Abstract
In mutualistic symbiosis, organisms often provide food to their partners. However, the processes and significance of food provisioning to hosts remain poorly understood. The anemonefish Amphiprion clarkii, which prefers larger hosts, has been suggested to provide food to its host the sea anemone Entacmaea quadricolor. In the present study, we investigated food provisioning by anemonefish and its effects on the symbiotic relationships. When given foods of various sizes and types in the field, anemonefish selectively consumed small animal food (krill, clams, squid, and fish) and green macroalgae of small size, while providing larger pieces of animal food to their hosts. Additionally, the anemonefish avoided either eating or providing brown macroalgae and sponges to the host anemone, which appeared to be unsuitable as food for both anemonefish and sea anemones. When repeatedly provided small pieces of animal food, the anemonefish initially consumed the food themselves, but upon satiety, increased provisioning to the host. Food provisioning positively influenced the growth of host anemones. These findings suggest that anemonefish actively provide food to host anemones based on the situation, adding to our knowledge of the mutual benefits of symbiosis among partners.
Similar content being viewed by others
Introduction
Mutualistic symbiosis is an interaction wherein different species living in the same location benefit from each other. In this interspecific relationship, nutritional exchanges between partners may develop to maintain interactions1. Most nutritional exchanges occur during endosymbiosis, during which both partners often provide nutrition to others. Corals and zooxanthellae2,3, rhizobia and plants4, and animals and their gut bacteria5,6 are typical examples. Meanwhile, in most cases of ectosymbiosis, one symbiont provides nutrition, whereas the other provides a different form of service7. There are only a few known examples in which both symbionts provide nutrition to each other. For instance, the symbiotic system between epiphytes and ants consists primarily of trophic interactions8,9,10,11; the larvae of the lycaenid butterfly provide nectar to the ants and, in turn, receive food from their partners12,13; and the attine ants that cultivate fungi use them as a nutrient resource14,15. Thus, although there are few known examples, the provisioning of nutrients plays an important role in maintaining interspecific relationships in ectosymbiosis, and elucidating the function of food and the direction of provisioning is a fundamental approach to understanding the maintenance and evolution of these relationships.
Coral reef areas are highly productive environments but nutrient-poor16,17,18. In such habitats, interspecific interactions often compensate for nutrient shortage. For instance, hermatypic corals benefit from the nutrients discharged by fish and boring bivalves to the coral19,20. Similarly, cleaner shrimp, which live in sea anemones and attract client fish near the anemone, may cause anemones to absorb nutrients from clients21. Additionally, in goby-shrimp relationships, the excrement of symbiont fish is used as a nutrient source for the shrimp host22. The anemone-anemonefish relationship, one of the oldest known examples of symbiosis in the ocean environment, may also involve trophic exchange between partners (reviewed by Karplus23). In these relationships, both benefit from each other. Host anemones provide anemonefish with refuge and spawning sites24,25,26,27,28,29. Anemonefish also benefit host anemones by protecting them from predation27,30,31,32,33, by aerating around the host34, and by removing waste products and unwanted material from the host27,35. The size of the host anemone also influences the social structure of the resident anemonefish, with Amphiprion percula females colonising larger anemones being able to grow and lay more eggs36. Recent studies using stable isotopes have shown that nutrients are exchanged between the anemones (and their endosymbionts, zooxanthellae) and anemonefish32,37,38,39,40,41. However, in these examples, the benefit to the hosts was from the uptake of nutrients passively released by the ectosymbionts. It is unclear whether ectosymbionts in coral reef environments actively provide food to their hosts, which may have implications for the evolution and maintenance of this mutualism.
Recently, an interesting behaviour of Clark’s anemonefish, A. clarkii was observed in our field research. When a piece of clam was given, the anemonefish sucked it into its mouth and attached it to the host anemone Entacmaea quadricolor tentacles (Supplementary Movies S1–S3). Indeed, this behaviour is known since the nineteenth century42 and can be easily induced in captivity23,43,44. The frequency of this behaviour may vary among species of anemonefish24,25, and is likely related to anemonefish hunger44, food size23,44,45, and the presence or absence of competitors46. Several hypotheses have been proposed regarding the significance of the food-provisioning behaviour of anemonefish to the host41,47,48. One possible explanation is that anemonefish use anemones to tear small pieces of food to eat42 or to eat food attached to the host later48. However, there is no clear evidence supporting these hypotheses23,25,49. Furthermore, because this behaviour is rarely observed in the wild48,49, it may be of little importance in symbiotic relationships23,30. Therefore, the significance of the food-provisioning behaviour of anemonefish to the host remains unclear, and the role of food provisioning in forming the relationship between anemones and anemonefish has not been examined.
Here, we hypothesised that anemonefish would benefit their host sea anemone by providing food and investigated the factors affecting this behaviour and its effects on the host sea anemone. Specifically, we investigated whether (i) host anemones consumed the food provided by the anemonefish, (ii) the size and type of food and (iii) the hunger level of the anemonefish affected food-provisioning behaviour, and (iv) food-provisioning behaviour affected the growth of host anemones. Because the reproductive success of anemonefish is correlated with the size of the host anemone36, increased growth of host anemones through food provisioning may benefit the anemonefish. To our knowledge, this is the first study to demonstrate the effects of food provisioning by anemonefish on host anemones.
