Abstract
Allergic rhinitis and asthma are common respiratory conditions with complex etiologies involving genetic, environmental, and physiological factors. In these conditions, the role of thyroid function remains underexplored. This study enrolled 116 participants with a mean age of 29.55 years: 66 with allergic rhinitis, 20 with concomitant asthma, and 30 healthy controls. BMI and serum IgE, T3, T4, and TSH levels were measured. A significant familial history of atopy was reported by 66.4% of participants. Skin prick tests revealed predominant sensitivity to house dust mites (55.2%). BMI was within normal ranges across all groups, serum IgE levels were significantly elevated in patients with respiratory allergies compared to controls (Mean = 36.85 IU/mL), especially those with allergic rhinitis and concomitant asthma (Mean = 218 IU/mL). Significant differences in serum T4 levels were observed, particularly among those with allergic rhinitis (p < 0.001 and p < 0.05). Pearson correlation analysis revealed significant associations between age, BMI, and thyroid hormones, with notable sex-specific differences (p < 0.05 and p < 0.01). The study highlights the complex interplay between metabolic, endocrine, and immune responses in respiratory allergies. Elevated serum IgE levels and alterations in thyroid function, particularly among males, suggest potential pathways for targeted therapeutic interventions. Further research is needed to elucidate these relationships and their underlying mechanisms.
Similar content being viewed by others
Introduction
Allergic rhinitis (AR) is the predominant IgE-mediated immune response within the spectrum of atopic disorders, exhibiting a diverse prevalence pattern worldwide1. Studies by Marine Savouré et al. have underscored this variability, reporting prevalence rates from 1 to 54% across different regions. Notably, prevalence rates vary within areas as well, with figures ranging from 3.6 to 22.8% in Africa, 3.5 to 54.5% in America, 1.0 to 47.9% in Asia, 1.0 to 43.9% in Europe, and 19.2 to 47.5% in Oceania2.
AR typically manifests a peak incidence during the second to fourth decade of life, followed by a gradual decline1,2. However, it’s noteworthy that AR also presents a substantial burden among the pediatric population3. Various risk factors contribute to the development of AR, including exposure to aeroallergens (both inhalant and occupational), a familial predisposition to atopy, elevated levels of allergen-specific IgE (> 100 IU/mL), and socioeconomic factors. These risk factors and the evolving landscape of environmental exposures contribute to temporal shifts in AR prevalence within similar populations4.
Nevertheless, certain risk factors associated with AR may also coincide with comorbidities such as asthma and atopic dermatitis. However, the correlation between these factors and AR remains inconclusive. For instance, factors like overweight/obesity or lifestyle changes exhibit inconsistent associations with AR across different studies5,6.
Moreover, the clinical presentation of AR, characterized by symptoms such as nasal congestion, sneezing, postnasal drip, nasal pruritus, and clear rhinorrhea, often overlaps with nonallergic rhinitis. Additionally, hormonal fluctuations, particularly in females, can further complicate the clinical picture7. Recent studies have uncovered intriguing associations between thyroid hormones and allergies among individuals with allergic rhinitis. Specifically, one study revealed that 24% of allergic patients also presented with hypothyroidism, whereas only 8% simultaneously had asthma. This indicates a potential link between these conditions8,9.
The intricate interplay between thyroid hormones (THs) and the immune system remains an active research area. Studies have elucidated the regulatory role of THs in modulating immune cell function, underscoring the bidirectional relationship between THs and immune responses10,11,12. Specifically, THs exert their influence through binding to thyroid hormone receptors (THRs) present in immune cells, thereby orchestrating various immunological processes13,14. Additionally, THs demonstrate diverse effects on immune cell proliferation, differentiation, and apoptosis, further highlighting their multifaceted roles in immune regulation.
Studies have shown that triiodothyronine (T3) promotes bone marrow maturation induction of dendritic cells (BMDC), associated with T cell stimulation in mice. However, THs also induce increased T cell apoptosis in human lymphocyte cell lines, indicating a dual role for THs in T cell immunity15,16. Similarly, B cell proliferation was observed in response to T3 stimulation of human peripheral blood and, it was suggested that thyroid-stimulating hormone (TSH) could act as a humoral mediator within the immune system, similar to respective cytokines17,18.
