Abstract
Cancer significantly contributes to the unfavorable prognosis of coronavirus disease 2019 (COVID-19) patients. The efficacy and safety of azvudine and nirmatrelvir/ritonavir (Paxlovid) in cancer patients with COVID-19 remain uncertain. Therefore, we designed a comprehensive retrospective study encompassing clinical data of 32,864 hospitalized COVID-19 patients, 691 of whom were cancer patients treated with azvudine and 200 were cancer patients treated with Paxlovid. After 2:1 propensity score matching, 397 patients in the azvudine group and 199 patients in the Paxlovid group were enrolled. Cox regression analysis revealed the risk of all-cause death (HR: 1.84, 95% CI: 1.059–3.182, P = 0.030) and composite disease progression (HR: 1.70, 95% CI: 1.043–2.757, P = 0.033) were greater in the Paxlovid group than in the azvudine group. Two sensitivity analyses confirmed the robustness of our findings. The safety analysis of adverse events revealed no statistically significant differences between the two groups. In conclusion, we carried out the first analysis to compare the efficacy and safety of azvudine and Paxlovid in cancer patients with COVID-19 and demonstrated that azvudine significantly reduced the risk of all-cause death and composite disease progression among cancer patients with COVID-19 compared with Paxlovid.
Similar content being viewed by others
Introduction
The prevalence of cancer poses a significant global challenge in the realm of public health and remains a prominent cause of mortality1. According to the 2020 Global Cancer Statistics, 19.3 million new cancer cases and nearly 10 million cancer-related fatalities are estimated to have occurred worldwide in 20202. Furthermore, given the rapid population growth, aging demographics, and cumulative impact of risk factor exposure, cancer mortality rates are anticipated to continue to escalate, which burdens numerous countries with both pathogen-related infections and a progressively westernized lifestyle3. Hence, safe and efficacious pharmaceutical interventions to ameliorate the global cancer landscape are urgently needed.
Cancer patients typically experience compromised health and are characterized by immune suppression due to malignant tumors, rendering them more susceptible to infections than noncancer patients4,5. Additionally, specific cancer treatments and longer hospital stays may further increase the risk of infection in cancer patients6,7. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a member of the coronavirus family characterized by its single-stranded RNA structure, which has led to the global outbreak of coronavirus disease 2019 (COVID-19) that precipitated an unprecedented global health crisis8,9. Multiple studies have demonstrated that compared with noncancer patients, cancer patients are more susceptible to COVID-19 infection and have severe clinical complications and a poorer prognosis10,11,12. Therefore, controlling SARS-CoV-2 infection is crucial for the treatment of cancer patients, and the use of antiviral drugs is the most effective way to achieve this goal.
Nirmatrelvir/ritonavir (Paxlovid) and azvudine are two prominent antiviral agents that have obtained approval for the treatment of COVID-19. Paxlovid can effectively reduce the risk of progression to severe illness among COVID-19 patients, which significantly reduces the mortality rate13,14. Azvudine, a nucleoside analog, exhibits dual-targeting capabilities against reverse transcriptase and viral infectivity factor and initially gained approval in China for the treatment of adult patients infected with HIV-115,16. Azvudine not only shortens the time of first nucleic acid negative conversion in patients with mild and moderate COVID-1917, but also has significant therapeutic effects on the average nucleic acid negative conversion time and recovery rate in patients with moderate to severe COVID-1918. Although both interventions have demonstrated certain efficacy in suppressing SARS-CoV-2, the current evidence remains insufficient to support their utilization in cancer patients with COVID-19.
To the best of our knowledge, no specific study has been conducted to compare the efficacy and safety of azvudine and Paxlovid in reducing the risk of all-cause death and composite disease progression in cancer patients with COVID-19. Therefore, in this study, we collected the clinical data of cancer patients with COVID-19 from nine hospitals in Henan, China, and conducted a retrospective analysis to investigate the efficacy and safety of oral azvudine and Paxlovid for cancer patients with COVID-19.
