Abstract
We assessed the diagnostic performance of the Narrow-Band Imaging (NBI) International Colorectal Endoscopic Classification (NICE) and the Japan NBI Expert Team classification (JNET) in predicting histological outcomes of advanced colorectal lesions. Additionally, we evaluated the sensitivity and positive predictive value (PPV) of the JNET and NICE classifications individually for high-grade lesions (including HGD adenomas, intramucosal carcinomas, and T1 carcinomas). This was a retrospective analysis of prospectively collected data, involving 211 patients (130 men, mean age 60 years) who underwent colonoscopy with endoscopic resection of advanced colorectal neoplasia (lesions ≥ 10 mm). Lesions were classified using both NICE and JNET criteria, and final histopathological results were used for comparison. Of the 257 lesions analyzed, the NICE classification accurately classifies a large proportion of lesions (93.8%). In JNET classification we observed 77.4% correctly classified lesions. Specifically, the sensitivity and positive predictive value (PPV) of the NICE classification for high-grade lesions were 100% and 24.4%, respectively. For the JNET classification, the sensitivity and PPV for high-grade lesions were 56.6% and 57.7%, respectively. The JNET classification, with a positive predictive value of 57.7% for high-grade colorectal lesions (including HGD adenomas, intramucosal carcinomas, and T1 carcinomas), should be used for decision-making regarding appropriate subsequent endoscopic therapy.
Similar content being viewed by others
Introduction
Colorectal cancer is the most common malignant tumor of the digestive system. It ranks third among the most common overall malignant tumors in both sexes, with an incidence of 1,926,118 and a mortality of 903,859 worldwide in the year 20221. Colonoscopy with polypectomy has been successful in preventing the occurrence of colorectal cancer and reducing mortality from this disease2. With the expansion of CRC screening, the finding of early colorectal neoplasia, including early colorectal cancer with superficial submucosal invasion, is being detected more frequently, and these can be effectively treated through endoscopic resection. Accurately characterising colorectal lesions using endoscopic imaging is crucial for appropriate management and treatment decisions. To avoid tissue scarring, real-time optical diagnosis using virtual chromoendoscopy is preferred over lesion biopsies. Accurate histological prediction enables determination of the optimal endoscopic technique for lesion resection, aiming for complete histological resection (R0 resection)3.
Therefore, over the last two decades, numerous techniques of virtual chromoendoscopy have been developed, eliminating the need for direct tissue colouring. These techniques rely on optical filters and digital image processing to replicate the appearance of classical chromoendoscopic images. Alongside the well-established technique called narrow band imaging (NBI)4, several other virtual chromoendoscopy methods have emerged, such as Blue Laser Imaging (BLI)5, Linked Colour Imaging (LCI)6, i-scan7, and Flexible spectral imaging colour enhancement (FICE)8.
These techniques play a vital role in assisting endoscopists in detecting and characterising lesions more accurately, thereby improving the diagnosis and treatment decisions for gastrointestinal lesions. However, it should be noted that the availability of these methods may vary depending on the endoscopy equipment and medical facilities. Among the various methods of virtual chromoendoscopy, NBI is one of the most widely used9. Studies have suggested that NBI technology is comparable to chromoendoscopy in differentiating the gross type of colorectal lesions10,11. To provide a standardised and systematic approach for the endoscopic assessment and characterisation of colorectal lesions observed using NBI, two widely used endoscopic classifications have been developed by expert groups: the NBI International Colorectal Endoscopic (NICE) classification for non-magnifying endoscopy in 200912, and the Japan NBI Expert Team (JNET) classification for magnifying endoscopy in 20149. These classifications aim to assist endoscopists in predicting the histological characteristics and assessing the risk of submucosal invasion in colorectal lesions. A three-point NICE classification is simpler for initial endoscopic assessment. A four-point JNET classification has been expected to enable proper decision making for expanded indications for endoscopic therapy of early high-grade colorectal neoplasia (up to T1 colorectal cancer). The primary objective of this study was to determine the diagnostic impact and applicability of the NICE and JNET classifications for clinical practice in predicting histology outcomes. Additionally, we evaluated the sensitivity and positive predictive value (PPV) of the JNET and NICE classifications individually for high-grade lesions (including HGD adenomas, intramucosal carcinomas, and T1 carcinomas)13.