Results
Consumption of provided feed by host anemone
To track the food provided by anemonefish to the host, bait krill tied to a 70 cm string was given to anemonefish at 26 hosts in total. In all cases, the fish attached the krill to the tentacles of the hosts immediately after receiving it. In all eight cases with no video recordings in 2020, the string stretched from the anemone’s mouth after 1 h. In 9 of 18 cases videotaped in 2021, host anemones likely brought the krill to the mouth using tentacles 101 ± 17 min (mean ± s.d.) with the shortest time of 4 min after receiving it. In eight and nine cases in 2020 and 2021, respectively, the host anemones likely consumed krill. In the remaining nine cases in 2021, no krill reached the host mouth because of disturbance by other fish or unknown organisms in five cases and pulling out of the string by the anemonefish in four cases (probably because the anemonefish judged the string as a foreign substance for the host anemone). Anemonefish pecked and/or picked up and returned krill attached to the hosts in 10 of the 18 cases in 2021; however, no fish removed or consumed krill (see the dataset for more details). These observations suggest that anemonefish provide food to their hosts.
Size and types of feed and food provisioning by anemonefish
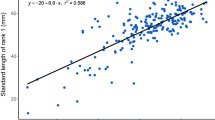
Seven different sizes (3, 5, 7, 10, 20, and 50 mm) of bait krill were randomly and continuously provided to the anemonefish (3–7 mm, five times each; 10–50 mm, twice each in maximum) and observed to determine whether the anemonefish consumed the krill by themselves or provided it to the host anemone. We found that feed size had a significant effect on food provisioning by anemonefish to the host. When the size of feed was smaller than 7 mm, the anemonefish were more likely to consume it themselves, whereas they were more likely to provide the feed if larger than 7 mm to the host anemone (binomial generalised linear mixed model [GLMM], χ12 = 112.52, p < 0.0001, n = 206 trials from 10 fish; Fig. 1). In particular, anemonefish never consumed feed of 20 or 50 mm in size and provided it to the host. Furthermore, no anemonefish abandoned the krill.
Size of feed and probability of food provisioning by anemonefish to the host. When the anemonefish consumed the feed, the probability is 0, and when the anemonefish provided the feed to the host anemone, the probability is 1. The probability of food provisioning by anemonefish to the host significantly increased with feed size (3, 5, 7, 10, 20, and 50 mm). The black line represents the regression line with the shaded 95% confidence interval from the binomial GLMM (n = 206 trials from 10 fish). The size of the circles indicates the number of occurrences.
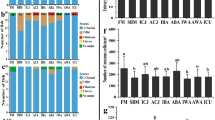
Seven different types of food were provided to each anemonefish to determine the type of feed to be provided to the host sea anemone. When the observer provided the anemonefish with the three types of animal food, a similar tendency was detected in the krill-providing experiment (see Fig. 1). All clams, squid, and almost all fish adjusted to 3 mm in size were consumed by the anemonefish itself, whereas those adjusted to 20 mm were mostly provided to the host sea anemone (Fisher’s exact test, df = 2, p < 0.0001 for all three food types; Fig. 2). Conversely, in over 60% of the cases, the anemonefish consumed small pieces (3 mm) of the soft-green macroalga Codium cylindricum themselves, but rarely consumed or provided the host with large pieces (20 mm) of green macroalgae (df = 2, p < 0.0001; Fig. 2). When large pieces (20 mm) of fresh hard tests of the sea urchin Echinometra mathaei with the epidermis and tube foot were provided to the anemonefish, in over 60% of cases, the fish gave them to the host. However, the tests were rarely consumed or provided when presented with small (3 mm) pieces (df = 2, p < 0.0001; Fig. 2). When the hard brown macroalga Sargassum hemiphyllum was administered, all anemonefish abandoned pieces of any size (df = 2, p > 0.99; Fig. 2). The results for the sponges were similar to those for the brown macroalgae, except that the anemonefish ate small pieces (3 mm) twice out of the 96 trials (df = 2, p = 0.50; Fig. 2).
Food types and food provisioning by anemonefish to the host. Feed was presented in three continuous trials of five types of feed of two different sizes per individual for clam, fish, squid, green macroalgae, and sea urchin test (n = 16 fish of different hosts, 480 trials in total) and six trials of two types of feed of two different sizes per individual for brown macroalgae and sponges (n = 8 fish of different hosts, 192 trials in total). Reactions of the anemonefish to the feed are shown in different colour bars.
Hunger level and food provisioning by anemonefish
To examine the relationship between “hunger level” and the occurrence of food provisioning by anemonefish to the host, krill adjusted to 3 mm were continuously given to the anemonefish. The results showed that as the number of feed presentations to the anemonefish increased, the frequency of food provisioning to the host also significantly increased (binomial GLMM, χ12 = 187.79, p < 0.0001, n = 1819 trials of 21 fish of different groups; Fig. 3). During the first dozen food presentations to the anemonefish, krill was consumed by the anemonefish, and no food provision for the host was observed until the 17th presentation. The first food provisioning was observed between 18 and 76 presentations depending on the individual fish (Fig. 3). The effects of the sex of anemonefish and the time of day of the experiment on the probability of their feeding to the host were not statistically significant (binomial GLMM, male vs female: χ12 = 1.88, p = 0.17; AM vs PM: χ12 = 0.71, p = 0.40).
Hunger level and food provisioning by anemonefish to the host. When the anemonefish consumed the feed krill of 3 mm in size, the probability is 0, and when the anemonefish provided the feed to the host anemone, the probability is 1. The probability of food provisioning by anemonefish to the host significantly increased with the number of feedings. The black line represents the regression line with the shaded 95% confidence interval from the binomial GLMM (n = 1819 trials from 21 fish). The size of the circles indicates the number of occurrences.