Methods
Study design and patient information
From December 5, 2022, to January 31, 2023, cancer patients infected with SARS-CoV-2 were selected from nine hospitals in Henan Province, including the First Affiliated Hospital of Zhengzhou University, Fengqiu County People’s Hospital, the Fifth People’s Hospital of Anyang, Henan Infectious Disease Hospital, Shangqiu Municipal Hospital, Luoyang Central Hospital, Nanyang Central Hospital, Guangshan County People’s Hospital, and Henan Provincial Chest Hospital. The demographic characteristics, medical history, diagnosis, prescriptions, admission and discharge dates, laboratory test results, imaging findings, intensive care unit admission dates, and dates of death were extracted from the hospital electronic medical records.
The inclusion criteria for the study were as follows: (a) hospitalized cancer patients with concurrent SARS-CoV-2 infection and (b) patients receiving oral azvudine or Paxlovid. The exclusion criteria were as follows: (a) patients under the age of 18 years; (b) patients without cancer; (c) patients receiving other antiviral regimens.
Group information
The enrolled patients were stratified into two cohorts based on their allocation to either the azvudine or the Paxlovid group. The azvudine cohort received a daily dose of 5 mg, whereas the Paxlovid cohort was administered nirmatrelvir at a dosage of 300 mg and ritonavir at a dosage of 100 mg twice daily.
Baseline covariates
The baseline covariates for the patients included age, sex, body mass index, severity of COVID-19 at admission, time from diagnosis to treatment exposure, concomitant systemic steroids, the primary tumor site and metastasis and the presence of clinical comorbidities, including diabetes, hypertension, liver diseases, cardio-cerebral diseases, and kidney diseases. Relevant laboratory indicators of clinical significance, such as neutrophil, lymphocyte, glucose, high-density lipoprotein, low-density lipoprotein, alanine aminotransferase, aspartate aminotransferase, creatine, glomerular filtration rate, c-reactive protein, procalcitonin, prothrombin time, activated partial thromboplastin time, cholesterol, triglyceride, alkaline phosphatase, gamma‒glutamyl transpeptidase, albumin and total bilirubin, were also incorporated.
Derivation of the propensity score
To reduce confounding bias, we identified covariates associated with treatment outcomes and subsequently employed logistic regression to calculate propensity scores of the azvudine and Paxlovid recipients. We then utilized greedy matching method with a ratio of 2:1 to match the azvudine to the Paxlovid treatments. Finally, we assessed balance after matching, with P > 0.05 and standardized mean difference (SMD) < 10% indicating the best balance between the two groups.
Outcomes
The main outcome of this study was all-cause death, and the secondary outcome focused on composite disease progression among cancer patients with COVID-19. All-cause death was determined based on discharge orders or records. Disease progression included the following aspects: (a) progression from mild or moderate to severe or critical disease or death. (b) progression from severe to critical conditions. Severe status was defined as the presence of shortness of breath, with a respiratory frequency of more than 30 breaths per minute, and observed on two separate occasions within a 12-hour period, the presence of dyspnea with a resting oxygen saturation less than 93%, a PaO2/FiO2 ratio less than 300 mmHg, or the progression of lung imaging lesions of more than 50% within 24–48 h. The patients were considered critical if they experienced respiratory failure that required mechanical ventilation, shock, or other organ dysfunction necessitating intensive care unit monitoring.
Subgroup analysis
The population was stratified by sex, age, disease severity, concomitant systemic steroids use, time from diagnosis to treatment, tumor metastasis status, primary tumor site, and presence of comorbidities, including diabetes, hypertension, liver diseases, cardio-cerebral diseases, kidney diseases for subsequent subgroup analysis.
Safety
The safety of azvudine and Paxlovid in cancer patients with COVID-19 was assessed according to the Common Terminology Criteria for Adverse Events version 5.0. Here, abnormal laboratory test results were graded, encompassing overall adverse events (AEs) as well as grade ≥ 3 AEs. Data collection spanned from drug administration until five half-lives after the final dose.