Materials and methods
Patients and endoscopy
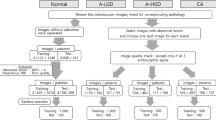
This study involved a retrospective analysis of data prospectively collected between 2017 and 2021 as part of studies registered in ClinicalTrials.gov under registration numbers NCT03434925 and NCT05929365. Data were gathered from 211 consecutive patients aged 18–75 years (130 men [62%] and 81 women [38%], with a median age of 60 years). Each patient underwent colonoscopy during the study period, which included the resection of advanced colorectal neoplasia, defined as lesions larger than 10 mm. White-light endoscopy, NBI imaging and magnifying NBI imaging were conducted simultaneously in the same tertiary endoscopic unit at the Military University Hospital in Prague, Czech Republic. Patients with inflammatory bowel disease, familial adenomatous polyposis, or incomplete clinical data were not included in the study.
After standard bowel preparation, six experienced endoscopists performed a complete colonoscopy using a high-definition colonoscope (CF-HQ190L, CF-H180AL, CF-HQ180L, CF-HQ190AL, CF-H190L, PCF-H190TL) and a standard videoendoscopic system (Olympus EXERA III, Tokyo, Japan). For each lesion, endoscopic images were captured and evaluated in the following sequence: conventional white-light endoscopy, non-magnifying NBI and magnifying NBI. The NICE and JNET assessments were performed for each lesion simultaneously in real-time, without prior knowledge of histology.
The size of each lesion was estimated using the open-biopsy forceps method, which involved measuring the open diameter of 7 mm with Radial Jaw 3 biopsy forceps from Boston Scientific Corp., Natick, MA, United States. The location of the lesions was categorised into two groups: (1) the right colon (cecum, ascending colon, and transverse colon) and (2) the left colon (descending colon, sigmoid colon, and rectum). Lesions were removed using various techniques using ERBE electrosurgical unit (Germany), including polypectomy (cold snare or hot snare electrocautery (Olympus, Tokyo, Japan), endoscopic mucosal resection (piecemeal or en-bloc), endoscopic submucosal dissection (ESD knife, Olympus, Tokyo, Japan), or endoscopic full-thickness resection (FTR, Ovesco Endoscopy, Germany).
Endoscopic evaluation using NICE and JNET classification
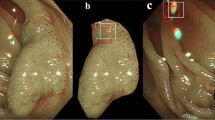
The NICE classification, proposed by the Colon Tumor NBI Interest Group is based on non-magnifying NBI observations14,15. According to the NICE classification, colorectal neoplasia is divided into three categories (Fig. 1) based on three characteristics: the colour of lesions, vascular pattern, and lining surface. Type 1 lesions correspond to hyperplastic lesions, type 2 to adenomas, and type 3 to invasive carcinomas12. Type 1 lesions should be monitored only, type 2 lesions should undergo polypectomy, and type 3 lesions should be removed endoscopically if possible (using endoscopic mucosectomy or ESD) or through surgical intervention.
The JNET classification uses NBI magnifying endoscopic visualisation9 and primarily focuses on vessel and surface patterns for diagnosing colorectal lesions. It categorises colorectal lesions into four types to provide better guidance for endoscopic treatment strategies (Fig. 2): Type 1 represents hyperplastic polyps (HP) or sessile serrated lesions with no dysplasia (SSL), Type 2 A corresponds to low-grade dysplasia (LGD) adenomas, Type 2B encompasses high-grade dysplasia (HGD), intramucosal cancer, and T1a carcinomas, while Type 3 indicates deep submucosal invasive carcinomas. In cases of mixed JNET classifications, specifically 2 A and 2B, within one colorectal lesion, the final determination of the JNET classification was guided by the presence of the higher grade in the classification system; thus, in this instance, the lesion was classified as JNET 2B.