Despite significant strides in understanding the immunomodulatory functions of THs, gaps persist in our comprehension of their impact on immune homeostasis. Perturbations in thyroid function, including hyperthyroidism and hypothyroidism, have been implicated in allergic diseases, underscoring the clinical relevance of thyroid status assessment in patients with allergic rhinitis8,19,20.
In clinical practice, assessing thyroid function entails evaluating total/free tetraiodothyronine (T4) and triiodothyronine (T3) levels, alongside thyroid-stimulating hormone (TSH) concentrations. Notably, TSH serves as a sensitive marker for overall thyroid function. Research suggests that even within the reference range, TSH concentration above 2.5 mIU/L could signify a risk factor for disease burden, warranting further investigation21,22,23.
Additionally, emerging evidence suggests a potential interplay between thyroid hormones, body mass index (BMI), and Th2 cell-mediated inflammation in AR. Leptin, a hormone in thermoregulation and metabolism, may influence THs and contribute to Th2 cell inflammation in AR24,25,26,27. Furthermore, elevated levels of IgE, a hallmark of allergic diseases, expectedly higher in allergic patients, may correlate with heightened TH levels, highlighting potential links between the thyroid axis and allergic inflammation28,29.
Despite these intriguing findings, our understanding of the intricate crosstalk between THs and the immune system remains incomplete, warranting further investigation. Hence, this study aims to explore associations between TH levels, IgE levels, and BMI in our AR patients, with or without asthma comorbidity.
Results
The study enrolled a total of 116 participants, categorized into distinct groups: 66 (56.9%) individuals with allergic rhinitis, 20 (17%) experiencing both allergic rhinitis and asthma symptoms, and 30 (25.9%) individuals comprising the healthy control group. The male–female ratio was 50:66, with a mean age of 29.55 years (standard deviation ± 11.5, age range 11–59 years). The average age of onset for allergy symptoms was determined to be 14.7 years (range 0–49 years). Furthermore, 66.4% of participants reported a positive familial history of atopy, indicating at least one family member was diagnosed by a physician with an allergic disease (Table 1).
Skin prick test
Given that all participants in the research group and a subset of the healthy control group exhibited a positive sensitivity reaction to various aeroallergens within the chosen panel, the findings underscore the predominant sensitivity to house dust mites, with a higher prevalence compared to pollens, animal dander and molds (with 55.2% to mites; 21% to pollens; 4.43% to animal dander and 4.25% to molds). Furthermore, a higher prevalence of positive skin prick test responses was observed among male subjects for all aeroallergens. However, the difference was slight only for dust mite allergens (Table 1).
BMI
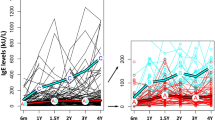
Patients with respiratory allergies exhibited normal ranges for BMI, with similar mean values observed in male and female subjects (BMI = 23.18 and 23.35 in males with Rh and RhA, compared to females with BMI = 22.9 and 22.88, respectively) (Fig. 1).
Total serum IgE levels
As expected, the total serum IgE levels in patients with respiratory allergies were significantly higher, compared to the healthy controls, as determined by the Kruskal–Wallis test (p < 0.05), within both the male and female groups. However, patients diagnosed only with allergic rhinitis had lower levels of IgE (mean IgE = 135.8 IU/L in males and IgE = 163.26 IU/L in females) compared to those with concomitant asthma symptoms (mean IgE = 218.2 IU/L in males and IgE = 212.26 IU/L in females) (Fig. 1).
Total serum T3, T4 and TSH levels
The results of our study indicate that over 90% of male and female participants in both the research and control groups had total serum T4 concentrations within the normal range (58–166 nmol/L). However, 3.1–10% of women with allergic rhinitis (AR) and asthma and 5.9% of men with AR showed serum T4 levels above the upper normal limit. Similarly, while 90% of female participants and 80% of male participants had T3 levels within the normal range (1.3–2.9 nmol/L), 3.1–10% of women and 17.6–20% of men with AR or AR + A showed T3 levels exceeding the upper normal limit. Regarding total serum TSH levels, 90–100% of female patients had normal levels, compared to 80–97% of male patients. Notably, 20% of men diagnosed with AR + A had TSH levels that exceeded the upper limit of normal (0.3–5.0 nIU/L), which puts them at risk for subclinical hypothyroidism. In contrast, only 10% of women within the same disease group had TSH levels that fell below the normal limit, making them prone to subclinical hyperthyroidism.
