Abstract
Polydopamine (PD), inspired by the wet adhesion mechanism of mussel foot proteins, has emerged as a promising adhesive material with wide-ranging applications. This study aimed to compare the adhesive properties of PD and Glass Ionomer Cement (GIC) on enamel and dentin substrates, evaluating PD’s potential as an alternative adhesive in dental practice. A total of 120 human premolars were prepared, with 80 teeth allocated for Scanning Electron Microscopy (SEM) analysis and 40 teeth reserved for shear bond strength testing. The 80 teeth for SEM were divided into four groups (n = 20 per group) based on the adhesive used (PD or GIC) and the substrate (enamel or dentin). The bond interfaces were analysed under SEM following adhesive self-polymerization. Statistical analysis using the Mann-Whitney U test (p < 0.05) revealed that GIC and PD showed more microcracks when bonded to dentin compared to enamel, with the PD-enamel group showing the fewest microcracks. For shear bond strength testing, the 40 remaining teeth were divided into four groups (n = 10 per group) according to the same adhesive-substrate combinations. The results analysed using the Kruskal-Wallis test (p < 0.001), indicated that PD bonded to enamel exhibited the highest bond strength, followed by PD-dentin, GIC-enamel, and GIC-dentin. These findings were consistent with the SEM analysis, demonstrating that PD provides superior bonding to enamel and outperforms GIC in both bond strength and interface quality. This study suggests that PD is a viable alternative to GIC, particularly for enamel bonding, and further research is recommended to assess its long-term clinical performance.
Similar content being viewed by others
Introduction
Over millennia, biological structures have undergone significant changes. Nature has been proactive in refining the characteristics of materials, resulting in an optimized structure-function relationship. Aligned with this prevailing pattern, the mussels’ capacity to securely cling to various moist surfaces, resilient enough to endure formidable oceanic currents, has sparked intrigue and incited exploration into the function of Polydopamine (PD). Mussels use the extensively replicated 3,4-dihydroxy-L-phenylalanine (DOPA) motif in their sticky foot protein Mefp-5 (Mytilus edulis foot protein 5) to achieve their adhesive properties1,2. PD has garnered significant interest due to its role in adhesion mechanisms observed in mussels3.
Dopamine serves as a neurotransmitter and finds extensive utility in the alteration of biomaterial surfaces, drawing inspiration from the adhesive behaviours observed in natural mussel adhesion processes. When exposed to aqueous solutions, dopamine undergoes oxidative polymerization, resulting in the formation of PD. This PD, akin to DOPA, exhibits remarkable adhesive capabilities to various substrates in wet conditions, achieved through both covalent and noncovalent bonding mechanisms. The efficacy of PD coatings extends to a diverse array of surfaces, including superhydrophobic materials that can be transformed into hydrophilic ones2,3,4.
This novel approach to surface modification has garnered considerable attention, particularly within the realms of materials science, biology, and biomedicine. Recent developments have highlighted the use of polymerized dopamine for straightforward, safe, successful, and cost-effective surface alteration in the context of bone tissue engineering. The generation of PD-coated interfaces on biomaterials is heavily influenced by the oxidation of dopamine within mildly alkaline solutions4. Within the domain of dentistry, the concept of wet adhesion inspired by mussels has gained substantial ground as a technique for enhancing surface tissue adhesion and modifying biomaterial surfaces. The wet bio-adhesive capacity, inhibitory effect on Matrix-metalloproteinases (MMP), and biomimetic remineralization capabilities render PD a valuable additive for dental adhesives5.
PD has also been used in medicine as a coating material for implantable medical devices, improving the biocompatibility and durability of the device6,7. PD coatings have been shown to have antimicrobial properties, which can help prevent infections on the surface of medical devices7,8.
One of the advantages of PD as an adhesive material is its biocompatibility. Being a naturally occurring compound found in the adhesive proteins produced by mussels, it is less likely to cause an immune response or other adverse reactions in the body9,10,11,12.
In the field of adhesive dentistry, polydopamine has gained attention due to its unique chemical properties and ability to create a strong and stable bond with tooth structure13. It has been found to have antibacterial properties that may help reduce the risk of secondary caries and other oral infections14. Developing adhesive materials with optimal bonding properties is essential for the long-term success of restorative procedures in dentistry. While glass ionomer cement (GIC) has been widely used as an adhesive material, it has certain limitations, such as reduced bonding strength and durability15. PD, a biomimetic material, has shown great potential as an adhesive material in various applications. However, its effectiveness as an adhesive in dental practice has not been fully evaluated.