Histopathological analysis
The lesions were subjected to histopathological analysis by two experienced pathologists at the same hospital. The histopathological diagnosis was based on the criteria set by the World Health Organization16. Following resection, the specimens were affixed with pins onto a cork mat and stored in a 10% formalin-filled container. The haematoxylin and eosin staining method was employed for the histological examination. The areas of suspected most of submucosal invasion were electronically marked and photographic documentation was sent to pathologists. In the location where suspicion was the greatest, the pathologist made thin cuts (1 mm) to obtain the most precise evaluation. For adenomas, the degree of dysplasia was assessed, while in carcinomas, parameters such as tumour depth, degree of differentiation, angio- and lymphangioinvasion, completeness of resection (R0), and the minimum distance from the resection margins were evaluated. High-grade colorectal lesions were defined as adenoma with high-grade dysplasia, intramucosal cancer (IMC), superficial low risk submucosal invasive cancer (T1a) with < 1000 μm17 of submucosal tumour invasion, or deep submucosal invasive cancer (T1b, depth of submucosal invasion ≥ 1000 μm).
Statistical analysis
The primary focus of the statistical analysis was to calculate the proportion of correctly classified colorectal lesions for both the NICE and JNET classifications. Additionally, we calculated the sensitivity and PPV of NICE and JNET classification for high grade lesions, including HGD adenomas, intramucosal carcinomas, T1 and T2 carcinomas. In this case, the classification NICE 2 and JNET Type 2B was considered as positive result. The final pathological findings were considered the gold standard for comparison. The statistical analysis was performed using SPSS Statistics version 28.0 (IBM Corp., Armonk, NY, United States).
Ethics and registration
The study involved a retrospective analysis of prospectively collected data from studies registered in ClinicalTrials.gov under registration numbers NCT03434925 and NCT05929365. These studies were designed to build upon each other, with the latter extending and refining the data collected in the former. The study was reviewed and approved by the Ethics Committee of Military University Hospital Prague, protocol number 108/17–73/2017. All procedures were conducted in accordance with relevant guidelines and regulations, including the Declaration of Helsinki. All patients were fully informed about the nature and purpose of the study, which involved the use of Narrow-Band Imaging (NBI) and the histological evaluation of colorectal lesions. Written informed consent was obtained prior to the examination, ensuring that participants were aware of the potential findings and subsequent procedures, such as lesion resection and histopathological assessment. No participant refused participation after imaging. The protection of patients’ personal data was ensured in accordance with the General Data Protection Regulation (GDPR) under the Regulation of the European Parliament and the Council of the European Union.
Results
A total of 257 cases of advanced colorectal neoplasia (defined as lesions ≥ 10 mm in size) were analysed. Bowel preparation was assessed using the Boston Bowel Preparation Scale, and all patients achieved a very good preparation score (≥ 8 points). A complete colonoscopy was performed up to the cecum for each patient. Out of 257 lesions, a total of 83 polypectomies were performed, accounting for 32.2% of all procedures, with an R0 resection rate of 88.0%. Piecemeal EMR was used in 5 cases (2.0%), achieving R0 resection in 60.0% of these cases. En-bloc EMR was the most frequently performed technique, with 139 cases (54.2%) and an R0 resection rate of 66.9%. ESD was utilized in 21 cases (8.1%), with an R0 resection rate of 66.7%, and FTR was performed in 9 cases (3.5%), achieving R0 resection in 55.6% of cases. Demographic data and characteristics of the lesions, such as size, location, and pathology, are presented in Table 1.
Among the 257 lesions, there were eight (3.1%) hyperplastic polyps, 47 (18.3%) sessile serrated lesions with no dysplasia, 152 (59.1%) adenomas with LGD, 36 (14.0%) high-grade dysplasia adenomas, 12 (4.7%) intramucosal carcinomas, 1 (0.4%) T1 carcinoma, and 1 (0.4%) T2 carcinoma.