The Kruskal–Wallis Test revealed a significant difference in T4 serum concentrations of males and females in the research and control groups (p < 0.001 and p < 0.05, respectively). These differences were particularly pronounced in male patients with AR compared to those with AR + A, in male patients with AR compared to controls (p < 0.05), and in females across both patient and control groups (p < 0.001). For total serum TSH levels, significant differences were observed only in male patients (p < 0.002), specifically between those with AR and AR + A (p < 0.001) (Table 2, Fig. 2).
Correlation
The results of the Pearson correlation test are intriguing, particularly after aplying the Holm method (adjustedPvalues = p.adjust(pvalues, method = “Holm”) # (under FWER control) to control the Family-Wise Error Rate (FWER). A significant positive correlation (p < 0.001) was found betweenage and BMI in both male and female subjects, highlightin their importance in hormonal changes. Additionally, age was closely associated with the onset of allergy symptoms in both sexes. Interestingly, no correlation was observed between BMI and THs across all subjects (Table 3). However, further significant correlations emerged when participants were categorized into disease and control groups. For instance, a positive correlation between age and BMI was observed exclusively in the study group (p < 0.001). In the rhinitis group, a significant positive correlation was found only between T3 and TSH (p < 0.001). Among patients with both rhinitis and asthma symptoms, a significant positive correlation was identified between T3 and T4 (p < 0.05). Conversely, the healthy control group exhibited a negative correlation between T4 and TSH (p < 0.01) (Table 4). No correlations were identified between IgE levels and other variables in the patient or control groups, except for a statistically significant positive correlation between IgE and age among all female subjects (Table 3).
Discussion
Allergic rhinitis (AR) is a common allergic condition affecting a significant portion of the global population. It often coexists with asthma, and this comorbidity can influence the prevalence and severity of both conditions. A systematic review by Bousquet et al.4 estimated that the global prevalence of allergic rhinitis in some countries can be as high as 50%. This prevalence varies by region, age group, and diagnostic criteria. Studies indicate that 20–50% of patients with allergic rhinitis also have asthma, while up to 80% of patients with asthma have concomitant allergic rhinitis30.
Considering AR is the most prevalent disease worldwide, several studies, have investigated the possible link to different risk factors. Our study stands out because it focuses on patients from a country with a notably lower allergy prevalence compared to its regional counterparts. This distinct perspective allows us to offer valuable insights that challenge common assumptions31,32. It is worth mentioning that obesity is also a major health problem due to physical inactivity and BMI has been associated with an increased risk of developing allergic rhinitis. For example, a study by Harugop et al.25 found that obesity is a risk factor for allergic rhinitis, particularly in adults. However, in a cross-sectional study of Taiwanese children, Li et al.33 reported that overweight and obesity negatively impact the severity of allergic rhinitis also in this group population. Obesity is a well-established risk factor for asthma, and meta-analysis by Parasuaraman et al.34 demonstrated that obesity significantly increases the risk of asthma incidents, especially in adults. The link between BMI and asthma may be due to systemic inflammation, mechanical effects on the respiratory system, and alterations in immune responses. In our study, however, participants with respiratory allergies exhibited normal BMI ranges, with no significant differences between males and females. The mean BMI values were comparable between those with allergic rhinitis and those with rhinitis and asthma. This finding is crucial as it indicates that, within this sample, BMI did not significantly influence the prevalence or severity of respiratory allergies. The only significant correlation between BMI and other variables was with participants’ biological age, supported by Nurhasanah et al.’s recent publication35.
Following the risk factors, elevated serum IgE levels are a hallmark of allergic diseases, including allergic rhinitis and asthma. High IgE levels indicate sensitization to various allergens and correlate with the severity of allergic symptoms.
A study by Gwalabe et al.36 demonstrated that patients with allergic rhinitis had significantly higher serum IgE levels than healthy controls, highlighting the importance of IgE as a biomarker for allergic diseases. Elevated IgE levels are associated with more severe allergic rhinitis and a higher likelihood of asthma comorbidity. Davila et al. found that patients with persistent allergic asthma had higher IgE levels that ranged from > 150 IU/ml to > 400 IU/ml37. As expected, total serum IgE levels were significantly elevated in our patients with respiratory allergies compared to healthy controls. Also, those with concurrent asthma symptoms had higher IgE levels than those with only allergic rhinitis. This difference suggests a more robust immune response in patients with both conditions, potentially indicating more severe or persistent allergic inflammation. This finding aligns with the study by Kostova et al.38 which reported higher IgE levels in patients with both allergic rhinitis and asthma compared to those with only allergic rhinitis.