Effects of food provisioning by anemonefish on host growth
To examine the effect of food provisioning by anemonefish on host anemone growth, the surface areas of the oral disks of sea anemones were compared among three treatments: (i) provisioning feed to anemonefish (n = 9), (ii) direct provisioning feed to hosts (n = 11), and (iii) no food provisioning (control, n = 11). Two pieces of fish (approximately 50 g in total), which were too large for anemonefish to swallow, were provided to each of the two fed groups at intervals of approximately every 3 days. At the start of the experiment, the surface area of the oral disk did not differ among the three treatments (Fig. 4a; Kruskal–Wallis test, χ22 = 1.15, p = 0.56). At the end of the experiment (91–106 days after the start of the experiment), sea anemones in the group fed with anemonefish and the group fed directly to the host were significantly larger than those at the start of the experiment (Fig. 4a; exact Wilcoxon signed-rank test, V = 0, p = 0.004, n = 9 and V = 0, p = 0.005, n = 11, respectively), whereas only slight changes in anemone size were found in the control groups (Fig. 4a; V = 20, p = 0.28, n = 11). Therefore, as predicted, at the end of the experiment, the surface area of the two fed treatments was significantly greater than that of the non-fed treatment (Fig. 4a; Kruskal–Wallis test, χ22 = 6.56, p = 0.04). Consequently, the fed anemones showed a higher growth rate than the non-fed anemones (Fig. 4b; Kruskal–Wallis test, χ22 = 8.62, p = 0.01). Daily growth was significantly higher in the group fed anemonefish (Fig. 4b; exact Wilcoxon rank-sum test, adjusted p = 0.009) and tended to be higher in the group fed directly to the host than in the no-feeding treatment (adjusted p = 0.07). Daily growth did not differ between the two fed treatments (adjusted p = 0.46).
Growth of sea anemones in the food-provisioning experiment. (a) Surface area (cm2) of oral disk at the start and at the end of the experiment (91–109 days after the start of the experiment). (b) Growth rate per day (cm2 day−1). **p < 0.01, *p < 0.05, #p = 0.07 by exact Wilcoxon rank-sum tests and Benjamin–Hochberg correction methods, or by exact Wilcoxon signed-rank tests.
Food provisioning by anemonefish to the host in natural situations
In the experiments, the observer artificially provided feed to the anemonefish; however, the occurrence of such a situation in nature remains unknown. However, we observed two cases in which anemonefish carried dead animals to host sea anemones in the wild. On 2nd June and 2 July 2022, the anemonefish carried a small portion (3–4 cm square) of a sea cucumber (Stichopus naso) body to their host at a depth of 5.5 m. During this period, many dead sea cucumbers were found at the study site, probably because of the high sea temperatures in summer. The frequency of concurrence was 2% (twice in 100 scuba dives).
Discussion
Little is known about the processes and significance of food provision to hosts in mutualistic symbiosis. Using a field experimental approach, we tested predictions based on the hypothesis that anemonefish benefit host sea anemones by providing food. We found that: (i) anemonefish provided food for the hosts, and the host anemones likely consumed the food; (ii) anemonefish provided larger pieces of animal food, but not brown macroalgae, to the host, whereas they fed on animal food and green marcoalgae small enough to their mouth size when foods of various sizes and types were given to the anemonefish; (iii) the probability of food provisioning to the host increased with the satiety of the anemonefish; and (iv) food provisioning resulted in increased growth of the host anemone. Our findings suggest that anemonefish are likely to actively provide food to their hosts depending on the food habits of the hosts and the conditions of the anemonefish. Furthermore, increased growth of host anemones through food provisioning will, in turn, benefit the anemonefish themselves, such as increasing the clutch size of the anemonefish and avoiding predation because of increased concealment among tentacles30,36,50.
We found that the anemonefish consumed smaller krill and provided larger krill to their hosts. Similarly, anemonefish fed on smaller clams, fish, and squids and provided larger pieces to the host, which agrees with the observations of previous studies44,45. These results suggest two alternative interpretations: anemonefish provide food that is too large for it to eat on its own, or select food that is sufficiently large to benefit the host. When anemonefish were given hard fresh sea urchins of two different sizes, they abandoned the smaller tests and provided the larger tests to the hosts. Sea urchin tests are very solid and are probably difficult for anemonefish to swallow and digest; however, host anemones might benefit from digesting the epidermis and tube foot in the test, although this is speculative. Indeed, during the field study, we occasionally found several body parts of sea urchins, such as spines and tests, in the gastrovascular cavities of host sea anemones (Y. Kobayashi, pers. obs.). This suggests that anemonefish choose larger feed items for their hosts, which are considered of greater benefit to the host. In addition, when the green macroalga C. cylindricum was provided to anemonefish, they consumed smaller pieces. Although there is no evidence that C. cylindricum forms a part of the natural diet of anemonefish, it is edible and digestible for omnivorous anemonefish that forage primarily on copepods and (filament) algae51,52,53. Conversely, the anemonefish mostly ignored larger pieces of green macroalgae and never provided them to the host, indicating that the anemonefish selected food for the carnivorous host, the sea anemone54. Anemonefish neither consumed nor provided sponges and brown macroalgae S. hemiphyllum to hosts of either size, which is presumably unsuitable as food for both anemonefish and their hosts. Collectively, anemonefish may select food items and provide the host with suitable feed that is beneficial to the host.
When small pieces of krill were repeatedly presented to the anemonefish, the anemonefish first ate the feed, but they began to provide krill to the host anemone later, as observed in a previous study44. This indicates that anemonefish behave selfishly and cooperate with the host depending on their body condition. Furthermore, this may be one of the reasons why food-provisioning behaviour is rarely observed in the wild because food provisioning to the host does not occur when fish are hungry. It is also important to note that the anemonefish never abandoned their food, even when their stomachs were full, reflecting the high strength of the connection between sea anemones and anemonefish. These results also suggest that anemonefish recognise the ultimate benefits of food provision to their hosts. The next step would be to present objects that could be harmful to the anemone and objects that could be beneficial to the anemone, but that the anemonefish had never seen before, and to observe the behaviour of the anemonefish.
