Abstract
Pembrolizumab plus Lenvatinib is regarded as a significant treatment option for advanced unresectable hepatocellular carcinoma (HCC). This study aims to meticulously monitor and identify adverse events (AEs) related to this combined therapy, enhance patient safety, and offer evidence-based recommendations for the appropriate use of these drugs. We gathered adverse drug reactions (ADRs)-related data from the FAERS database for HCC patients who received Pembrolizumab, both alone and in combination with Lenvatinib from the first quarter of 2014 to the fourth quarter of 2023. ADRs signal detection was performed using the ROR, PRR, BCPNN, MHRA, and MGPS methods. We gathered data on 459 and 358 AEs from patients with HCC treated with pembrolizumab alone and in combination with lenvatinib, respectively. Using four signal quantification techniques, we identified 50 and 38 distinct AEs, which were classified into 15 different System organ class (SOC) categories. Notably, the most common AEs associated with pembrolizumab were gastrointestinal disorders and hepatobiliary disorders. In both patient groups, the most frequently reported AEs were hepatic encephalopathy, blood bilirubin increased and diarrhea. We also observed some unexpected significant AEs, such as dehydration, skin ulcers, and intestinal perforation. The countries reporting the highest number of AEs were the United States, followed by China, France, and Japan. The median onset time for AEs related to pembrolizumab alone and its combination with lenvatinib was 80.5 days (interquartile range 20.0–217.3 days) and 77.5 days (interquartile range 19.7–212.3 days), respectively. This study offers new insights into the monitoring and management of ADRs in HCC patients receiving pembrolizumab alone or in combination with lenvatinib. It is crucial to closely monitor the safety of this treatment regimen in HCC patients to avoid serious AEs.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) remains a significant global health challenge, with increasing incidence rates and high mortality1. Traditional treatment options, including surgical resection and local therapies, often yield limited efficacy, especially in advanced stages of the disease. The combination of local treatments and emerging systemic therapies may offer new hope for HCC treatment, such as TACE in combination with targeted therapies or immunotherapy2. Recent advancements in immunotherapy and targeted therapies have transformed the treatment landscape for HCC, with the combination of pembrolizumab, an immune checkpoint inhibitor, and lenvatinib, a multi-tyrosine kinase inhibitor, has been widely used in clinical trials3. This combination aims to enhance antitumor immune responses while simultaneously targeting the tumor vasculature4.
In fact, pembrolizumab combined with lenvatinib has shown promising results in patients with advanced, unresectable HCC5. Despite the therapeutic potential of this regimen, the emergence of adverse drug reactions (ADRs) remains a critical concern. Pembrolizumab, which targets the programmed cell death protein 1 (PD-1), enhances T-cell activation and proliferation, while lenvatinib inhibits multiple signaling pathways involved in tumor growth and angiogenesis4. The unique mechanisms of action of these drugs can lead to a complex interplay of immune-mediated and targeted therapy-related side effects6,7. Understanding the safety profile of this combination therapy is vital for optimizing treatment outcomes and improving patient quality of life8.
Preliminary studies have indicated that the combination of pembrolizumab and lenvatinib may increase the overall incidence of ADRs compared to pembrolizumab monotherapy9. Therefore, the potential for severe and sometimes life-threatening events, such as immune-related adverse effects and hepatic dysfunction, necessitates vigilant monitoring10. Immune-related reactions can manifest in various forms, including dermatitis, colitis, and endocrinopathies, which may require prompt intervention and management strategies11,12. Furthermore, lenvatinib’s known side effects, such as hypertension, fatigue, and gastrointestinal disturbances, can complicate the clinical picture13,14,15.
The FDA Adverse Event Reporting System (FAERS) database is maintained by the U.S. Food and Drug Administration (FDA) and is used to collect and analyze reports of adverse events (AEs) related to drugs. This database contains various information reported by patients regarding adverse reactions, drug interactions, and therapeutic effects that occur during the use of medications on the market. This study uses real-world data from the FAERS database to investigate the safety and inherent risks of pembrolizumab alone and in combination with lenvatinib for the treatment of HCC, with the aim of developing effective management strategies and ultimately improving the therapeutic index for patients battling this challenging malignancy.