The objective of this study is to comprehensively evaluate the adhesive efficacy of PD in comparison to GIC on dental hard tissues, specifically enamel and dentin. Two testing methodologies were utilized: scanning electron microscopy (SEM) for detailed microstructural analysis of bonding interfaces, and shear bond strength testing to quantitatively assess the adhesive performance of both materials. This research aims to fill existing knowledge gaps and provide critical insights into the feasibility of PD as an alternative adhesive in dental practice. Ultimately, this study seeks to contribute to advancements in dental materials and methods, offering innovative solutions that may enhance clinical outcomes and improve restorative practices in dentistry.
Materials and methods
Ethical approval
The study protocol received approval from the Institutional Ethics Committee of AB Shetty Memorial Institute of Dental Sciences, Nitte Deemed to be University (Approval No. ABSMIDS/277/2022).
Sample preparation
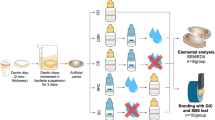
A total of 120 freshly extracted, non-carious, unrestored permanent human premolars were collected for this study. Of these, 80 were allocated for Scanning Electron Microscopy (SEM) analysis, while 40 were designated for shear bond strength testing. The roots of all teeth were sectioned perpendicular to the long axis using a low-speed diamond disk under constant water irrigation, exposing both enamel and dentin surfaces.
For SEM analysis, the samples were divided into two groups based on the substrate type: enamel or dentin. Each group was further subdivided according to the adhesive material used, creating four groups of 20 teeth each:
-
1.
EG: Enamel with GIC,
-
2.
EP: Enamel with PD,
-
3.
DG: Dentin with GIC,
-
4.
DP: Dentin with PD.
For shear bond strength testing, the remaining 40 samples were similarly divided into the same four groups, with each group containing 10 teeth.
SEM analysis
Surface treatment and adhesive application
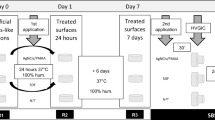
The enamel surfaces were etched with 37% phosphoric acid for 15 s, and dentin surfaces were treated with 2.5% sodium hypochlorite for 15 s to remove the smear layer. The treated surfaces were rinsed with distilled water and dried.
PD was synthesized via oxidative polymerization of dopamine hydrochloride (Sigma Aldrich, Germany) under alkaline conditions, forming a PD coating. This coating was applied to both enamel and dentin, and self-polymerization was allowed to proceed for 24 h. Conventional GIC (Fuji II, GC Japan) was used for the GIC groups, applied in luting consistency following the manufacturer’s instructions. After setting, all samples were stored in distilled water at 37 °C for 24 h before further testing.
Sample preparation for SEM
Following curing, the samples were prepared for SEM analysis by sectioning with a low-speed diamond saw under continuous water cooling to minimize damage. The sectioned samples were dehydrated in a graded ethanol series (50%, 70%, 90%, 100%) to eliminate moisture. Dehydrated samples were mounted on aluminium stubs with conductive carbon tape and sputter-coated with gold to enhance surface conductivity. The bond interfaces were examined under a scanning electron microscope at 1000x magnification to evaluate microstructural features and identify microcracks or defects. SEM images were processed using ImageJ software (v1.53c) for microcrack quantification. To ensure accuracy, a single experienced operator performed all measurements. Three readings per image were taken, and median values were calculated to minimize the influence of outliers and ensure representative results.
Shear bond strength testing
For shear bond strength testing, the occlusal surfaces were ground flat using 320, 600, and 1200-grit silicon carbide abrasive disks under constant water flow to standardize the bonding surface. Each tooth was mounted on poly(methyl methacrylate) resin blocks with the exposed enamel or dentin surface facing upward.
As per the group designations, the respective adhesives (PD or GIC) were applied to the substrates, and a cylindrical buildup of 5 mm in height was created16,17. Glass ionomer groups were conditioned with Ketac Conditioner (3 M ESPE) for 10 s, followed by rinsing with water-soaked cotton pellets and drying with clean ones, according to the Atraumatic Restorative Treatment (ART) protocol17.
The shear bond strength was evaluated using a universal testing machine (ZWICK/ROELL Z020 20KHN). A pre-load force of 0.5 N was applied, followed by a crosshead speed of 0.5 mm/min. Force was applied perpendicular to the bonding interface until debonding occurred, and the load required for debonding was recorded for each sample.