The reason why anemonefish provide feed to their hosts is not well understood despite studies being conducted over a long time23. Previous studies have proposed that the host is used to make the food finer for consumption by anemonefish42 or that anemonefish forage later48. However, these assumptions were rejected by our food-tracking experiment: the host sea anemone likely consumed feed provided by the anemonefish, and the anemonefish did not remove or consume feed and frequently returned krill to the host tentacles when they picked them up. These observations strongly suggest that anemonefish actively provide food for their hosts. Furthermore, our host growth experiment revealed that food-provisioning behaviour by anemonefish increased the growth rate of the host anemone. The host anemone provides refuge and nest sites to lay eggs for the anemonefishes24,25,29,50,55. Females of the clown anemonefish, A. percula which settle on larger hosts, grow larger and lay more eggs36. These results suggest that host size is important for the resident anemonefish, A. clarkii and influences their fitness. Thus, it is plausible that anemonefish increase their fitness by growing their hosts through the provision of food. Furthermore, anemonefish are long-lived dwellers56,57, and their symbiotic relationship with sea anemones, which appears to continue for a long period, makes food provisioning meaningful. To date, there are only a few examples of symbionts that provide feed to their hosts to improve habitat quality, such as Fijian ants and Squamellaria spp.10. Therefore, this study provides a rare example of the relationship between organisms through the provision of food.
Clark’s anemonefish, A. clarkii, occasionally forages on the tentacles and eggs of the host sea anemone41,58 (Y. Kobayashi and S. Awata, unpublished data). Furthermore, zooxanthella-derived nutrients (i.e., from tentacles) accumulate in the liver of anemonefishes41. If anemonefish utilise the host anemone as a food resource, this behaviour is costly to the host. Food provisioning may compensate for these costs by allowing the growth of anemones. In this case, the relationship between the two is similar to that in cultivation. Cultivation is well known for its connection between ants and fungi14 and is also found in algal-farming damselfish and algae59,60 and mysid-shrimp-domesticating damselfish and algae61. In these examples, the symbiont partner acts only as a food resource, but the host anemone also serves as a “home” for the anemonefish. No such complex relationships exist in other mutualistic symbioses. This study suggests that anemonefish improve host quality by providing food to their host anemones in a situation-dependent and proactive manner depending on the host’s food habits and their own conditions. The predation of host anemones by resident anemonefish is an interesting topic for understanding the evolution of cultivation.
Until now, food provisioning by anemonefish for the host anemone has rarely been observed in the wild and is regarded as having little importance in their symbiotic relationships23,30,48,55. However, dead animals may frequently appear after bad weather, such as storms and typhoons, or during cold winters and hot summers. Furthermore, these animals are often consumed by other animals. Normally, field studies are conducted during good weather and temperatures; therefore, food-provisioning behaviour is rarely observed in nature. In the present study, we observed such behaviour in the wild at an occurrence rate of 2% in summer, during which time many dead sea cucumbers were found at the study site, probably because of the high sea temperature. If field studies are carried out after storms and drastic changes in water temperature occur, food-provisioning behaviour by anemonefish to the host may be more frequently observed.
Future studies on the relationship between feeding behaviour and external factors, such as the presence or absence of competitors for food and the condition of the symbiotic partner (size and condition of the host), will contribute to the understanding of food provisioning among anemonefish and their host sea anemones.
Methods
Study site and animals
We conducted fieldwork with scuba from August to October 2020 and from June to October 2021 at Morode Beach, Ainan, Japan (33°00′16″N, 132°30′19″E). The study site consists mainly of a boulder zone containing coral communities and a sandy bottom, with a high density of anemone-anemonefish clusters in the boulder zone at depths of 3–10 m. Breeding pairs of the Clark’s anemonefish Amphiprion clarkii were associated with bubble-tip anemone, Entacmaea quadricolor, and no other Amphiprion species were found at the study site. Only young anemonefish inhabited another species of sea anemone, Antheopsis koseirensis. At the study site, the social group of anemonefish mainly consisted of a breeding pair, and one juvenile (rank 3, see Buston 200362) that does not participate in reproduction was found in approximately 10% of the breeding groups. A total of 112 social groups were used in the experiment over the two seasons, including groups with and without juveniles. The same groups were never used again during the same season, although several groups overlapped between the seasons.
Tracking of food provided by anemonefish to the host
There are two possible reasons why anemonefish carry food to their sea anemone hosts: host feed and food storage48. First, if anemonefish carry food to feed the host, the host will eventually consume it. Second, if anemonefish take food to the host for storage, they will consume the food after attaching it to the host. To assess these two alternative explanations, we monitored whether the host anemone consumed food carried by the anemonefish. One end of the string (thickness: 4 mm; length: 0.7 m) was tied to thawed frozen krill (purchased from a supermarket), whereas the other end was not tied to anything. An observer presented the bait to the anemonefish. In all cases, the anemonefish immediately carried the bait to the host and attached it to the tentacles. In 2020, we checked the baits after one hour by eye (n = 8). In 2021, we video-shot anemones with bait (n = 18) using a tripod-mounted handycam (JVCKENWOOD, GZ-RX680, Yokohama, Japan), GoPro Hero 7, or 8. The shooting time was 1–2 h. The videos were analysed in the laboratory using the video annotation software ELAN. From the videos, we recorded the time when the string was found to stretch from the anemone’s mouth after it was provided by the anemonefish, and the number of times the anemonefish pecked, picked up, or returned the krill to the host.