Materials and methods
Data collection and processing
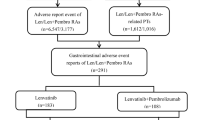
The data for this study was downloaded from the FAERS database. We extracted AEs report data related to drug reactions from the first quarter of 2014 to the fourth quarter of 2023 and analyzed it using MySQL. After removing duplicates, we focused on adverse event reports primarily associated with pembrolizumab alone or pembrolizumab in combined with lenvatinib. The FAERS database is made up of seven sections: demographic and administrative information (DEMO), adverse drug reaction data (REAC), patient outcome information (OUTC), drug information (DRUG), drug therapy start and end dates (THER), report source information (RPSR), and indications for use/diagnosis (INDI). We performed a fuzzy search on the “DRUGNAME” field in the DRUG table using both the trade name and generic name of the drugs. The search terms used were “pembrolizumab,” “Keytruda,” “lenvatinib,” and “Lenvima”. Concurrently, the terms ‘hepatocellular carcinoma’, ‘advanced hepatocellular carcinoma’, ‘hepatocellular carcinoma male’, ‘hepatocellular carcinoma female’, ‘hepatocellular carcinoma metastatic’, ‘hepatocellular carcinoma nos’, ‘hepatocellular carcinoma recurrent’ were utilized as search terms for HCC. This allowed us to identify drugs as primary suspect (PS), secondary suspect (SS), and as a drug (C), as well as to capture drug interactions (I) for data completeness. Through this process, we successfully filtered a total of 80,206 reports related to pembrolizumab. After specifying the indication as HCC, we obtained 459 reports related to the use of pembrolizumab alone, and 358 reports concerning the combined use of lenvatinib and pembrolizumab (Fig. 1). All AEs reports for pembrolizumab and lenvatinib are categorized at the levels of system organ class (SOC) and preferred term (PT).
Statistical analysis
This study uses disproportionality analysis based on reporting odds ratios (ROR), proportional reporting ratios (PRR), Bayesian confidence propagation neural networks (BCPNN), and empirical Bayesian geometric means (EBGM) to explore the relationship between pembrolizumab, lenvatinib, and AEs. ROR is useful for reducing bias in cases with fewer reported events16. In contrast, PRR emphasizes identifying and evaluating the specific impact of various risk factors, allowing for better detection of potential risks17. BCPNN is particularly strong at integrating and cross-validating data from multiple sources, effectively managing uncertainty and missing data to improve prediction accuracy18. MGPS is especially effective in detecting signals from infrequent events19,20. By combining ROR, PRR, BCPNN, and MGPS, this study leverages their strengths to broaden the scope of detection and validation from multiple angles. Therefore, this study combined four methods to obtain signals with significant associations. In this process, if at least one of the four algorithms meets the criteria, the drug-related AEs is considered a positive signal. Conversely, when all four algorithms meet the criteria, it indicates a strong association between AEs, effectively reducing the risk of potential false positive signals. These methods are based on a 2 × 2 contingency table, as shown in Supplementary Table 1. The formulas for each method and the conditions for signal generation are presented in Supplementary Table 2 (Supplementary Table 2). MySQL 8.0, Microsoft Excel 2019, and R 4.4.2 were used for data processing and statistical analysis.
Results
Descriptive analysis
After excluding duplicate data, reports of adverse reactions related to pembrolizumab treatment for HCC patients were extracted from the FAERS database, covering records from the first quarter of 2014 to the fourth quarter of 2023. A total of 459 AEs were identified for pembrolizumab monotherapy and 358 for its combination with lenvatinib. Approximately 80% of these events occurred in male patients. Age stratification analysis revealed that more than 50% of patients were aged 60 to 74 years. The majority of these reports originated from the United States, followed by China, France, and Japan. Furthermore, the number of reported cases showed a year-on-year increasing trend. Notably, pembrolizumab alone and in combination with lenvatinib resulted in 579 and 450 serious outcomes, respectively, including death, life-threatening outcomes, disability, and permanent damage. Among these, there were 84 deaths related to pembrolizumab monotherapy and 54 deaths related to its combination with Lenvatinib (Table 1).
Signal detection
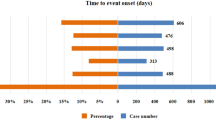
We conducted a statistical analysis using four algorithms: ROR, PRR, BCPNN, and MGPS. For patients receiving only pembrolizumab, we identified 63, 63, 59, and 162 preferred terms (PTs) with each algorithm, and 50 PTs were consistent across all four. In patients treated with pembrolizumab plus lenvatinib, the algorithms identified 43, 44, 41, and 120 PTs, with 38 PTs meeting the criteria across all algorithms (Table 2). Additionally, we analyzed the onset times of each PT, as illustrated in Fig. 2. We found that the PTs in HCC patients receiving only pembrolizumab and those receiving the combination therapy were primarily concentrated in the first month of treatment, with PTs occurring within the first 90 days at rates of 55.6% and 59.5%, respectively (Fig. 2A, B). The median time to PTs for patients on pembrolizumab alone was 80.5 days (interquartile range: 20.0–217.3 days), while for those receiving the combination with lenvatinib, it was 77.5 days (interquartile range: 19.7–212.3 days).
Signals of system organ class
The positive signals for PTs were categorized according to the SOC in MedDRA version 24.0. The results showed that 50 PTs associated with pembrolizumab monotherapy and 38 PTs associated with the combination of pembrolizumab and lenvatinib in the treatment of HCC involved 15 different organ systems, respectively. The top 30 most common PTs and their corresponding SOCs are presented in Tables 3 and 4. Detailed information on others PTs can be found in Supplementary Tables 3 and 4. The most frequently reported PTs for pembrolizumab monotherapy were “hepatic encephalopathy”, “blood bilirubin increased”, “diarrhea”, “malignant neoplasm progression”, and “ascites”. Interestingly, in the combination therapy with pembrolizumab and lenvatinib, “acute kidney injury” appeared among the top five PTs, while the remaining four PTs were the same as those seen with pembrolizumab monotherapy. Of note, unexpected significant AEs, including “dehydration”, “skin ulcers”, and “intestinal perforation” and so on, were observed in the label.