Statistical analysis
All data were compiled and analysed using SPSS version 25. The Mann-Whitney U Test was employed for the SEM data, while the Kruskal-Wallis test was used to analyse the shear bond strength data. A p-value of less than 0.05 was considered statistically significant.
Results
SEM analysis
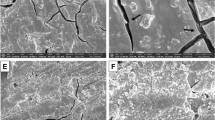
SEM analysis revealed variations in the bond quality between the adhesive materials and the dental hard tissues. Microcracks were observed in several samples at the interface between both PD and GIC with enamel and dentin. In certain instances, high-quality adhesion and close contact were noted between the materials and hard dental tissues, particularly in the PD groups (Figs. 1, 2). Table 1 illustrates the results, showing that the least microcracks were observed between enamel and PD (EP: 2.19 μm), while the most were noted between dentin and PD (DP: 90.95 μm). The GIC groups showed larger microcracks compared to the PD groups, with median values of 56.99 μm for enamel and GIC (EG) and 43.89 μm for dentin and GIC (DG). (Figs. 3, 4).
Statistical comparisons using the Mann-Whitney U test revealed significant differences between Group 1 (EG) and Group 2 (EP) (p = 0.009), and between Group 2 (EP) and Group 4 (DP) (p = 0.009). These findings suggest that PD forms a significantly better bond with enamel than with dentin, and that the bond quality of PD to enamel is superior to that of GIC (Table 1).
Shear bond strength analysis
Shear bond strength testing revealed that PD exhibited the highest bond strength when applied to enamel (EP: 12.10 N). PD also demonstrated stronger adhesion to dentin (DP: 9.81 N) compared to GIC. The GIC groups showed significantly lower bond strengths, with enamel (EG: 5.03 N) and dentin (DG: 4.31 N) both exhibiting weaker adhesion compared to the PD groups. (Fig. 5).
A Kruskal-Wallis test confirmed significant differences between the groups (p < 0.001). Post-hoc analysis showed that the bond strength of PD to enamel (EP) was significantly higher than in all other groups (Table 2), particularly when compared to GIC on both enamel and dentin. The comparison between PD on enamel (EP) and dentin (DP) showed no significant difference in bond strength.
Pairwise comparisons confirmed significant differences between EP and DP (p < 0.001), and between EP and EG (p < 0.001), indicating that PD outperforms GIC in terms of bonding strength to enamel (Table 3). No significant difference was found between EP and DP (p = 0.818), suggesting that PD exhibits similar bonding strength to both enamel and dentin.
The study demonstrates that PD exhibits superior adhesive properties when compared to GIC, particularly with enamel. SEM analysis showed fewer microcracks between PD and enamel compared to other combinations, and shear bond strength testing confirmed that PD provides significantly stronger adhesion to both enamel and dentin compared to GIC.
Discussion
PD is a polymer inspired by nature, derived from dopamine, a neurotransmitter naturally found in the human body. PD has demonstrated exceptional adhesive properties to a variety of surfaces, including dental hard tissues such as enamel and dentin18. Upon application, PD undergoes a self-polymerization process, forming a thin adhesive film capable of robust bonding with dental surfaces4. This unique property of PD has attracted attention for its potential applications in dentistry, where strong and durable adhesion to enamel and dentin is critical for the longevity of restorations. Currently available adhesive materials, while widely used, often face limitations such as insufficient bonding strength and lower durability18,19. PD’s ability to form covalent bonds with dental tissues presents a significant advantage over traditional adhesives20,21.
In contrast, GIC is a commonly used dental material for both restorative and adhesive purposes. It consists of glass particles that, through an acid-base reaction, form a cement-like material with good adhesive properties to both enamel and dentin22. GIC’s adhesion relies on both chemical and mechanical bonding mechanisms, GIC is well-established in restorative dentistry, however its limitations—such as lower bond strength and susceptibility to microcracks highlight the need for alternative adhesive materials with enhanced performance22,23.
The SEM analysis highlighted clear differences in the adhesion quality between dental hard tissues and both GIC and PD. Microcrack formation, used as a marker of bond integrity, was most prominent at the PD-dentin interface, while the least microcracks were found between PD and enamel. Statistical analysis of P-values confirmed a significant difference in bond strength, with PD showing stronger adhesion to enamel than to dentin. These results suggest that PD forms a more durable bond with enamel compared to dentin.