Feed size and food provisioning by anemonefish to the host
To investigate the relationship between feed size and food provisioning by anemonefish to the host, we presented feed of different sizes to 10 individual anemonefish living symbiotically on different hosts. Freshly thawed krill (approximately 50 mm long) purchased from a supermarket was cut into 3, 5, 7, 10, and 20 mm long pieces. Cut krill (3–20 mm) and whole krill (50 mm) were presented to each anemonefish in random order, five times each for 3, 5, and 7 mm, and twice each for 10, 20, and 50 mm (21 trials in total per fish). We recorded whether the anemonefish consumed feed or carried the krill bait to their hosts. One fish left the host during the experiment; therefore, in this case, data from 17 trials were used for analysis.
Food type and food provisioning of anemonefish to the host
Anemonefish feed primarily on copepods and (filament) algae51,52,53, while their host sea anemones are known to be capable of preying on fish and other animals54. If anemonefish benefit from the host anemone through food provisioning, they would select food for the host anemone according to the host’s feeding habits. Seven different types of food were provided to each anemonefish to determine the type of feed to be provided to the host sea anemones. Clam (Ruditapes philippinarum), fish (Saurida macrolepis), and squid (Todarodes pacificus) were purchased from supermarkets. Sea urchins (Diadema setosum), two types of algae (green macroalgae: Codium cylindricum and brown macroalgae: Sargassum hemiphyllum), and purple sponges (Haliclona permollis) were collected at the study site. The feed was frozen until the start of the experiments. Sea urchin spines were removed from the body and crushed, and fresh tests with the epidermis and tube foot were used for the experiments. All prepared feeds were cut into two different sizes, with the longest sides of 3 mm and 20 mm. Feed was presented by the observer to the anemonefish continuously for six trials of two types of feed with two different sizes per individual anemonefish (n = 8 fish from different hosts, 192 trials in total) for brown macroalgae and sponges, and three trials of five types of feeds with two different sizes per individual anemonefish (n = 16 fish from different hosts, 480 trials in total) for other food items. The behaviour of the anemonefish was recorded.
Sea anemones are carnivores54, whereas anemonefish are omnivorous, feeding on both zooplankton and (filament) algae25,51,52,53. If an anemonefish carries feed to its host, the following results can be predicted: (i) As clams, fish, and squids are animal foods that can be used as food resources by both anemonefish and anemones, the anemonefish would provide these large-sized feeds to the host and would consume them if the feeds were small. (ii) C. cylindricum is a soft-green macroalgae. Although there is no evidence that C. cylindricum forms a part of the natural diet of anemonefish, it is edible and digestible to omnivorous anemonefish51,52. Therefore, the anemonefish is likely to eat C. cylindricum, but would not provide it to the host because green macroalgae are probably not suitable as a food source for anemones. (iii) As the fleshy test of the sea urchin is considered too difficult for anemonefish to eat, whereas the host anemone can digest the epidermis and tubefoot, the anemonefish may provide the sea urchin test to the host. Occasionally, sea urchin spines and tests have been observed in the gastrovascular cavity of host sea anemones (Y. Kobayashi, pers. obs.). (iv) S. hemiphyllum is a brown macroalga. Because S. hemiphyllum is hard and contains carbohydrates63, it is indigestible by anemonefish. Therefore, S. hemiphyllum is not a food resource for anemonefish or carnivorous sea anemones. (v) Sponges generally have chemical defences against predatory animals such as fish, crustaceans, and sea urchins (reviewed by Paul and Puglisi64), and both anemonefish and their hosts are predicted to be inedible.
Hunger level and food provisioning of anemonefish
To investigate the relationship between “hunger level” and the occurrence of food-provisioning behaviour, an experiment was conducted in which krill with the longest side adjusted to 3 mm were continuously presented to the anemonefish. The observer repeatedly presented the fish with krill until it stopped responding and recorded whether it consumed the food itself or provided food to the host anemone. The experiment was conducted using 21 anemonefish inhabiting different hosts.
Effects of food provisioning by anemonefish on host growth
If food provisioning by the anemonefish benefits the host anemone, the growth rate of the latter is expected to increase. To test this hypothesis, we examined the growth rate of anemones by manipulating the food provisioning by anemonefish to host sea anemones. Before the experiments, the host anemones were numbered individually. Numbered anemones were assigned to one of the three treatments. The three treatments were as follows: (i) observer-provisioning feed to anemonefish (n = 9), (ii) direct provisioning of feed to the hosts (n = 11), and (iii) no food provisioning (control, n = 11). The two fed groups were provided with two pieces of fish (approximately 50 g in total) of jack mackerel (Trachurus japonicus) or lizard fish (Saurida macrolepis), which were too large for anemonefish to swallow, at intervals of approximately every 3 days. The experimental period was 103 days (± 4 s.d., range = 91–106 days). During the experiment, one juvenile anemonefish was observed in four of the 31 numbered anemones. The nutrients excreted by resident anemonefish nourish host anemones37,41; however, the presence or absence of juveniles likely did not affect host growth (see the dataset), and the data of these four anemones were included in the analyses. The body sizes of the anemones were measured at the start and end of the experiment. The body size of the host anemone was determined by measuring the long and short diameters (cm) of the anemone’s oral disk with a fibreglass folding rule (Shinwa Rules, Niigata, Japan) and calculating the surface area (cm2) according to Hattori and Yanagisawa65 (long diameter × short diameter × 4−1 × π). The growth rate of the anemones per day was calculated using the surface area of the sea anemones at the start and end of the experiment and on the experimental days (cm2 day−1).
Food provisioning by anemonefish to the host in nature
In 2022, during the fieldwork of another study, we observed the food-provisioning behaviour of anemonefish under natural conditions at the same study site. During this period, the anemonefish carried dead animals to the host anemones without manipulation. In this study, we describe the occurrence of such events.