Sensitivity analysis
For sensitivity analysis, firstly, we performed a 2:1 propensity score matching (PSM) using Probit regression model instead of logistic regression. Secondly, we did not perform PSM analysis and included all eligible cancer patients with COVID-19, in the analysis, including patients who met the inclusion criteria but were not included in the primary analysis set.
Statistical analysis
First, missing baseline data were imputed using multiple imputations, followed by 2:1 PSM to control for differences in baseline covariates. The achievement of group balance was considered when the p value between the azvudine group and the Paxlovid group exceeded 0.05. Second, cumulative event curves were generated using the Kaplan‒Meier method to examine survival differences between the azvudine and Paxlovid groups, which were tested using log-rank analysis. Additionally, Cox proportional hazards regression models were employed to evaluate the hazard ratio (HR) and corresponding 95% confidence intervals (CIs) for all-cause death and composite disease progression.
The statistical analysis was performed using R software version 4.0.3. A two-sided p value of less than 0.05 was considered statistically significant. Continuous variables with a normal distribution are presented as the means ± standard deviations and were analyzed via t tests. Nonnormally distributed continuous variables are presented as medians (interquartile ranges) and were analyzed by the Mann‒Whitney U test. Categorical variables are reported as absolute numbers (percentages) and were analyzed by the chi-square test.
Ethics statement
Our study was designed in accordance with the Declaration of Helsinki. The study received approval from the Institutional Review Board of the First Affiliated Hospital of Zhengzhou University (2023-KY-0865-001). The study was also registered with ClinicalTrials.gov (NCT06349655). Because the private information of all patients was not disclosed in this retrospective study, the Ethics Committee of the First Affiliated Hospital of Zhengzhou University approved the waiver of obtaining informed consent.
Results
Baseline characteristics
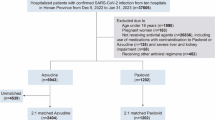
The study collected clinical information of 32,864 patients from nine hospitals in Henan Province. Based on the inclusion and exclusion criteria, we identified 691 cancer patients receiving azvudine treatment and 200 cancer patients receiving Paxlovid treatment for analysis (Fig. 1). The PSM technique was subsequently employed at a ratio of 2:1 to control for confounding factors, resulting in the inclusion of 397 patients in the azvudine group and 199 patients in the Paxlovid group.
The baseline characteristics of the included patients before and after PSM are presented in Table 1. Prior to PSM, compared with the Paxlovid group, the azvudine group presented a significantly longer duration from diagnosis to treatment initiation (0–5 days) (P = 0.002), higher proportions of patients receiving concomitant systemic steroids (P = 0.001), greater numbers of patients with kidney diseases (P < 0.001), and lower numbers of patients with liver diseases (P = 0.01) and cardio-cerebral diseases (P < 0.001). With respect to laboratory tests, the Paxlovid group presented greater lymphocyte counts (P = 0.027), shorter prothrombin time (P < 0.001) and activated partial thromboplastin time (P = 0.011), lower albumin (P = 0.006) and procalcitonin (P = 0.044) concentrations. Following matching, the characteristics of the two groups no longer significantly differed (all P > 0.05) (Fig. S1).
All-cause death
During the 31-day follow-up period, a total of 63 all-cause death events were recorded, with 33 occurring in the azvudine group and 30 in the Paxlovid group. The Kaplan-Meier analysis revealed that patients receiving Paxlovid treatment had a significantly greater 31-day all-cause death risk compared to those receiving azvudine treatment (P = 0.02), as determined by the log-rank test (Fig. 2). After adjusting for confounding factors using Cox regression analysis, the Paxlovid group presented a greater risk of all-cause death than the azvudine group (HR: 1.84, 95% CI: 1.059–3.182; P = 0.03) (Fig. 3). Furthermore, patients with severe disease (HR: 3.28, 95% CI: 1.092–9.839, P = 0.034), cardio-cerebral diseases (HR: 2.20, 95% CI: 1.193–4.041, P = 0.012), and kidney diseases (HR: 2.96, 95% CI: 1.350–6.494, P = 0.007) had significantly elevated risks of all-cause death (Fig. 3).