Using a combination of the NICE classification (NICE 1, 2, and 3) and the JNET classification (JNET 1, 2 A, 2B, 3), four groups were formed with the following numbers of lesions: NICE 1, JNET 1 (N = 49); NICE 2, JNET 2 A (N = 153); NICE 2, JNET 2B (N = 46); NICE 3, JNET 3 (N = 9). Out of the total 257 lesions, 199 (77.4%) were correctly classified according to the JNET classification, while 241 lesions (93.8%) were correctly classified according to the NICE classification. There were 15 lesions which were misclassified according to both classification systems. The most common issue was misclassifying high-grade adenomas and intramucosal carcinomas as pertaining to the “NICE 3, JNET 3” category. Correct diagnostics of colorectal lesions according to NICE and JNET classification are shown in Fig. 3. Use of the NICE classification was associated with a high proportion of correctly predicted low risk colorectal neoplasia. Due to the variable distribution of different histological types of colorectal lesions in the NICE 2 group, an analysis of sensitivity and positive predictive value (PPV) for high-grade lesions (HGD adenomas, intramucosal carcinomas and T1 carcinomas) for the NICE and the JNET classification was added. The sensitivity and positive predictive value (PPV) of JNET and NICE classification for these high-grade lesions are shown in the Table 2. The sensitivity of the NICE classification (considering NICE 2 as a positive finding) for high-grade lesions was 100%, but the PPV was only 24.4%. The sensitivity and PPV of the JNET classification (considering JNET 2B as a positive finding) for high-grade lesions were 56.6% and 57.7%, respectively.
Discussion
In this study, two endoscopic classifications of surface patterns (NICE and JNET systems) were retrospectively analyzed using data prospectively collected for colorectal lesions larger than 10 mm. Based on our findings, we have observed that the NICE classification system accurately classifies a larger proportion of lesions but is not able to distinguish between low-grade and high-grade colorectal adenomas. JNET classification has been more suitable for determining the appropriate therapy for patients with LGD and HGD adenomas. We demonstrated that if more advanced endoscopic therapy was applied to all lesions classified as NICE 2, only 24.4% of these lesions were actually high-grade lesions (HGD adenomas, intramucosal carcinomas or T1 carcinomas). The remaining majority consisted of LGD lesions, making the use of more advanced therapy unnecessarily risky. Conversely, using more advanced endoscopic therapy for lesions categorised as JNET 2B could reduce the risk of overtreatment, as 57.7% of these lesions were indeed HGD adenomas, intramucosal carcinomas or T1a carcinomas.
The primary objective of this study was to determine the diagnostic impact and applicability of the NICE and JNET classifications for clinical practice in predicting histology outcomes. The NICE classification accurately classifies a large proportion of lesions (93.8%). In JNET classification we observed 77.4% correctly classified lesions. This discrepancy is attributed to the fact that the NICE classification includes more histological types of colorectal neoplasia in the NICE 2 group. The NICE 2 classification does not allow for distinction between adenomas and superficial mucosal carcinomas, which hinders its effectiveness in guiding endoscopic treatment strategies. With the increasing availability of endoscopic devices equipped with magnifying capabilities, the combination of NBI and magnifying endoscopy is gaining in popularity9. This approach enhances diagnostic efficiency and plays a crucial role in assessing the depth of lesion invasion.
The NICE 2 group includes lesions belonging to the JNET 2 A or JNET 2B group. From the point of view of subsequent therapy, this group of lesions must be distinguished. Whereas type 2 A polyps can be resected by piecemeal EMR, type 2B polyps should be resected en-bloc by EMR or ESD to obtain a precise histologic diagnosis concerning the invasion depth and to determine endoscopic curability.
When the infiltration of the lesion is confined to the first 1000 μm of the submucosa (low risk T1a CRC), the risk of lymph node spread is almost negligible18. Generally, there is a consensus that adenomas with LGD, HGD, sessile serrated polyps (SSP), and superficial submucosal low risk invasive T1a carcinomas are suitable for endoscopic resection, while observation is recommended for solitary hyperplastic polyps. Commonly accepted criteria for low risk T1a CRC are curative resection including R0 resection with horizontal and vertical clear margins (R0), absence of lympho-vascular or vessel infiltration (L0, V0), a low to moderate histological grading (G1/2) and limited (< 1000 μm) infiltration into the submucosa17.
