10.1136/gutjnl-2022-327745 (2022).
Zhou, L. et al. 2022 Chinese national clinical practice guideline on Helicobacter pylori eradication treatment. Chin. Med. J. (Engl) 135, 2899–2910. https://doi.org/10.1097/cm9.0000000000002546 (2022).
Demir, M., Göktürk, S., Oztürk, N. A., Serin, E. & Yilmaz, U. Bismuth-based first-line therapy for Helicobacter pylori eradication in type 2 diabetes mellitus patients. Digestion 82, 47–53. https://doi.org/10.1159/000236024 (2010).
Maev, I. V., Mkrtumyan, A. M., Bektemirova, L. G., Andreev, D. N. & Dicheva, D. T. The effectiveness of eradication therapy of the 1st line of Helicobacter pylori infection in patients with type 2 diabetes mellitus. Ter. Arkh. 94, 209–215. https://doi.org/10.26442/00403660.2022.02.201372 (2022).
Vafaeimanesh, J. et al. Effect of Helicobacter pylori eradication on glycaemia control in patients with type 2 diabetes mellitus and comparison of two therapeutic regimens. Arab J. Gastroenterol. 14, 55–58. https://doi.org/10.1016/j.ajg.2013.03.002 (2013).
Peng, X. et al. Combination of vonoprazan and amoxicillin as the first-line Helicobacter pylori eradication therapy: A multicenter, prospective, randomized, parallel-controlled study. Clin. Exp. Med. 23, 4011–4019. https://doi.org/10.1007/s10238-023-01074-5 (2023).
Peng, X. et al. Efficacy and safety of vonoprazan–amoxicillin dual regimen with varying dose and duration for Helicobacter pylori eradication: A multicenter, prospective, randomized study. Clin. Gastroenterol. Hepatol. 22, 1210–1216. https://doi.org/10.1016/j.cgh.2024.01.022 (2024).
Wang, X., Teng, G., Dong, X., Dai, Y. & Wang, W. Efficacy and safety of vonoprazan–amoxicillin dual therapy for Helicobacter pylori first-line treatment: A single-center, randomized, controlled trial. Therap. Adv. Gastroenterol. 16, 17562848231190976. https://doi.org/10.1177/17562848231190976 (2023).
Yu, J. et al. Safety and effectiveness of vonoprazan-based rescue therapy for Helicobacter pylori infection. World J. Gastroenterol. 29, 3133–3144. https://doi.org/10.3748/wjg.v29.i20.3133 (2023).
Sargýn, M. et al. Type 2 diabetes mellitus affects eradication rate of Helicobacter pylori. World J. Gastroenterol. 9, 1126–1128. https://doi.org/10.3748/wjg.v9.i5.1126 (2003).
Yao, C. C. et al. First-line Helicobacter pylori eradication rates are significantly lower in patients with than those without type 2 diabetes mellitus. Infect. Drug Resist. 12, 1425–1431. https://doi.org/10.2147/idr.S194584 (2019).
Megraud, F. et al. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut 70, 1815–1822. https://doi.org/10.1136/gutjnl-2021-324032 (2021).
Chawla, A., Chawla, R. & Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum?. Indian J. Endocrinol. Metab. 20, 546–551. https://doi.org/10.4103/2230-8210.183480 (2016).
Liu, D. S. et al. Primary antibiotic resistance of Helicobacter pylori in Chinese patients: A multiregion prospective 7-year study. Clin. Microbiol. Infect. 24(780), e785-780.e788. https://doi.org/10.1016/j.cmi.2017.11.010 (2018).
Zhuang, Q. et al. Comparative efficacy of P-CAB vs proton pump inhibitors for grade C/D esophagitis: A systematic review and network meta-analysis. Am. J. Gastroenterol. 119, 803–813. https://doi.org/10.14309/ajg.0000000000002714 (2024).
Nam, S. J. et al. Helicobacter pylori eradication in patients with type 2 diabetes mellitus: Multicenter prospective observational study. SAGE Open Med. 7, 2050312119832093. https://doi.org/10.1177/2050312119832093 (2019).
Jafar, N., Edriss, H. & Nugent, K. The effect of short-term hyperglycemia on the innate immune system. Am. J. Med. Sci. 351, 201–211. https://doi.org/10.1016/j.amjms.2015.11.011 (2016).
Endoh, K., Kauffman, G. L. Jr. & Leung, F. W. Mechanism of aggravation of mucosal injury by intravenous nicotine in rat stomach. Am. J. Physiol. 261, G1037-1042. https://doi.org/10.1152/ajpgi.1991.261.6.G1037 (1991).
Parente, F., Lazzaroni, M., Sangaletti, O., Baroni, S. & Bianchi Porro, G. Cigarette smoking, gastric acid secretion, and serum pepsinogen I concentrations in duodenal ulcer patients. Gut 26, 1327–1332. https://doi.org/10.1136/gut.26.12.1327 (1985).
Funding
The study was supported by The Top Talent of Changzhou “The 14th Five-Year Plan” High-Level Health Talents Training Project (No. 2022CZBJ051), The Clinical Research Project of Changzhou Medical Center of Nanjing Medical University (No. CMCC202309), and The Science and Technology Project of Changzhou Health Commission (No. ZD202336).
Author information
Authors and Affiliations
Contributions
JZ and XC: acquisition of data, analysis and interpretation of data, and drafting of the manuscript; KM, YJ and XQ: interpretation of data and revision of the manuscript; XW: conception and design of the study, critical revision, acquisition of data, analysis and interpretation of data, and drafting of the manuscript. All authors had reviewed, edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, J., Cao, X., Ma, K. et al. Safety and effectiveness of dual therapy for Helicobacter pylori infection and the effect on the glycated hemoglobin level in type 2 diabetes. Sci Rep 15, 1537 (2025). https://doi.org/10.1038/s41598-025-85628-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85628-5