To further validate the robustness of our findings, we conducted a sensitivity analysis using Probit method and observed that the all-cause death rate was significantly greater in the Paxlovid group than in the azvudine group (P = 0.0013) (Fig. S2). Additionally, multivariate Cox proportional hazards regression analysis confirmed that patients treated with Paxlovid had a higher all-cause death rate (HR: 2.33, 95% CI: 1.295–4.200; P = 0.005) (Fig. S3). The baseline characteristics of patients before and after PSM using Probit method are presented in Table S1. Subsequently, we did not perform PSM analysis and included all eligible 691 azvudine recipients and 200 Paxlovid recipients. The Kaplan-Meier analysis confirmed a significantly higher all-cause death risk in the Paxlovid group (P = 0.0032) (Fig. S4). The results of the Cox proportional hazards regression analysis were consistent with the aforementioned findings (HR: 1.78, 95% CI: 1.062–2.988, P = 0.029) (Fig. S5).
Composite disease progression
Within 31 days of treatment, composite disease progression was observed in 84 patients, with 47 (55.95%) receiving azvudine and 37 (44.05%) receiving Paxlovid. The Kaplan‒Meier analysis revealed that the efficacy of Paxlovid in reducing the risk of composite disease progression in patients was lower than that of azvudine, as determined by the log-rank test (P = 0.028) (Fig. 4). According to the results of Cox regression analysis, the Paxlovid group had a significantly greater risk of composite disease progression than the azvudine group did (HR: 1.70, 95% CI: 1.043–2.757, P = 0.033) (Fig. 5). Furthermore, the risk of composite disease progression was markedly increased in patients with severe illness at admission (HR: 6.96, 95% CI: 2.415–20.036, P = 0.000) and those with kidney diseases (HR: 2.58, 95% CI: 1.293–5.151, P = 0.007) (Fig. 5).
A sensitivity analysis was subsequently performed to validate our findings via Probit method, which revealed a significantly greater composite disease progression rate in the Paxlovid group than in the azvudine group (P = 0.011) (Fig. S6). The Cox regression analysis further revealed that Paxlovid exhibited reduced efficacy compared with azvudine in mitigating composite disease progression (HR: 1.85, 95% CI: 1.127–3.024; P = 0.015) (Fig. S7). Additionally, we validated the results in all eligible patients. The findings indicated that there was no significant difference in the reduction of composite disease progression between azvudine and Paxlovid, as evidenced by the Kaplan-Meier analysis (P = 0.14) and the Cox proportional hazards regression model (HR: 1.39, 95% CI: 0.898–2.158, P = 0.139) (Figs. S8, S9).
Dynamic changes in leukocyte subsets
To investigate the impact of azvudine and Paxlovid on immune regulation in patients, we comprehensively analyzed the dynamic changes in subsets of leukocytes during the follow-up period. The results demonstrated that there were intermittent differences in neutrophil counts between the two groups following a 15-day treatment period. Specifically, a significant increase (P < 0.05) in the neutrophil counts was observed on Days 2 in the azvudine group and Days 9 in the Paxlovid group (Fig. 6A). The lymphocyte counts did not significantly differ between the two groups throughout the course of antiviral therapy (Fig. 6B). Notably, while no statistically significant difference was observed in basophil counts at the end of follow-up, the azvudine group consistently presented greater basophil counts throughout the course of antiviral therapy (Fig. 6C). The levels of eosinophils and monocytes were generally within the normal range in both patient groups, with occasional discrepancies observed between the two groups (Fig. 6D, E).