Likewise, sensitization to house dust mites (HDM) is one of the most common triggers of allergic rhinitis and asthma. Studies have shown a high prevalence of HDM sensitivity among patients with these conditions. For instance, Rodniova et al. reported that HDM sensitization is strongly associated with allergic rhinitis and asthma in children and adults, exceeding 20% in European countries and reaching 40% in communities of North America39. Also, pollen allergens are significant triggers for seasonal allergic rhinitis. Exposure to pollens from trees, grasses, and weeds can lead to the development and exacerbation of allergic rhinitis and asthma. D'Amato et al. highlighted the role of pollens in the onset and exacerbation of respiratory allergies, particularly in urban areas with high pollen counts40. Moreover, sensitization to animal dander, such as cat and dog allergens, is another important risk factor for allergic rhinitis and asthma. A study done by Pinot de Moira et al. found that early exposure to pets may increase the risks associated with a specific pet’s allergic sensitization41. Ongoing, Mold exposure is linked to allergic rhinitis and asthma, especially in damp indoor environments. Forkan et al. reviewed the impact of indoor mold exposure on respiratory health and concluded that it significantly increases the risk of allergic rhinitis and asthma42. The skin prick test results of our patients highlighted a predominant sensitivity to house dust mites (55.2%), followed by pollens (21%), animal dander (4.43%), and molds (4.25%). This pattern aligns with known epidemiological data, emphasizing house dust mites as a major allergen in respiratory allergic conditions. A recent study by Romero-Sanchez et al. corroborated these findings, indicating that house dust mites are a common sensitizing allergen in patients with allergic rhinitis and asthma43. The higher prevalence of positive skin prick test responses among male subjects suggests potential sex-based differences in allergen sensitivity. The results were validated by the study conducted by Karl-Christian Bergman44.
Interestingly, recent studies have identified a possible link between thyroid dysfunction and allergic rhinitis. Both hypothyroidism and hyperthyroidism can influence immune responses and exacerbate allergic conditions. Research by Fawzan al. demonstrated patients with allergic rhinitis were more likely to have thyroid dysfunction, suggesting that thyroid screening could benefit patients presenting with allergic symptoms. This connection is likely due to the role of thyroid hormones (THs) in regulating immune cell maturation, proliferation, apoptosis, and polarization8,45. Triiodothyronine (T3) and thyroxine (T4) play a crucial role in immune system regulation, and the iodine trace element is their base component. Alterations in thyroid hormone levels can impact the severity and management of allergic rhinitis, as was shown in a pilot study by Fawzan et al. depicting 20% of AR patients with concomitant hypothyroidism, with a suggestion of dietary iodine deficiency8,45. Moreover, the “allergic rhinitis research model” by Nakamura et al. demonstrated that dietary iodine could alleviate AR’s symptoms by stimulating ferroptosis in activated B cells and inhibiting allergen-specific IgE, without raising serumTH levels despite higher iodine concentrations45. In line with the above, our study revealed that although over 90% of participants had normal serum T4 levels (a slightly lower percentage of males had normal T3 levels), significant differences in T4 concentrations were observed in males and females, particularly among patients with allergic rhinitis as compared to controls. These differences were higher by an average of 39 nmol/L in females and 33 nmol/L in males with AR, deviating by about 30% from the control group. Detection of major alterations in thyroid function is an easy way to confirm by measuring and interpreting thyroid function tests, however, minor alterations could be challenging and raise questions about whether allergologists should screen for thyroid function in patients without specific indications for thyroid testing46. In our study, we adopted the reference values established by the test kit manufacturer (Beckman Coulter) due to the absence of defined reference values in our laboratories, which necessitates research at the general population level. Also, given the circadian rhythm of TSH secretion—peaking between 2:00 and 4:00 a.m. and reaching its lowest levels between 3:00 and 8:00 p.m.—and a decrease of about 30% of TSH levels at 2 h after calorie intake, blood samples drawn only once between 8:00 and 9:00 a.m. minimized the risk of misinterpreting TSH levels as indicative of subclinical hyperthyroidism47. In a study by Andersen et al., the thyroid gland subclinical dysfunction could be diagnosed based on the normal reference range for total T4 and within or above the range for TSH levels46. As for TSH levels, significant differences were observed in male patients between those with allergic rhinitis and those with concomitant asthma symptoms. These findings suggest potential alterations in thyroid function in the context of respiratory allergies in both genders as a recent study by Zhang et al.48 concluded the usefulness of screening for thyroid function and autoimmunity in patients with allergic symptoms. Moreover, the common pathway between allergy and autoimmunity is further supported by Molnar et al.49 who concluded the modification of thyroid hormone levels caused by antigen mimicry between thyroid allergens and aeroallergens.