The results suggest that bonding to dentin is more challenging, as larger microcracks were observed at the material-dentin interface compared to enamel. This is likely due to the structural differences between the two tissues, with dentin being more heterogeneous and porous24.
The shear bond strength analysis indicated that PD exhibited the highest bond strength to enamel among all groups. Furthermore, PD displayed significantly stronger adhesion to both enamel and dentin when compared to glass ionomer cement (Fig. 5). In contrast, GIC demonstrated notably lower bond strength, with values of 5.03 N for enamel and 4.31 N for dentin, supporting previous findings that emphasize the limitations of GIC in high-strength applications15,22.
While PD exhibited higher shear bond strength with enamel than dentin, there was no statistically significant difference in its adhesion between the two substrates. This observation aligns with previous studies, which have consistently shown that dental materials tend to bond more strongly to enamel than to dentin, reflecting the inherent differences in their structural and compositional properties25,26.
The adhesion of PD can be affected by the different surface properties and chemical compositions of enamel and dentin. Enamel is primarily composed of highly mineralized hydroxyapatite crystals, while dentin contains a higher proportion of organic material, such as collagen fibres, and is less mineralized.
PD contains surface-anchored catecholamine moieties that enrich the interface with calcium ions, promoting the formation of hydroxyapatite crystals that align to the c-axes parallel to the PD layer, similar to natural hydroxyapatites found in mineralized tissues. This alignment of hydroxyapatite crystals can facilitate the formation of calcium-rich bonds with the surface-anchored catecholamine moieties present in PD, enhancing adhesion. Furthermore, the amino and catechol groups in the polydopamine structure can also promote the formation of hydrogen bonds and covalent bonds with the substrate, further enhancing adhesion7,27.
Based on the SEM analysis, while PD demonstrated excellent adhesion to enamel, its bonding effectiveness to dentin was observed to be lower. This may be attributed to PD’s adhesive mechanism, which promotes the formation of hydroxyapatite on surfaces due to the abundant catecholamine groups in its structure27. However, the heterogeneous composition of dentin, with hydroxyapatite crystals interspersed among collagen fibres, presents a challenge to this process28,29. Additionally, factors such as hydrostatic pressure within the pulp, dentinal fluid flow, and the higher moisture content of dentin can interfere with the close interaction between polydopamine and dentin, impacting the level of adhesion achieved30.
Contrary to the SEM findings, there was no statistically significant difference in the shear bond strength values of PD with enamel and dentin, suggesting effective mechanical bonding to both substrates. This can be attributed to PD’s unique mechanical properties, including its highly oriented layered structure, which provides mechanical robustness with a Young’s modulus of 13 ± 4 GPa and hardness of 0.21 ± 0.03 GPa31 Additionally, PD enhances tensile and compressive strength through strong interfacial interactions, combining chemical bonding and physical interlocking mechanisms, such as hydrogen bonds, π–π interactions, and increased surface roughness32. These properties likely contribute to the high shear bond strength observed in our study and align with findings from other studies, supporting PD’s efficacy in bonding to both enamel and dentin20,33.
To sum it up, while the presence of microcracks may imply a weaker bond at the dentin interface, the shear bond strength results demonstrate that both enamel and dentin can achieve effective adhesion, albeit through different underlying mechanisms. This complexity underscores the necessity for further research to explore the relationship between microstructural integrity and bond strength.
Based on the findings of the current study, PD shows great promise for dental applications due to its strong adhesion to dental substrates. However, as indicated by our SEM analysis, bonding to dentin presents challenges that require further investigation and innovative solutions. One potential strategy could involve incorporating polydopamine into existing adhesive systems to establish a stronger, biomineralized bond20. Additionally, surface modification techniques such as plasma treatment and laser irradiation may optimize the bonding surface, enhancing adhesion and durability34,35.
Conclusion
This study demonstrates that PD exhibits significantly stronger adhesion to enamel compared to dentin and outperforms GIC in bond strength and quality. The findings indicate that PD could serve as a viable alternative adhesive in restorative dentistry. Further research is needed to assess the long-term clinical efficacy of PD in dental applications.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Silverman, H. G. & Roberto, F. F. Understanding marine mussel adhesion. Mar. Biotechnol. 9, 661–681 (2007).
Lee, H., Scherer, N. F. & Messersmith, P. B. Mussel-inspired surface chemistry for multifunctional coatings. Science 318, 426–430. https://doi.org/10.1126/science.1147241 (2007).