Subgroup analysis
We stratified the population based on sex, age, disease severity, concomitant systemic steroids, time interval from diagnosis to treatment initiation, comorbidities (diabetes, hypertension, liver diseases, kidney diseases, cardio-cerebral diseases), tumor metastasis status, and primary tumor site and then conducted subgroup analysis based on the aforementioned variables. Cox regression analysis revealed that the effect of azvudine versus Paxlovid on all-cause death was consistent across all subgroups (all P for interaction values > 0.05 (Fig. 7). Regarding the composite disease progression, the results showed a differential effect of azvudine versus Paxlovid in the following subgroups: concomitant systemic steroids (P for interaction: 0.018) and liver diseases (P for interaction = 0.017) (Fig. S10). Among patients without concomitant systemic steroids, the risk of composite disease progression was increased by 132% in the Paxlovid group compared with the azvudine group (HR: 2.32, 95% CI: 1.38–3.91, P for interaction: 0.018). The patients with liver diseases treated with Paxlovid had a 359% increased risk of composite disease progression (HR: 4.59, 95% CI: 1.65–12.76, P for interaction = 0.017). However, other factors, such as tumor metastasis, primary tumor site, did not significantly influence the efficacy of either drug in reducing composite disease progression.
Safety assessment
To evaluate the safety of azvudine and Paxlovid in the population, we systematically collected data on AEs during the follow-up period for both drug treatment cohorts (Table S2). The findings revealed that, across all categories of all-grade AEs and the incidence of grade ≥ 3 AEs, there was no statistically significant difference between the azvudine group and the Paxlovid group.
Discussion
The outbreak and pandemic of COVID-19 have presented substantial threats to global public health. The management of chronic diseases poses a formidable challenge amidst the ongoing pandemic. Cancer, one of the four major chronic non-communicable diseases, is highlighted by current research as a significant risk factor for the deterioration of clinical outcomes in patients with SARS-CoV-2 infection19,20. Base on this, our study established a multicenter, large sample cohort comprising 32,864 patients, with 691 cancer patients receiving azvudine treatment and 200 cancer patients receiving Paxlovid treatment. After balancing the confounding variables between the two groups, we incorporated 397 patients who were administered azvudine and 199 patients who received Paxlovid into a cohort of cancer patients with COVID-19. Our findings indicated that azvudine was significantly superior to Paxlovid in terms of reducing the risk of all-cause death among cancer patients with COVID-19 and that it may also have an advantage in mitigating the cumulative risk of composite disease progression. To the best of our knowledge, this is the first study to investigate the efficacy and safety of azvudine and Paxlovid in cancer patients with COVID-19. These results provided insights into the selection of antiviral drugs for cancer patients with COVID-19.
The study has demonstrated that azvudine and Paxlovid exhibited similar effectiveness in reducing mortality rates, negative PCR conversion time and hospital stay. However, azvudine showed better effectiveness in improving other outcomes21. Another study comparing azvudine and nirmatrelvir-ritonavir found significant differences in mortality rate, ICU admission rates, and need for mechanical ventilation, but the two treatments were not significantly different in negative PCR conversion time, and hospital stay22. To our knowledge, previous retrospective evidences on the use of azvudine and Paxlovid in cancer patients has been limited, with only a single analytical approach employed23,24. To ensure the reliability of our findings, we conducted two sensitivity analyses to rigorously validate the robustness of the results. First, we applied PSM using Probit method to control confounding factors. Second, to account for the potential impact of sample size, we included all eligible COVID-19 cancer patients without applying PSM. The analysis of Probit method corroborated our findings. However, the analysis without PSM revealed that azvudine did not significantly differ from Paxlovid in reducing the risk of composite disease progression. It is important to note that while the Paxlovid group exhibited a higher all-cause death risk, the proportion of deaths in the azvudine group increased significantly after the 20th days. We carefully analyzed the potential possible reasons: First, the number of patients who were still at risk after 20 days was small, with only 71 azvudine recipients and 42 Paxlovid recipients. The small sample size will magnify the impact of any event. Therefore, although this study found that for cancer patients with COVID-19, the effectiveness of azvudine in the later stage of observation, especially after 20 days, may be inferior to that of Paxlovid, the influence of bias cannot be ruled out. Second, the follow-up time of this study was too short, only 31 days, and we could not observe whether the mortality rate of azvudine still increased proportionally after 31 days. Therefore, it is impossible to determine whether this is a coincidence or a continuous event. In summary, future follow-up studies with larger sample sizes and longer periods of time are needed to observe the effectiveness of azvudine compared with Paxlovid in cancer patients with COVID-19.