Moreover, the Pearson correlation analysis indicated significant positive correlations between age and BMI in male and female subjects, highlighting these factors as potential risk indicators for hormonal changes. Also, males exhibited a negative correlation between BMI and T4, which was not observed in females. This sex-specific difference in correlation patterns may point to distinct metabolic or endocrine responses in allergic conditions.
In disease-specific groups, the correlation patterns varied. For instance, in the rhinitis group, age and BMI were positively correlated, and there was a significant negative correlation between T3 and TSH. These correlations were also present in patients with rhinitis and asthma, considering age and BMI, but regarding hormones, significant positive correlations were recorded between T3 and T4. Interestingly, the control group only showed a significant negative correlation between T4 and TSH, indicating that the presence of allergic diseases might influence thyroid hormone interplay. A study by Zhengzhou et al. reported similar correlation patterns between thyroid function and BMI50.
Ultimately, 66.4% of atopic cases reported among the participants’ families highlight the genetic basis of respiratory allergies, which are hypersensitivity reactions of the immune system. These allergies clinically manifest through the interplay of familial predisposition and environmental factors51,52. Furthermore, autoimmune diseases—another form of immune system dysregulation—share common genetic components with allergies, as evidenced by findings from genome-wide association studies (GWAS)53,54. Notably, this connection suggests a higher prevalence of hidden autoimmune thyroiditis in allergic patients compared to the control group. This shared genetic overlap underscores the complex interplay between immune-related disorders, paving the way for more targeted research and therapeutic interventions.
Our study may have potential limitations, including its single-center and region-specific nature. Most participants were urban residents with easier access to the outpatient Allergy service, whereas rural residents were more likely to use local outpatient services for allergy symptoms. Additionally, a relatively small sample size within each research subgroup could be another limitation.
Measurements of serum thyroid hormones and TSH were conducted using radioimmunoassay (RIA) kits, which are known for their high sensitivity. This is one of the strengths of the study in terms of accuracy. Additionally, the study exclusively included patients diagnosed with respiratory allergies, excluding those with any prior known thyroid dysfunction, which further strengthens its findings.
Future research directions include investigating the levels of serum thyroglobulin antibodies (TgAb) and thyroid peroxidase antibodies (TPOAb) to explore the potential link between autoimmune thyroid disease and allergic rhinitis in our patients. This research could provide insights into whether aggravations of respiratory allergy symptoms are associated with autoimmune thyroid disease or if severe allergy symptoms could exacerbate thyroid autoimmune conditions. Additionally, establishing reference values for thyroid hormones (THs) specific to the Kosovar population could provide further insight into the significance of our findings.
Conclusion
This study underscores the complexity of respiratory allergies and their association with various physiological parameters. The findings highlight the predominant sensitivity to house dust mites among allergic individuals and the potential sex-based differences in allergen sensitivity. Elevated serum IgE levels in patients with respiratory allergies affirm their role as a biomarker for allergic inflammation. The observed correlations between BMI, age, and thyroid hormones suggest that metabolic and endocrine factors may play a role in the pathophysiology of allergic diseases, especially considering significantly higher thyroid hormone levels among patients with allergic rhinitis. Further research is needed to elucidate these relationships and explore the underlying mechanisms. These insights can inform clinical practices and guide targeted interventions for managing respiratory allergies.
Patients and methods
Inclusion criteria
Patients aged 5–65 with diagnosed AR, with or without asthma symptoms, and positive reactions to at least one aeroallergen via skin prick tests were eligible.
Exclusion criteria
Excluded were individuals outside the age range, those with dermatographism, hyper/hypothyroidism diagnoses, or ongoing treatments for these conditions, and pregnant females.
Healthy controls included subjects with no history of allergic symptoms or hormonal diseases.
Data collection
Before the commencement of the investigation, informed written consent was obtained from each participant. Socio-demographic information, including age, gender, onset of allergy symptoms, and family history of atopy, was meticulously recorded. Details regarding presenting symptoms and any associated acute and chronic comorbidities were also documented.