Liu, Y., Ai, K. & Lu, L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 114, 5057–5115. https://doi.org/10.1021/cr400407a (2014).
Yang, H., Luo, J., Lv, Y., Shen, P. & Xu, Z. Surface engineering of polymer membranes via mussel-inspired chemistry. J. Membr. Sci. 483, 42–59. https://doi.org/10.1016/j.memsci.2015.02.027 (2015).
Yao, C. et al. High-performance bioinspired microspheres for boosting dental adhesion. Small e2310251 https://doi.org/10.1002/smll.202310251 (2024).
Cheng, W. et al. Versatile polydopamine platforms: synthesis and promising applications for surface modification and advanced nanomedicine. ACS Nano 13, 8537–8565. https://doi.org/10.1021/acsnano.9b04436 (2019).
Ryu, J., Ku, S. H., Lee, H. & Park, C. B. Mussel-inspired polydopamine coating as a universal route to hydroxyapatite crystallization. Adv. Funct. Mater. 20, 2132–2139. https://doi.org/10.1002/adfm.200902347 (2010).
Liu, Y., Zhang, Z., Lv, H., Qin, Y. & Deng, L. Surface modification of chitosan film via polydopamine coating to promote biomineralization in bone tissue engineering. J. Bioact Compat. Polym. 33, 134–145. https://doi.org/10.1177/08839115177132 (2018).
Wang, H. et al. Mussel-inspired polydopamine coating: a general strategy to enhance osteogenic differentiation and osseointegration for diverse implants. ACS Appl. Mater. Interfaces 11, 7615–7625. https://doi.org/10.1021/acsami.8b21558 (2019).
Kwon, I. S. & Bettinger, C. J. Polydopamine nanostructures as biomaterials for medical applications. J. Mater. Chem. B 6, 6895–6903. https://doi.org/10.1039/C8TB02310G (2018).
Huang, Z. et al. Enhanced in vitro biocompatibility and osteogenesis of titanium substrates immobilized with dopamine-assisted superparamagnetic Fe3O4 nanoparticles for hBMSCs. R Soc. Open. Sci. 5, 172033. https://doi.org/10.1098/rsos.172033 (2018).
Wang, T. et al. Polydopamine-coated chitosan hydrogel beads for synthesis and immobilization of silver nanoparticles to simultaneously enhance antimicrobial activity and adsorption kinetics. Adv. Compos. Hybrid. Mater. 4, 696–706. https://doi.org/10.1007/s42114-021-00305-1 (2021).
Zhang, J. et al. A novel dental adhesive containing Ag/polydopamine-modified HA fillers with both antibacterial and mineralization properties. J. Dent. 111, 103710. https://doi.org/10.1016/j.jdent.2021.103710 (2021).
Ramburrun, P. et al. Recent advances in the development of antimicrobial and antifouling biocompatible materials for dental applications. Materials 14, 3167. https://doi.org/10.3390/ma14123167 (2021).
El Wakeel, A. M., Elkassas, D. W. & Yousry, M. M. Bonding of contemporary glass ionomer cements to different tooth substrates; Microshear bond strength and scanning electron microscope study. Eur. J. Dent. 9, 176–182. https://doi.org/10.4103/1305-7456.156799 (2015).
Deng, Z., Shang, B. & Peng, B. Polydopamine based colloidal materials: synthesis and applications. Chem. Rec. 18, 410. https://doi.org/10.1002/tcr.201700051 (2018).
Frencken, J. E., Pilot, T., Songpaisan, Y. & Phantumvanit, P. Atraumatic restorative treatment (ART): Rationale, technique, and development. J. Public Health Dent. 56, 135–140. https://doi.org/10.1111/j.1752-7325.1996.tb02423.x (1996).
Teng, R. et al. Combination of polydopamine coating and plasma pre-treatment to improve bond ability between PEEK and primary teeth. Front. Bioeng. Biotechnol. 8, 630094. https://doi.org/10.3389/fbioe.2020.630094 (2021).
Chen, C. et al. Antibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticles. Dent. Mater. 30, 891–901. https://doi.org/10.1016/j.dental.2014.03.008 (2014).
Devarajan, S. S. et al. Effect of polydopamine incorporated dentin adhesives on bond durability. J. Adhes. Sci. Technol. 35, 185–198. https://doi.org/10.1080/01694243.2020.1792136 (2021).