Lymphocytes play a pivotal role in the regulation of viral infections. Research findings have demonstrated a positive correlation between the severity of infection and the extent of lymphocyte reduction, and depletion of lymphocytes is closely associated with disease progression25. Given that azvudine was found to be significantly more effective than Paxlovid in reducing the risk of all-cause death, we hypothesized that this superiority might be attributed to enhanced immune function induced by azvudine. To test this hypothesis, we analyzed the dynamic changes in lymphocyte counts. However, our results indicated that there was no significant difference in lymphocyte counts between the azvudine group and Paxlovid group during the treatment period. This lack of difference may be attributable to the limited sample size or short follow-up duration. Notably, Paxlovid interacts with commonly used anticancer medications, including tyrosine kinase inhibitors26, taxanes27, and vinca alkaloids28, thereby increasing the risk of drug‒drug interactions and cumulative toxicity. Therefore, we reasonably suspect that the combination of Paxlovid and anticancer drugs may contribute to the observed poor efficacy in cancer patients due to potential toxic side effects. This finding provides a plausible explanation for the aforementioned outcome.
To further investigate the impact of additional factors on the reduction in all-cause death and composite disease progression, subgroup analyses were conducted. We found a significant correlation between concomitant systemic steroids and composite disease progression. Corticosteroids, widely employed anti-inflammatory and immunosuppressive agents, elicit divergent results regarding their efficacy in the management of patients with COVID-1929. A case report demonstrated that targeted methylprednisolone intervention during the acute phase of COVID-19 resulted in reduced clinical symptoms among critically ill patients, suggesting that short-term administration of medium-dose corticosteroids may facilitate patient recovery30. Acute respiratory distress syndrome is a major cause of fatal COVID-19. Fortunately, a recent study revealed that corticosteroids treatment alleviated severe inflammatory storms, prevented further multiorgan damage and shock, and reduced the 28-day all-cause death rate in patients with acute respiratory distress syndrome31. However, corticosteroids exert an inhibitory effect on cytokine storms32, and they also induce a prolonged period of coronavirus clearance33. A study revealed that, compared with non-severe COVID-19 patients who did not receive corticosteroids treatment, patients who received corticosteroids had significantly prolonged viral clearance times and hospitalization durations, as well as an increased frequency of antibiotic utilization34. In summary, the efficacy of corticosteroids in COVID-19 patients may be associated with disease severity, treatment dosage and duration of treatment, and further research is warranted to determine their specific application in this patient population. The observed 132% increase in the risk of composite disease progression among patients who did not receive systemic steroids after Paxlovid administration attracted our interest. This finding suggested that COVID-19 patients receiving systemic steroids may have experienced fewer adverse outcomes following Paxlovid treatment.
Some research has demonstrated a correlation between the severity of illness and the risk of death in cancer patients with COVID-19, which is contingent upon the specific cancer type and metastatic status35. Our analysis revealed that the presence of metastasis and the location of the primary tumor had no discernible impact on all-cause death or composite disease progression in either the azvudine or paxlovid group. First, the limited sample size may have prevented the results from accurately reflecting the true differences in drug efficacy across various cancer types and metastatic stages. Second, the disease progression of cancer patients with COVID-19 can be influenced by multiple factors, including underlying health conditions and immune function, which may obscure the true impact of the drugs. Finally, limitations in the study design, such as an insufficient observation period, could also contribute to potential biases in the results. The safety of drugs is a critical consideration in clinical practice. Therefore, we conducted a comprehensive analysis and classification of various clinical AEs. The results demonstrated that there was no statistically significant difference in AEs between the two groups. Additionally, the overall safety of both drugs remains within acceptable limits.