Body Mass Index (BMI) was calculated for each participant using measurements obtained with a stadiometer and an electronic scale to determine body weight in kilograms divided by the square of the height in meters (kg/m2). BMI classifications were based on guidelines from the National Institutes of Health following WHO standards, categorizing individuals as normal (BMI 18.5–24.9 kg/m2), low (< 18.5 kg/m2), overweight (25–29.9 kg/m2), or obese (≥ 30 kg/m2) (https://www.nice.org.uk/guidance/cg189/chapter/Recommendations).
Blood samples (5 ml) were drawn from all participants in the morning, between 8:00 and 9:00 am, to prevent the error of interpreting low TSH values. Samples were allowed to clot at room temperature and then centrifuged to separate the serum, which was subsequently stored at − 80 °C until use. Laboratory analyses for serum total IgE, T3, T4, and TSH levels were conducted using RIA and IRMA kits (Beckman Coulter Immunotech, France). The normal reference ranges were < 100 IU/mL for IgE levels (REF 1699), 1.3–2.9 nmol/L for T3 (REF 33830), 58–166 nmol/L for T4 (Median 101.9 nmol/L, REF IM1447/IM3286) and 0.3–5 mIU/L for TSH concentrations (REF IM3713/13) (Beckman Coulter Immunotech, France). The research Ethics Committee at the Faculty of Medicine, University of Prishtina, “Hasan Prishtina” (ref. nr. 3126/2011) approved the study, and all methods were performed following relevant guidelines and regulations.
Skin prick test (SPT)
The appropriate selection of specific aeroallergens from a standard test panel (G aeroallergens, Allergopharma, Germany), along with positive (histamine 1 mg/ml) and negative (saline) controls, was employed. Patients were classified as having a positive reaction to at least one aeroallergen if a wheal with a diameter of ≥ 3 mm was observed after 15–20 min.
Statistical analysis
Statistical Package for Social Sciences (SPSS) version 25 was used for data analysis. Qualitative data were presented as frequencies and percentages, while quantitative variables were summarized using mean and standard deviation (SD). To identify significant differences between variables, an independent sample t-test was employed, with a significance level set at P < 0.05.
Data availability
Data will be available from the corresponding author upon proper request from the editor.
References
Akhouri, S. & House, S. A. Allergic Rhinitis. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK538186/ (2023).
Savouré, M. et al. Worldwide prevalence of rhinitis in adults: A review of definitions and temporal evolution. Clin. Transl. Allergy. 12, e12130 (2022).
Mir, E., Panjabi, C. & Shah, A. Impact of allergic rhinitis in school-going children. Asia Pac. Allergy. 2, 93–100 (2012).
Bousquet, J. et al. Allergic rhinitis. Nat. Rev. Dis. Primers. 6, 95 (2020).
Tajima, H. & Pawankar, R. Obesity and adiposity indicators in asthma and allergic rhinitis in children. Curr. Opin. Allergy Clin. Immunol. 19, 7–11 (2019).
Wise, S. K. et al. International consensus statement on allergy and rhinology: Allergic rhinitis. Int. Forum Allergy Rhinol. 8, 108–352 (2018).
Liva, G. A., Karatzanis, A. D. & Prokopakis, E. P. Review of rhinitis: Classification, types, pathophysiology. J. Clin. Med. 10, 3183 (2021).
Fawzan, A. E., Assiri, S. A., Althaqafi, R. M. M., Alsufyani, A. & Alghamdi, A. S. A. Association of allergic rhinitis with hypothyroidism, asthma, and chronic sinusitis: clinical and radiological features. World J. Otorhinolaryngol. Head Neck Surg. 8, 262–268 (2022).
Weare-Regales, N., Chiarella, S. E., Cardet, J. C., Prakash, Y. S. & Lockey, R. F. Hormonal effects on asthma, rhinitis, and eczema. J.A.C.I Pract. 10, 2066 (2022).
Wenzek, C. et al. The interplay of thyroid hormones and the immune system—Where we stand and why we need to know about it. Eur. J. Endocrinol. 186, R65 (2022).
Balázs, C., Leövey, A., Szabó, M. & Bakó, G. Stimulating effect of triiodothyronine on cell-mediated immunity. Eur. J. Clin. Pharmacol. 17, 19–23 (1980).