Additionally, we evaluated the sensitivity and positive predictive value (PPV) of JNET and NICE classification for high-grade lesions (including HGD adenomas, intramucosal carcinomas and T1a carcinomas). The sensitivity of the NICE classification for high-grade lesions was 100%, but the PPV of NICE 2 was only 24.4%. The sensitivity of the JNET classification for high-grade lesions was 56.6%, while PPV of JNET Type 2B for these lesions reached 57.7%. These results are consistent with findings reported in previous studies19,20,21.
The sensitivity of JNET 2B lesions for diagnosing HGD-T1a carcinoma has been previously reported to range from 44.9–61.9%22,23. Among the various types of JNET type 2B demonstrated the weakest diagnostic ability, likely due to the presence of histological features spanning from adenomas to deep submucosal cancers. This finding aligns with current knowledge, where the gold standard for JNET type 2B lesions involves pit pattern diagnosis using dye-based magnifying chromoendoscopy to guide final treatment decisions24,25.
Several comparative studies have been conducted to assess the NICE and JNET classifications (Table 3). Studies have shown that the specificity and sensitivity of both classifications are comparable for their respective categories. NICE 1 corresponds to JNET Type 1 with high specificity (> 95%) for both experienced and less experienced endoscopists20. NICE 3 matches JNET Type 3, both indicating invasive cancer with high specificity (> 95%) but varying sensitivity based on the endoscopist’s experience22. According to ESGE guidelines, the JNET classification is indicated for selected lesions, such as non-ulcerated NICE type 3 lesions or when a demarcated area (nodule, redness, or depression) is present in a NICE type 2 lesion26.
The overall diagnostic accuracy of the NICE classification system was found to range from 59.5 to 84.2%20. Optical recognition yielded significantly better results with larger polyps, high-risk lesions (HGD), and neoplastic lesions. However, the NICE classification system demonstrated inferior performance compared to histopathological analysis. In a recent retrospective study, among lesions classified as NICE 2, 21.6% (21/91) showed low-grade dysplasia, 56.7% (55/91) demonstrated HGD, 16.5% (16/91) demonstrated sm1 invasion, and 5.5% (5/91) demonstrated ≥ sm2 invasion. In NICE 3 lesions, 14.3% (2/14) were T1a carcinomas, and the remainder (85.7%, 12/14) were T1b carcinomas27.
For the JNET classification, the sensitivity and specificity of Type 1 lesions in differentiating between non-neoplastic and neoplastic lesions were 78.1% and 98.6%, respectively. In Type 2 A lesions, the sensitivity and specificity for distinguishing LGD from others were 98% and 76.5%, respectively. Type 3 lesions showed a sensitivity of 99.5% and specificity of 83.3% in differentiating deep submucosal invasive carcinoma from other lesions28.
JNET type 2B lesions remain the biggest challenge for endoscopists. These lesions often exhibit features that overlap with both less severe (JNET 2 A) and more severe (JNET 3) categories, making it difficult to distinguish between benign lesions, high-grade dysplasia, and invasive cancer. Previous studies have shown that the sensitivity of the JNET classification for Type 2B lesions in diagnosing HGD and T1a CRC was 44.9–61.9%22,23. Compared with other types of JNET classification, the diagnostic ability of type 2B is the weakest. Therefore, the authors suggest that direct observation of the Kudo pit pattern with crystal violet should be performed in JNET 2B lesions. In cases of rectal lesions, endoscopic ultrasound (EUS) can be used to provide detailed images of the layers of the gastrointestinal wall.
Overall, several issues about NICE and JNET classification systems can be summarised. First, the NICE classification does not include SSPs due to the lack of well-established histopathological diagnostic criteria for SSPs29.