Among all the analysis methods, the results on the reduction of composite disease progression by the azvudine and the Paxlovid were not entirely inconsistent, and this discrepancy may be attributed to the limitations of our study. First, as our study was a retrospective analysis, despite obtaining data from nine hospitals, unavoidable selection bias remained. Second, PSM could only balance known confounders, unknown confounders affecting outcome events could not be balanced. Although the p-values of all variables between the two groups after PSM were less than 0.05, it still cannot completely control confounding bias and cannot completely simulate the effect of a randomized controlled study. In the future, randomized clinical controlled trials are still needed to explore the effectiveness and safety of azvudine compared with Paxlovid in the treatment of cancer patients with COVID-19. Third, regional variations in treatment preferences may introduce additional bias. Fourth, following the lifting of anti-COVID-19 restrictions, nucleic acid testing for SARS-CoV-2 was no longer mandatory, and thus the time of the first nucleic acid negative conversion was not used to assess effectiveness. Finally, different anticancer treatments might interact with azvudine or Paxlovid, and clinical outcomes could also be influenced by cancer stage and classification. Unfortunately, we did not conduct a detailed classification of clinical information for cancer patients in this study, which might impact our research findings.
Conclusion
Our study represents the first large-scale, multicenter retrospective analysis to assess and compare the efficacy and safety of azvudine and Paxlovid in cancer patients with COVID-19. Our findings demonstrated that azvudine significantly reduced all-cause death compared with Paxlovid and exhibited potential superiority in mitigating cumulative composite disease progression risk. This research offers valuable insights and therapeutic options for antiviral treatment among cancer patients with COVID-19.
Data availability
The data that support the findings of this study are not openly available for reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Bray, F., Laversanne, M., Weiderpass, E. & Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 127, 3029–3030 (2021).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 71, 209–249 (2021).
Vineis, P. & Wild, C. P. Global cancer patterns: causes and prevention. Lancet 383, 549–557 (2014).
Pardoll, D. Cancer and the immune system: basic concepts and targets for intervention. Semin. Oncol. 42, 523–538 (2015).
Sica, A. & Massarotti, M. Myeloid suppressor cells in cancer and autoimmunity. J. Autoimmun. 85, 117–125 (2017).
Lee, L. Y. et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet (Lond. Engl.) 395, 1919–1926 (2020).
Kamboj, M. & Sepkowitz, K. A. Nosocomial infections in patients with cancer. Lancet Oncol. 10, 589–597 (2009).
Yang, H. & Rao, Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat. Rev. Microbiol. 19, 685–700 (2021).
V’kovski, P., Kratzel, A., Steiner, S., Stalder, H. & Thiel, V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 19, 155–170 (2021).
Guan, W. J. et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720 (2020).
Liang, W. et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 21, 335–337 (2020).
Zhang, L. et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 31, 894–901 (2020).
Hammond, J. et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N Engl. J. Med. 386, 1397–1408 (2022).
Najjar-Debbiny, R. et al. Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin. Infect. Dis. 76, e342–e349 (2023).
Sun, L. et al. Mechanistic insight into antiretroviral potency of 2’-deoxy-2’-β-fluoro-4’-azidocytidine (FNC) with a long-lasting effect on HIV-1 prevention. J. Med. Chem. 63, 8554–8566 (2020).
National Medical Products Administratior. The National Medical Products Administration has conditionally approved the marketing of Azvudine Tablets (2024, accessed 26 Dec 2024). https://www.nmpa.gov.cn/zhuanti/cxylqx/cxypxx/20210721142223181.html.
Ren, Z. et al. A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv. Sci. (Weinh.) 7, e2001435 (2020).
Zhang, J. L. et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal. Transduct. Target. Therapy 6, 414 (2021).
Dai, M. et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 10, 783–791 (2020).
Ma, J., Yin, J., Qian, Y. & Wu, Y. Clinical characteristics and prognosis in cancer patients with COVID-19: a single center’s retrospective study. J. Infect. 81, 318–356 (2020).