Fernandez, V. & Videla, L. A. On the mechanism of thyroid hormone-induced respiratory burst activity in rat polymorphonuclear leukocytes. Free Radic. Biol. Med. 19, 359–363 (1995).
Boelen, A. et al. Induction of type 3 deiodinase activity in inflammatory cells of mice with chronic local inflammation. Endocrinol. 146, 5128–5134 (2005).
Gigena, N. et al. Dissecting thyroid hormone transport and metabolism in dendritic cells. J. Endocrinol. 232, 337–350 (2017).
Mascanfroni, I. et al. Control of dendritic cell maturation and function by triiodothyronine. FASEB J. 22, 1032–1042 (2008).
Mihara, S. et al. Effects of thyroid hormones on apoptotic cell death of human lymphocytes. J. Clin. Endocrinol. Metab. 84, 1378–1385 (1999).
Paavonen, T. Enhancement of human B lymphocyte differentiation in vitro by thyroid hormone. Scand. J. Immunol. 15, 211–215 (1982).
Adamczewski, Z. et al. Recombinant human thyroid-stimulating hormone increases the percentages of natural killer T cells and B lymphocytes in human peripheral blood in vivo. Front. Endocrinol. 11, 543845 (2020).
Degirmenci, P. B. et al. Allergic rhinitis and its relationship with autoimmune thyroid diseases. Am. J. Rhinol. 29, 257–261 (2015).
Joshi, T. P. et al. Association of atopic dermatitis with Graves’ disease and Hashimoto’s thyroiditis: A case-control study in the All of Us research program. J. Am. Acad. Dermatol. 89, e175–e176 (2023).
Hadlow, N. C. et al. The relationship between TSH and free T(4) in a large population is complex and nonlinear and differs by age and sex. J. Clin. Endocrinol. Metab. 98, 2936–2943 (2013).
Baloch, Z. et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 13, 3–126 (2003).
Surks, M. I. & Hollowell, J. G. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: Implications for the prevalence of subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 92, 4575–4582 (2007).
Vatankhah, V., Khazraei, H., Iranpoor, H. & Lotfizadeh, M. Impact of high body mass index on allergic rhinitis patients. Rev. Fr. Allergol. 57, 370–374 (2017).
Harugop, A. S., Walia, A., Havaldar, R. R. & Mudhol, R. S. Correlation between allergic rhinitis and body mass index: An observational study. Indian J. Otolaryngol. Head Neck Surg. 74, 1033–1036 (2022).
Kalpaklıoglu, A. F., Baccioglu, A. & Yalim, S. A. Does serum leptin levels differ between patients with rhinitis of allergic versus nonallergic etiology?. Clin. Respir. J. 15, 1352–1358 (2021).
Fu, J. et al. Association between body mass index and thyroid function in euthyroid chinese adults. Med. Sci. Monit. 27, e930865 (2021).
Yamada, T. et al. An elevation of serum immunoglobulin E Provides a new aspect of hyperthyroid graves’ disease. J. Clin. Endocrinol. Metab. 85, 2775–2778 (2000).
Kalkan, İ, Atmaca, H. U. & Akbaş, F. The relationship of thyroid nodules with total serum IgE level and metabolic parameters in patients with hashimoto thyroiditis. İstanbul Med. J. 22, 218–222 (2021).
Leynaert, B., Neukirch, C., Liard, R., Bousquet, J. & Neukirch, F. Quality of life in allergic rhinitis and asthma. A population-based study of young adults. Am. J. Respir. Crit. Care Med. 162, 1391–1396 (2000).
Mesonjesi, E., Piluri, Z. E., Gupta, R., Strachan, D. & Priftanji, A. The prevalence and time trend of asthma in Albanian children in 2011 – Alb ISAAC. Clin. Transl. Allergy. 5, P8 (2015).
Gashi, V., Ahmetaj, L. N. & Ahmeti, B. Allergic rhinitis and eczema in a population of school children from the city of Gjilan in Kosovo. Experimed. 9, 113–119 (2019).
Li, R. et al. Allergic rhinitis children with obesity are more vulnerable to air pollution: A cross-sectional study. Sci. Rep. 13, 1–8 (2023).
Parasuaraman, G. et al. The association between body mass index, abdominal fatness, and weight change and the risk of adult asthma: A systematic review and meta-analysis of cohort studies. Sci. Rep. 13, 1–14 (2023).