Statistics and reproducibility
Statistical analyses were performed using R version 4.0.3 (https://www.r-project.org). The significance level was set at p < 0.05 for two-tailed tests. The relationship between food provisioning by anemonefish to the host and feed size or hunger level was analysed using a binomial GLMM because multiple trials were conducted on the same individual fish. The feed size model was constructed with feed size as an explanatory variable, food provisioning by the anemonefish to the hosts (provided to the hosts: 1; eaten by the anemonefish: 0) as a response variable, and individual ID as a random effect (206 trials from 10 individual fish). The hunger level model was constructed with the number of feedings by the observer as the explanatory variable, food provisioning by anemonefish to the hosts (provided to the hosts: 1; eaten by the anemonefish: 0) as the response variable, and individual ID as a random effect (1819 trials from 21 individual fish). Furthermore, because a female dominance hierarchy in anemonefish66 and the daily rhythm67,68 may affect individual anemonefish activity, we also examined whether the sex of the anemonefish and the time of day were related to food provisioning. We constructed models that included sex and time of day (morning or afternoon) as explanatory variables.
To examine the type of feed the anemonefish provided to the host anemone, we examined whether the proportion of the three behaviours (fish providing the feed to the host anemone, consuming the feed themselves, and ignoring or spitting out the feed) differed between the small (3 mm) and large (20 mm) feeds. Fisher’s exact test (3 × 2 cross-tabulation of the reaction of anemonefish and feed size) was used to analyse each of the seven types of food.
To determine the effect of food provisioning by anemonefish on the growth rate of the host, we compared the surface area (cm2) of sea anemones at the start and end of the experiment and the growth rate (cm2 day−1) among the three treatment groups using the Kruskal–Wallis test: (i) provisioning feed to anemonefish (n = 9), (ii) direct provisioning feed to the hosts (n = 11), and (iii) no food provisioning (control, n = 11). The exact Wilcoxon rank-sum test and Benjamin–Hochberg correction method were used for multiple comparisons. Furthermore, we compared the surface areas of the sea anemones before and after the experiment within each treatment group using the exact Wilcoxon signed-rank test.
Data availability
The datasets analysed during the current study are available in Zenodo (https://zenodo.org/records/11055773).
References
Bronstein, J. L. (ed.) Mutualism (Oxford University Press, 2015).
Davies, P. S. The role of zooxanthellae in the nutritional energy requirements of Pocillopora eydouxi. Coral Reefs 2, 181–186 (1984).
Houlbréque, F. & Ferrier-Pagés, C. Heterotrophy in tropical scleractinian corals. Biol. Rev. 84, 1–17 (2009).
Denison, R. F. Legume sanctions and the evolution of symbiotic cooperation by rhizobia. Am. Nat. 156, 567–576 (2000).
Levenson, S. M., Nagler, A. L., Geever, E. F. & Seifter, E. Acute choline deficiency in germfree, conventionalized and open-animal-room rats: effects of neomycin, chlortetracycline, vitamin B12 and coprophagy prevention. J. Nutr. 95, 247–270 (1986).
The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Noë, R. & Hammerstein, P. Biological markets: supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behav. Ecol. Sociobiol. 35, 1–11 (1994).
Treseder, K. K., Davidson, D. W. & Ehleringer, J. R. Absorption of ant-provided carbon dioxide and nitrogen by a tropical epiphyte. Nature 375, 137–139 (1995).
Chomicki, G. & Renner, S. S. Phylogenetics and molecular clocks reveal the repeated evolution of ant-plants after the late Miocene in Africa and the early Miocene in Australasia and the Neotropics. New Phytol. 207, 411–424 (2015).
Chomicki, G. & Renner, S. S. Obligate plant farming by a specialized ant. Nat. Plants 2, 16181. https://doi.org/10.1038/nplants.2016.181 (2016).
Chomicki, G., Kiers, T. & Renner, S. S. The evolution of mutualistic dependence. Annu. Rev. Ecol. Evol. Syst. 51, 409–432 (2020).
Wada, A., Isobe, Y., Yamaguchi, S., Yamaoka, R. & Ozaki, M. Taste-enhancing effects of glycine on the sweetness of glucose: a gustatory aspect of symbiosis between the ant, Camponptus japonicus, and the larvae of the Lycaenid butterfly, Niphanda fusca. Chem. Senses 26, 983–992 (2001).
Pierce, N. E. & Dankowicz, E. Behavioral, ecological and evolutionary mechanisms underlying caterpillar-ant symbioses. Curr. Opin. Insect Sci. 52, 100898. https://doi.org/10.1016/j.cois.2022.100898 (2020).
Murakami, T. & Higashi, S. Social organization in two primitive attine ants, Cyphomyrmex rimosus and Myrmicocrypta ednaella, with reference to their fungus substrates and food sources. J. Ethol. 15, 17–25 (1997).
Mueller, U. G., Gerardo, N. M., Aanen, D. K., Six, D. L. & Schultz, T. R. The evolution of agriculture in insects. Annu. Rev. Ecol. Evol. Syst. 36, 563–595 (2005).
Barnes, D. J. (ed.) Perspectives in Coral Reefs (Brian Clouston Publ, 1983).
Chadwick-Furman, N. E. Reef coral diversity and global change. Glob. Change Biol. 2, 559–568 (1996).
Davies, J. A. Marine nitrogen: phosphorus stoichiometry and the global N:P cycle. Biogeochemistry 37, 237–252 (1997).
Meyer, J. L. & Schultz, E. T. Tissue condition and growth rate of corals associated with schooling fish. Limnol. Oceanogr. 30, 157–166 (1985).
Mokady, O., Loya, Y. & Lazar, B. Ammonium contribution from boring bivalves to their coral host–a mutualistic symbiosis?. Mar. Ecol. Prog. Ser. 169, 195–301 (1998).