Second, the NICE 2 category encompasses two distinct types of lesions: benign low-grade adenomas and submucosal invasive cancers with malignant behaviour. These two types should be individually diagnosed to determine the appropriate treatment strategy. Low-grade adenomas are typically treated through polypectomy or piecemeal EMR resection, while high-grade adenomas or superficial submucosal carcinoma require en-bloc EMR or ESD to accurately assess the depth of cancer invasion, which is crucial for determining curative or non-curative resection. In this case it is preferable to use the JNET classification, where type 2 category is divided into type 2 A (low-grade adenoma) and type 2B (high-grade adenoma, intramucosal carcinoma, and superficial submucosal cancer).
Thirdly, although JNET appears to be clinically useful for selectively diagnosing HGD or superficial T1a carcinoma, JNET 2B exhibits low sensitivity (56.6%) and low PPV (57.7%)30,31,32,33,34. This is likely due to including various histological features within this group. Special attention is given to T1b carcinoma; it is characterized by a higher metastatic risk for node involvement (6%) and, according to current guidelines, is indicated for surgical treatment35. In our study, we were unable to evaluate lesions with deeper invasion than T1b due to the very low number of T1 carcinomas in the patient group. However, the diagnostic accuracy of T1b colorectal cancer in JNET 2B and JNET 3 lesions is highly variable. Iwatate et al.23 performed an evaluation of the diagnostic accuracy of JNET for a T1b lesion and reported that more than 50% of colorectal lesions diagnosed as Type 2B are found to have deep submucosal invasion on histologic examination. In this context, a retrospective study assessing 2933 images reported that 60% of the lesions classified as JNET 2B were diagnosed as carcinomas with deep submucosal invasion (T1b), while 55.4% of lesions classified as JNET Type 3 were found to have the same characteristic22. Therefore, the authors suggest that direct observation of the Kudo pit pattern with crystal violet should be performed in these lesions36,37,38. Magnifying chromoendoscopy with pit pattern classification had 73.3% sensitivity and 100% specificity39.
Lastly, JNET classification for colorectal tumors using magnifying NBI showed moderate interobserver agreement (κ = 0.52) and excellent intraobserver agreement (κ = 0.88) among experienced endoscopists30.
Based on these research findings, the application of the NICE classification in clinical practice is beneficial to quickly evaluate neoplastic and non-neoplastic lesions. The NICE 1 classification corresponds with JNET type 1 lesions, suspected to be hyperplastic or sessile serrated polyp (SSPs), which may have been followed up (SSPs in right colon and with diameter over 10 mm in size need to be endoscopically removed). In case of NICE 2 and NICE 3 findings the JNET classification should be added, allowing to distinguish between a benign (low-grade) neoplastic lesion and a potentially malignant (high-grade or submucosal invasion) neoplastic lesion. In case of JNET Type 2 A lesions, which are suspected as low-grade dysplasia, the piece meal EMR technique can be used, and there might not be any requirement to supplement with the chromoendoscopy technique. In JNET type 2B lesions, magnifying dye-based chromoendoscopy should be performed, and resection should be done in one piece with EMR or ESD to adequately obtain a histological diagnosis of the depth of invasion and to assess the potential for endoscopic curability. In JNET type 3 lesions, which are suspected to be deep submucosal invasive carcinomas, forceps biopsy and surgical treatment are recommended.
We acknowledge potential limitations of our study. Firstly, the data were analyzed retrospectively, and the study was conducted at a single endoscopic center, involving endoscopists exclusively from the same hospital and adhering to similar guidelines. Secondly, the low prevalence of T1 and deep submucosal invasive carcinomas in our study may have impacted the findings. Lastly, we did not examined intraobserver and interobserver agreement regarding JNET and NICE classification.
Conclusion
The JNET classification, with a positive predictive value of 57.7% for high-grade colorectal lesions (including HGD adenomas, intramucosal carcinomas, and T1 carcinomas), should be used for decision-making regarding appropriate subsequent endoscopic therapy.
Data availability
Research data will be shared upon reasonable request to the corresponding author.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74(3), 229–263 (2024).