Nurhasanah, W.S. et al. Correlation between body mass index and biological age in young adults. N. S. T. Proceedings, 63–65 (2022).
Gwalabe, S. A., Adamu, A., Kirfi, A. M., Dunga, J. A. & Maigari, I. M. Serum immunoglobulin E level and its relationship with eosinophil count among patients with allergic rhinitis in tertiary hospital in bauchi, Northeastern Nigeria: A cross-sectional study. Niger. J. Clin. Pract. 27, 389–393 (2024).
Dávila, I. et al. Relationship between serum total IgE and disease severity in patients with allergic asthma in Spain. J. I. A. C. Immunol. 25, 120–127 (2015).
Kostova, P. et al. Elevated IgE levels—An allergy or an underlying inborn error of immunity in children with recurrent infections?. Antibodies 12, 70 (2023).
Rodinkova, V. V. et al. Molecular profile sensitization to house dust mites as an important aspect for predicting the efficiency of allergen immunotherapy. Front. Immunol. 13, 848616 (2022).
D’Amato, G. et al. The effects of climate change on respiratory allergy and asthma induced by pollen and mold allergens. Allergy 75, 2219–2228 (2020).
Pinot de Moira, A. et al. Associations of early-life pet ownership with asthma and allergic sensitization: A meta-analysis of more than 77,000 children from the EU Child Cohort Network. J. A. C. Immunol. 150, 82–92 (2022).
Forkel, S. et al. Sensitization against fungi in patients with airway allergies over 20 years in Germany. Int. Arch. Allergy Immunol. 182, 515–523 (2021).
Romero-Sánchez, L. et al. Der p 23 sensitization in patients with house dust mite respiratory allergy. Eur. Ann. Allergy Clin. Immunol. 56, 79–85 (2024).
Bergmann, K. C. Frequency of sensitizations and allergies to house dust mites. Allergo. J. Int. 31, 279–283 (2022).
Nakamura, Y. et al. Dietary iodine attenuates allergic rhinitis by inducing ferroptosis in activated B cells. Sci. Rep. 13, 1–12 (2023).
Andersen, S. et al. Interpretation of TSH and T4 for diagnosing minor alterations in thyroid function: A comparative analysis of two separate longitudinal cohorts. Thyroid Res. 15, 19 (2022).
Dong, A., Huang, Y., Huang, Y. & Jia, B. Effects of calorie intake and sampling time on thyroid stimulating hormone concentration. B.M.C. Endocr. Disord. 22, 85 (2022).
Zhang, C. et al. Correlations of thyroid autoantibodies with allergic diseases: A case-control study of 434 Chinese patients. Medicine 101, e29871 (2022).
Molnar, I., Kelemen, E. & Somogyine-Var, I. E. Antigen mimicry between aeroallergens and thyroid antigens can modify the levels of thyroid hormones and antibodies in thyroid autoimmunity. Endocr. Abstr. 29, 1606 (2012).
Zhengzhou, P. et al. The association between body mass index and subclinical thyroid dysfunction in different sexes of Chinese. Endocr. Pract. 25, 1166–1175 (2019).
Dávila, I. et al. Genetic aspects of allergic rhinitis. J. Investig. Allergol. Clin. Immunol. 19, 25–31 (2009).
Choi, B. Y., Han, M., Kwak, J. W. & Kim, T. H. Genetics and epigenetics in allergic rhinitis. Genes (Basel). 12, 2004 (2021).
Shirai, Y. et al. Multi-trait and cross-population genome-wide association studies across autoimmune and allergic diseases identify shared and distinct genetic components. Ann. Rheum. Dis. 81, 301–1312 (2022).
Xue, K., Yang, J., Zhao, Y., Cheng, J. & Wang, Z. Identification of susceptibility genes to allergic rhinitis by gene expression data sets. Clin. Transl. Sci. 13, 169–178 (2020).
Author information
Authors and Affiliations
Contributions
V.L.B. collected the data and designed and created a study concept for the manuscript. V.L.B. and B.G.L. wrote and prepared the manuscript for review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lokaj-Berisha, V., Gacaferri Lumezi, B. Increased thyroxine levels of patients with allergic rhinitis. Sci Rep 15, 2667 (2025). https://doi.org/10.1038/s41598-025-85762-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-85762-0
Keywords
This article is cited by
-
Pharmacovigilance analysis of drug-induced hypertrophic rhinitis using FAERS data
Scientific Reports (2025)