Cantrell, C. E., Henry, R. P. & Chadwick, N. E. Nitrogen transfer in a Caribbean mutualistic network. Mar. Biol. 162, 2327–2338 (2015).
Kohda, M., Yamanouchi, H., Hirata, T., Satoh, S. & Ota, K. A novel aspect of goby–shrimp symbiosis: gobies provide droppings in their burrows as vital food for their partner shrimps. Mar. Biol. 164, 22 (2017).
Karplus, I. Symbiosis in Fishes: The Biology of Interspecific Partnerships (Wiley, 2014).
Verwey, J. Coral reef studies. I. The symbiosis between damselfishes and sea anemones in Batavia Bay. Treubia 12, 305–366 (1930).
Mariscal, R. N. The nature of the symbiosis between Indo-Pacific anemone fishes and sea anemones. Mar. Biol. 6, 58–65 (1970).
Fautin, D. G. & Allen, G. R. Field Guide to Anemonefishes and Their Host Sea Anemones (Western Australian Museum, 1992).
Fautin, D. G. & Allen, G. R. Anemonefishes and Their Host Sea Anemones, Revised Edition (Western Australian Museum, 1997).
Frazão, B., Vasconcelos, V. & Antunes, A. Sea anemone (Cnidaria, Anthozoa, Actiniaria) toxins: an overview. Mar. Drugs 10, 1812–1851 (2012).
Nedosyko, A. M., Young, J. E., Edwards, J. W. & da Sliva, K. B. Searching for a toxic key to unlock the mystery of anemonefish and anemone symbiosis. PLos One 9, e98449. https://doi.org/10.1371/journal.pone.0098449 (2014).
Fautin, D. G. The anemonefish symbiosis: what is known and what is not. Symbiosis 10, 23–46 (1991).
Godwin, J. & Fautin, D. G. Defense of host actinians by anemonefish. Copeia 3, 902–908 (1992).
Porat, D. & Chawick-Furman, N. E. Effects of anemonefish on giant sea anemones: expansion behavior, growth, and survival. Hydrobiologia 530(531), 513–520 (2004).
Holbrook, S. & Schmitt, R. Growth, reproduction and survival of a tropical sea anemone (Actiniaria): Benefits of hosting anemonefish. Coral Reefs 24, 67–73 (2005).
Szczebak, J. T., Henry, R. P., Al-Horani, F. A. & Chadwick, N. E. Anemonefish oxygenate their anemone hosts at night. J. Exp. Biol. 216, 970–976 (2013).
Goldshmid, R., Holzman, R., Weihs, D. & Genin, A. Aeration of corals by sleep-swimming fish. Limnol. Oceanogr. 49, 1832–1839 (2004).
Barbasch, T. A. et al. Substantial plasticity of reproduction and parental care in response to local resource availability in a wild clownfish population. Oikos 129, 1844–1855 (2020).
Cleveland, A., Verde, A. E. & Lee, R. W. Nutritional exchange in a tropical tripartite symbiosis: Direct evidence for the transfer of nutrients from anemonefish to host anemone and zooxanthellae. Mar. Biol. 158, 589–602 (2011).
Roopin, M., Thornhill, D. J., Santos, S. R. & Chadwick, N. E. Ammonia flux, physiological parameters, and Symbiodinium diversity in the anemonefish symbiosis on Red Sea coral reefs. Symbiosis 53, 63–74 (2011).
Tremblay, P., Grover, R., Maguer, J. F., Legendre, L. & Ferrier-Pages, C. Autotrophic carbon in coral tissue: a new 13C-based model of photosynthate translocation. J. Exp. Biol. 215, 1384–1393 (2012).
Kopp, C. et al. Subcellular investigation of photosynthesis-driven carbon assimilation in the symbiotic reef coral Pocillopora damicornis. mBio 6, 2–14 (2015).
Verde, E. A., Cleveland, A. & Lee, R. W. Nutritional exchange in a tropical tripartite symbiosis II: direct evidence for the transfer of nutrients from host anemone and zooxanthellae to anemonefish. Mar. Biol. 162, 2409–2429 (2015).
Sluiter, C. P. Ein merkwürdiger Fall von Mutualismus. Zool. Anz. 11, 240–243 (1888) (in German).
Graefe, G. Zur Anemonen-Fisch-Symbiose, nach Freilanduntersuchungen bei Eilat/Rotes Meer. Z. Tierpsychol. 21, 468–485 (1964) (in German).
Fishelson, L. Observations and experiments on the Red Sea anemones and their symbiotic fish Amphiprion bicinctus. Bull. Sea Fish. Res. Stat. Haifa 39, 1–14 (1964).
Blösch, M. Untersuchungen über das Zusammenleben von Korallenfischen (Amphiprion) mit Seeanemonen (Eberhard-Karls University, 1965) (in German).
Hodgson, V. S. Conditioning as a factor in the symbiotic feeding relationship of sea anemones and anemonefishes. Proc. 4th Int. Coral Reefs Symp. 2, 553–561 (1981).
Saville-Kent, W. The Great Barrier Reef of Australia: its Products and Potensialities (W.H. Allen and Co., 1893).
Fricke, H. W. Öko-Ethologie des monogamen Anemonefisches Amphiprion bicinctus (Freiwasseruntersuchung aus dem Roten Meer). Z. Tierpsychol. 36, 429–512 (1974) (in German).
Abel, E. F. Zur kenntnis des verhaltens und der ökologie von fischen an Korallenriffen bei Ghardaqa (Rotes Meer). Zeitschrift fur Morphologie und Oekologie der Tiere 49, 430–503 (1960) (in German).