Kahi, C. J., Imperiale, T. F., Juliar, B. E. & Rex, D. K. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin. Gastroenterol. Hepatol. 7, 770–775 (2009).
Ferlitsch, M. et al. Colorectal polypectomy and endoscopic mucosal resection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2024. Endoscopy 56, 516–545 (2024).
Hosotani, K. et al. Can advanced endoscopic imaging help us avoid surgery for endoscopically resectable colorectal neoplasms? A proof-of-concept study. Dig. Dis. Sci. 65, 1829–1837 (2019).
Subramaniam, S. et al. Optical diagnosis of colorectal polyps with Blue Light Imaging using a new international classification. United Eur. Gastroenterol. J. 7, 316–325 (2019).
Shinozaki, S., Osawa, H., Hayashi, Y., Lefor, A. K. & Yamamoto, H. Linked color imaging for the detection of early gastrointestinal neoplasms. Th. Adv. Gastroenterol. 12, 1756284819885246 (2019).
Basford, P. J., Longcroft-Wheaton, G., Higgins, B. & Bhandari, P. High-definition endoscopy with i-Scan for evaluation of small colon polyps: the HiSCOPE study. Gastrointest. Endosc. 79, 111–118 (2014).
Picot, J. et al. Virtual chromoendoscopy for the real-time assessment of colorectal polyps in vivo: a systematic review and economic evaluation. Health Technol. Assess. 21, 1–308 (2017).
Sano, Y. et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig. Endosc. 28, 526–533 (2016).
Iwatate, M. et al. NBI and NBI Combined with Magnifying Colonoscopy. Diagn. Ther. Endosc. 173269 (2012).
Kobayashi, S. et al. Diagnostic yield of the Japan NBI Expert Team (JNET) classification for endoscopic diagnosis of superficial colorectal neoplasms in a large-scale clinical practice database. United Eur. Gastroenterol. J. 7, 914–923 (2019).
Hayashi, N. et al. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest. Endosc. 78, 625–632 (2013).
Schlemper, R. J. et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 47, 2:251–255 (2000).
Puig, I., Mármol, C. & Bustamante, M. Endoscopic imaging techniques for detecting early colorectal cancer. Curr. Opin. Gastroenterol. 35, 432–439 (2019).
Tanaka, S. & Sano, Y. Aim to unify the narrow band imaging (NBI) magnifying classification for colorectal tumors: current status in Japan from a summary of the consensus symposium in the 79th Annual Meeting of the Japan Gastroenterological Endoscopy Society. Dig. Endosc. 23 (1), 131–139 (2011).
Nagtegaal, I. D. et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 76, 182–188 (2020).
Hassan, C. et al. Endoscopic surveillance after surgical or endoscopic resection for colorectal cancer: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Digestive Oncology (ESDO) Guideline. Endoscopy 51, 266–277 (2019).
Yasue, C. et al. Pathological risk factors and predictive endoscopic factors for lymph node metastasis of T1 colorectal cancer: a single-center study of 846 lesions. J. Gastroenterol. (2019).
Cocomazzi, F. et al. Accuracy and inter-observer agreement of the NICE and KUDO classifications of superficial colonic lesions: a comparative study. Int. J. Colorectal Dis. 36, 1561–1568 (2021).
Wang, Y., Li, W. K., Wang, Y. D., Liu, K. L. & Wu, J. Diagnostic performance of narrow-band imaging international colorectal endoscopic and Japanese narrow-band imaging expert team classification systems for colorectal cancer and precancerous lesions. World J. Gastrointest. Oncol. 13, 58–68 (2021).
Patrun, J., Okresa, L., Ivekovic, H. & Rustemovic, N. Diagnostic accuracy of NICE classification system for optical recognition of predictive morphology of colorectal polyps. Gastroenterol. Res. Pract. 7531368 (2018).
Sumimoto, K. et al. Clinical impact and characteristics of the narrow-band imaging magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Gastrointest. Endosc. 85, 816–821 (2017).