Zhang, C. et al. Deposition and adhesion of polydopamine on the surfaces of varying wettability. ACS Appl. Mater. Interfaces 9, 30943–30950. https://doi.org/10.1021/acsami.7b09774 (2017).
McLean, J. W. The clinical use of glass-ionomer cements. Dent. Clin. North Am. 36, 693–711. (1992).
Ferrari, M., Cagidiaco, M. C., Goracci, C., Vichi, A. & Mason, P. N. Bonding to root canal: Structural characteristics of the substrate. Am. J. Dent. 13, 81–86. (2000).
Goldberg, M., Kulkarni, A. B., Young, M., Boskey, A. & Dentin structure, composition and mineralization. Front. Biosci. (Elite Ed.) 3, 711–735. https://doi.org/10.2741/e281 (2011).
Andermatt, L. & Özcan, M. Micro-shear bond strength of resin composite cement to coronal enamel/dentin, cervical enamel, cementoenamel junction, and root cementum with different adhesive systems. J. Adhes. Sci. Technol. 35, 2079–2093. https://doi.org/10.1080/01694243.2021.1872195 (2021).
Carvalho, T. S., van Amerongen, W. E., de Gee, A., Bönecker, M. & Sampaio, F. C. Shear bond strengths of three glass ionomer cements to enamel and dentine. Med. Oral Patol. Oral Cir. Bucal. 16, e406–e410. https://doi.org/10.4317/medoral.16.e406 (2011).
Ghorbani, F., Zamanian, A., Behnamghader, A. & Daliri-Joupari, M. Bone-like hydroxyapatite mineralization on bio-inspired PDA nanoparticles using microwave irradiation. Surf. Interfaces 15, 38–42. https://doi.org/10.1016/j.surfin.2019.01.007 (2019).
Moradian-Oldak, J. & George, A. Biomineralization of enamel and dentin mediated by matrix proteins. J. Dent. Res. 100, 1020–1029. https://doi.org/10.1177/00220345211018405 (2021).
Manuja, N., Nagpal, R. & Pandit, I. K. Dental adhesion: mechanism, techniques, and durability. J. Clin. Pediatr. Dent. 36, 223–234. https://doi.org/10.17796/jcpd.36.3.68805rl1r037m063 (2012).
Hashimoto, M. et al. Fluid movement across the resin-dentin interface during and after bonding. J. Dent. Res. 83, 843–848. https://doi.org/10.1177/154405910408301104 (2004).
Coy, E., Iatsunskyi, I., Colmenares, J. C., Kim, Y. & Mrówczyński, R. Polydopamine films with 2D-like layered structure and high mechanical resilience. ACS Appl. Mater. Interfaces 13, 23113–23120. https://doi.org/10.1021/acsami.1c02483 (2021).
Qin, Z. et al. Recent advances in polydopamine for surface modification and enhancement of energetic materials: a mini-review. Crystals 13, 976. https://doi.org/10.3390/cryst13060976 (2023).
Çoban Kanyılmaz, A. N., Belli, S. & Neelakantan, P. The mussel-inspired polydopamine surface treatment influences bond strength of glass fiber posts to radicular dentin. Int. J. Adhes. Adhes. 105, 102791. https://doi.org/10.1016/j.ijadhadh.2020.102791 (2021).
Dong, X., Li, H., Chen, M., Wang, Y. & Yu, Q. Plasma treatment of dentin surfaces for improving self-etching adhesive/dentin interface bonding. Clin. Plasma Med. 3, 10–16. https://doi.org/10.1016/j.cpme.2015.05.002 (2015).
Wang, J., Yang, K. & Zhang, B. Effects of Er laser pre-treatment on dentin structure and bonding strength of primary teeth: an in vitro study. BMC Oral Health 20, 316. https://doi.org/10.1186/s12903-020-01315-z (2020).
Acknowledgements
The researchers would like to acknowledge the Deanship of Scientific Research, Taif University, for funding this work.
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rao, L.N., Sarkar, S., Shetty, A. et al. A comparative study of polydopamine vs. glass ionomer cement for adhesion mechanisms on enamel and dentin using SEM and shear bond strength evaluation. Sci Rep 15, 2243 (2025). https://doi.org/10.1038/s41598-025-85735-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85735-3
Keywords
This article is cited by
-
Mussel-inspired remineralizing agent: effects on morphology and permeability of dentin after erosion and abrasion cycling protocol
Clinical Oral Investigations (2025)