Huebner, L. K., Dailey, B., Titus, B. M., Khalaf, M. & Chadwick, N. E. Host preference and habitat segregation among Red Sea anemonefish: effects of sea anemone traits and fish life stages. Mar. Ecol. Prog. Ser. 464, 1–15 (2012).
Galetto, M. J. & Bellwood, D. R. Digestion of algae by Stegastes nigricans and Amphiprion akindynos (Pisces: Pomacentridae), with an evaluation of methods used in digestibility studies. J. Fish. Biol. 44, 415–428 (1994).
Nakamura, Y., Horinouchi, M., Nakai, T. & Sano, M. Food habits of fishes in a seagrass bed on a fringing coral reef at Iriomote Island, southern Japan. Ichthyol. Res. 50, 15–22 (2003).
Khoo, M. L., Das, S. K. & Ghaffar, M. A. Growth pattern, diet and reproductive biology of the clownfish Amphiprion ocellaris in waters of Pulau Tioman, Malaysia. Egypt. J. Aquat. Res. 44, 233–239 (2018).
Aditya, F., Patria, M. P. & Soedjiarti, T. Feeding behaviour of bubble-tip anemones Entacmaea quadricolor (Leuckart, 1828). IOP Conf. Ser. Earth Environ. Sci. 241, 012040. https://doi.org/10.1088/1755-1315/241/1/012040 (2018).
Allen, G. R. The Anemonefishes: Their Classification and Biology (T.F.H. Publications, 1972).
Moyer, J. T. Longevity of the anemonefish Amphiprion clarkii at Miyake-jima, Japan with notes on four other species. Copeia 1986, 135–139 (1986).
Buston, P. M. & Garcia, M. B. An extraordinary life span estimate for the clown anemonefish Amphiprion percula. J. Fish Biol. 70, 1710–1719 (2007).
Scott, A. & Francisco, B. Observations on the feeding behaviour of resident anemonefish during host sea anemone spawning. Coral Reefs 25, 451 (2006).
Hata, H. & Kato, M. Weeding by the herbivorous damselfish Stegastes nigricans in nearly monocultural algae farms. Mar. Ecol. Prog. Ser. 237, 227–231 (2002).
Hata, H. & Kato, M. A novel obligate cultivation mutualism between damselfish and Polysiphonia algae. Biol. Lett. 2, 593–596 (2006).
Brooker, R. M. et al. Domestication via the commensal pathway in a fish-invertebrate mutualism. Nat. Commun. 11, 6253. https://doi.org/10.1038/s41467-020-19958-5 (2020).
Buston, P. Size and growth modification in clownfish. Nature 424, 145–146 (2003).
Fang, R. E., Wei, Y. J., Fang, S. Y. & Huang, C. H. Effects of Sargassum-derived oligosaccharides, polysaccharides and residues on ameliorating enteritis and dysbiosis in a murine model of food allergy. J. Funct. Foods 110, 105844. https://doi.org/10.1016/j.jff.2023.105844 (2023).
Paul, V. J. & Puglisi, M. P. Chemical mediation of interactions among marine organisms. Nat. Prod. Rep. 21, 189–209 (2004).
Hattori, A. & Yanagisawa, Y. Life-history pathways in relation to gonadal sex differentiation in the anemonefish, Amphiprion clarkii, in temperate waters of Japan. Environ. Biol. Fish. 31, 139–155 (1991).
Fricke, H. & Fricke, S. Monogamy and sex change by aggressive dominance in coral reef fish. Nature 266, 830–832 (1977).
Kohbara, J. et al. Self-feeding behavior of yellowtail, Seriola quinqueradiata, in net cages: diel and seasonal patterns and influences of environmental factors. Aquaculture 22, 581–594 (2003).
Kohbara, J., Kitade, M., Kuriyama, I. & Tsuchihashi, Y. Diel self-feeding rhythms of the sevenband grouper Epinephelus septemfasciatus. Aquacult. Sci. 62, 13–22 (2014).
Acknowledgements
We thank Tomonori Hirata and Shiori Hirata for their support in the field, members of the Laboratory of Animal Sociology at Osaka City University (Osaka Metropolitan University) for their fruitful discussions, and Seiya Okuno and Taiga Saeki for their help in preparing the figures. We also thank Editage (http://www.editage.jp) for their English language editing services. This research was supported by JSPS KAKENHI (23KJ1838 to Y. Kobayashi and 22H02703 and 23H03868 to S.A.), JST SPRING (JPMJSP2139-RS22A027 to Y. Kobayashi), Sasakawa Scientific Research Grant from the Japan Science Society (2021-4082 to Y. Kobayashi), and OCU Strategic Research Grant (2019 and 2021: OCU-SRG2021_BR10 to S.A.).
Author information
Authors and Affiliations
Contributions
Conceptualisation, Y. Kobayashi, S.A.; Field experiments, Y. Kobayashi; Data analysis, Y. Kobayashi, Y. Kondo, S.A.; Writing—original draft, Y. Kobayashi, Y. Kondo, S.A.; Writing—review and editing, all authors; Funding acquisition, Y. Kobayashi, S.A.; Resources, S.A.; Supervision, S.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
No fish or sea anemones were captured, tagged, or sacrificed in this study, and this study relied on behavioural data collected non-invasively from animals. The fieldwork adhered to the guidelines for the Use of Animals in Research and was approved by the Osaka City University Animal Care Committee and local fisheries cooperative associations. The protocol for this study was not subject to the university’s ethical regulations; therefore, approval from the ethics committee was not required, and the study was carried out using a method that did not cause stress to the target organisms.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kobayashi, Y., Kondo, Y., Kohda, M. et al. Active provisioning of food to host sea anemones by anemonefish. Sci Rep 15, 4115 (2025). https://doi.org/10.1038/s41598-025-85767-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85767-9