Iwatate, M. et al. Validation study for development of the Japan NBI Expert Team classification of colorectal lesions. Dig. Endosc. 30, 642–651 (2018).
Puig, I. et al. Accuracy of the narrow-band imaging international colorectal endoscopic classification system in identification of deep invasion in colorectal polyps. Gastroenterology 156, 75–87 (2019).
Tanaka, S. et al. JGES guidelines for colorectal endoscopic submucosal dissection / endoscopic mucosal resection. Dig. Endosc. 27, 417–434 (2015).
Bisschops, R. et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2019. Endoscopy 51, 12:1155–1179 (2019).
Ahmed, N. & Bechara, R. Endoscopic submucosal dissection and JNET classification for colorectal neoplasia: a north American academic center experience. DEN Open 27 (1), e322 (2023).
Koyama, Y. et al. New scoring system to distinguish deep invasive submucosal and muscularis propria colorectal cancer during colonoscopy: a development and global multicenter external validation study (e-T2 score). Gastrointest. Endosc. 96, 321–329e2 (2022).
Tanaka, S. & Saitoh, Y. Endoscopic Management of Colorectal T1(SM) Carcinoma Springer. (2020).
Komeda, Y. et al. Magnifying narrow band imaging (NBI) for the diagnosis of localized colorectal lesions using the Japan NBI expert team (JNET) classification. Oncology 93, 49–54 (2017).
Higurashi, T. et al. Comparison of the diagnostic performance of NBI, Laser-BLI and LED-BLI: a randomized controlled noninferiority trial. Surg. Endosc. 36, 7577–7587 (2022).
Hirata, D. et al. Effective use of the Japan narrow Band Imaging Expert Team classification based on diagnostic performance and confidence level. World J. Clin. Cases. 7, 2658–2665 (2019).
Pu, Z. C. T. Comparison of different virtual chromoendoscopy classification systems for the characterization of colorectal lesions. JGH Open. 4, 818–826 (2020).
Wagner, A. et al. Systematic review on optical diagnosis of early gastrointestinal neoplasia. J. Clin. Med. 10, 2794 (2021).
Pimentel-Nunes, P. et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2022. Endoscopy 54, 6:591–622 (2022).
Minoda, Y. et al. Objective validity of the Japan narrow-band imaging Expert Team classification system for the differential diagnosis of colorectal polyps. Dig. Endosc. 31, 544–551 (2019).
Sumimoto, K. et al. Diagnostic performance of Japan NBI Expert Team classification for differentiation among noninvasive, superficially invasive, and deeply invasive colorectal neoplasia. Gastrointest. Endosc. 86, 700–709 (2017).
Sakamoto, T. et al. Comparison of the diagnostic performance between magnifying chromoendoscopy and magnifying narrow-band imaging for superficial colorectal neoplasms: an online survey. Gastrointest. Endosc. 87, 1318–1323 (2018).
Kawaguti, F. S. et al. Role of magnification chromoendoscopy in the management of colorectal neoplastic lesions suspicious for submucosal invasion. Dis. Colon Rectum. 62, 422–428 (2019).
Acknowledgements
Supported by programme projects of the Ministry of Health of the Czech Republic with Reg. No. NU22-08-00424, DZRVO - MO1012 and Cooperatio.
Author information
Authors and Affiliations
Contributions
Tomas Grega, Klara Kmochova and Katerina Hejcmanova wrote the initial draft of the manuscript; Tomas Grega, Nadija Brodyuk and Stepan Suchanek conducted endoscopic resection of colorectal lesions and collected the colonoscopy examination data; Ondrej Ngo, and Ondrej Majek were responsible for statistical analysis; Jan Bures, Petr Urbanek and Miroslav Zavoral were responsible for the overall scientific direction.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Grega, T., Kmochova, K., Hejcmanova, K. et al. Impact of narrow band imaging in prediction of histology of advanced colorectal neoplasia. Sci Rep 15, 1414 (2025). https://doi.org/10.1038/s41598-025-85669-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85669-w