Amani, B. & Amani, B. Azvudine versus paxlovid in COVID-19: a systematic review and meta-analysis. Rev. Med. Virol. 34, e2551 (2024).
Amani, B. & Amani, B. Effectiveness and safety of azvudine in COVID-19: a systematic review and meta-analysis. PLoS One 19, e0298772 (2024).
Li, F. et al. A retrospective analysis of azvudine in patients with COVID-19 and pre-existing cancer. J. Cancer 15, 2442–2447 (2024).
Guermazi, D., Arvanitis, P., Vieira, K., Warner, J. L. & Farmakiotis, D. Oral antivirals for COVID-19 among patients with cancer. Res. Square. https://doi.org/10.21203/rs.3.rs-3876022/v1 (2024).
Zhang, C. et al. NKG2A is a NK cell exhaustion checkpoint for HCV persistence. Nat. Commun. 10, 1507 (2019).
Anwar, K., Nguyen, L., Nagasaka, M., Ou, S. H. I. & Chan, A. Overview of drug-drug interactions between ritonavir-boosted nirmatrelvir (paxlovid) and targeted therapy and supportive care for lung cancer. JTO Clin. Res. Rep. 4, 100452 (2023).
Vaishampayan, U., Parchment, R. E., Jasti, B. R. & Hussain, M. Taxanes: an overview of the pharmacokinetics and pharmacodynamics. Urology 54, 22–29 (1999).
Yao, D., Ding, S., Burchell, B., Wolf, C. R. & Friedberg, T. Detoxication of vinca alkaloids by human P450 CYP3A4-mediated metabolism: implications for the development of drug resistance. J. Pharmacol. Exp. Ther. 294, 387–395 (2000).
Tobaiqy, M. et al. Therapeutic management of patients with COVID-19: a systematic review. Infect. Prev. Pract. 2, 100061 (2020).
Wang, K. et al. Therapeutic response to corticosteroids in a critically ill patient with COVID-19: a case report. Med. (Baltim). 99, e21597 (2020).
WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. Jama 324, 1330–1341 (2020).
Jung, K. et al. Altered pathogenesis of porcine respiratory coronavirus in pigs due to immunosuppressive effects of dexamethasone: implications for corticosteroid use in treatment of severe acute respiratory syndrome coronavirus. J. Virol. 81, 13681–13693 (2007).
Lee, N. et al. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J. Clin. Virol. 31, 304–309 (2004).
Ma, Y. et al. Corticosteroid use in the treatment of COVID-19: a Multicenter Retrospective Study in Hunan, China. Front. Pharmacol. 11, 1198 (2020).
Sun, Y. et al. Oral azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study. EClinicalMedicine 59, 101981 (2023).
Acknowledgements
We extend our sincere gratitude to all the participants involved in this study.
Funding
This work was supported by the National Key Research and Development Program of China (2023YFC3043514 and 2022YFC2303100), 2024 Special Project of the National Key Laboratory of Innovative Drugs for Antiviral Infectious Diseases, Funding for Scientific Research and Innovation Team of The First Affiliated Hospital of Zhengzhou University (QNCXTD2023002 and ZYCXTD2023002).
Author information
Authors and Affiliations
Contributions
Bohan Jia, Junyi Sun, Di Zhu, Ling Wang and Xiaobo Hu contributed equally to this work. Hongxia Liang, Zujiang Yu and Zhigang Ren conceived and designed the study; Guowu Qian, Donghua Zhang, Silin Li, Hong Luo, Shixi Zhang, Guotao Li and Guangming Li managed the patients and collected the data; Di Zhu, Haiyu Wang, Ling Wang and Xiaobo Hu analyzed the data; Bohan Jia, Junyi Sun wrote the manuscript; All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jia, B., Sun, J., Zhu, D. et al. Efficacy and safety of azvudine versus nirmatrelvir/ritonavir in cancer patients with COVID-19. Sci Rep 15, 11022 (2025). https://doi.org/10.1038/s41598-025-85677-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85677-w