Using the lower limit of the ROR confidence interval, we selected the top 20 high-risk signals for pembrolizumab monotherapy and its combination with lenvatinib, presenting them in a forest plot (Fig. 3A, B). As shown, reactive capillary proliferation is the highest-risk signal for pembrolizumab monotherapy, whereas this particular AEs was not observed with the therapy of pembrolizumab plus lenvatinib.
Regarding the types of SOCs, the most commonly affected categories for both pembrolizumab monotherapy and the combination therapy with lenvatinib were “gastrointestinal disorders” (8 types each), “hepatobiliary disorders” (5 types for pembrolizumab monotherapy and 7 types for the combination therapy), and “investigations” (4 types for pembrolizumab monotherapy and 6 types for the combination therapy) (Fig. 4).
Discussion
Pembrolizumab, a monoclonal antibody that targets programmed cell death receptor-1 (PD-1), has shown significant clinical efficacy in treating various cancers, including non-small cell lung cancer, melanoma, gastric cancer, and head and neck cancers21,22,23,24. It has also been approved for the treatment of HCC. For advanced patients, pembrolizumab offers a promising treatment by overcoming immune evasion mechanisms. As our understanding of the PD-1/PD-L1 immune evasion pathway improves, pembrolizumab’s role in HCC treatment has expanded, not only as a monotherapy but also in combination with other drugs to enhance its efficacy25,26. Several clinical trials in HCC have shown that the combination of pembrolizumab and lenvatinib provides better results than using either a targeted therapy or an immunotherapy alone5. The KEYNOTE-524 study, an open-label, single-arm, Phase Ib, multicenter trial, reported encouraging therapeutic outcomes3. In 100 patients with advanced HCC, the median overall survival was 22.0 months (95% CI 20.4 months–not reached), and the median progression-free survival was 8.6 months (95% CI 7.1–9.7 months). The disease control rate was 88%. Based on mRECIST criteria, the objective response rate was 46% (95% CI: 36.0-56.3%), with 11 complete responses and 35 partial responses. The duration of response was 8.6 months (95% CI: 6.9 months–not reached). However, the results of the global, multicenter Phase III clinical trial (LEAP-002) showed that the combination of lenvatinib and pembrolizumab did not achieve the predefined significance in improving OS and PFS compared to lenvatinib plus placebo. However, the combination therapy did not increase the incidence of Grade 3–4 adverse events or death. This contrasts with the findings of a real-world study, which concluded that combination therapy could improve the prognosis of HCC patients27,28. It is worth noting that another global, multicenter, double-blind, phase 3 study (LEAP-012) showed that patients receiving TACE combined with pembrolizumab and lenvatinib had significantly longer PFS and OS compared to those receiving TACE + placebo29. However, this study lacked detailed results on the occurrence of AEs.
Our study, on the other hand, focused on monitoring and identifying AEs, providing valuable information for clinicians regarding the safety of this combination therapy, and helping to better balance efficacy with safety. In LEAP-002, common AEs associated with the combination therapy included hypertension, diarrhea, liver function abnormalities, and fatigue. The incidence of these AEs was higher compared to monotherapy, but most patients were able to manage them effectively through symptomatic treatment or medication adjustments. In our study, the most common AEs were hepatic encephalopathy, increased bilirubin levels, and diarrhea, which align with the observations in LEAP-002 and LEAP-012. However, we also identified some unexpected serious AEs, such as dehydration, skin ulcers, and intestinal perforation, which were not widely reported or highlighted in LEAP-002 and LEAP-012.
Many previous studies have examined the relationship between pembrolizumab and individual AEs, but a comprehensive summary is still lacking. In this study, we provide an in-depth understanding of the safety of combination therapy, offering important data on the safety of Pembrolizumab and Lenvatinib in the treatment of HCC. This helps clinicians better assess treatment risks in real-world practice and develop personalized management plans. We explored AEs associated with pembrolizumab monotherapy and its combination with lenvatinib for the treatment of HCC, leveraging the largest available real-world data from the FAERS database. We identified and analyzed significant new ADRs, with the goal of informing product labeling and supporting evidence-based clinical decision-making.
Our findings show that the incidence of AEs with pembrolizumab monotherapy and its combination with lenvatinib is higher in male patients (79.08% and 81.01%) compared to female patients (18.08% and 17.88%). This may be due to the greater number of male HCC patients30. Age distribution indicates that AEs are most commonly reported in patients aged 60–74, likely reflecting the higher morbidity and the number of pembrolizumab users in this age group31. It’s important to note that these AEs resulted in serious outcomes. Among patients treated with pembrolizumab monotherapy and its combination with lenvatinib, 14.51% and 12.03%, respectively, experienced fatal outcomes, while 59.59% and 68.82% required hospitalization or prolonged stays. As immunotherapy continues to gain broader use and pembrolizumab’s clinical application expands, clinicians should be especially cautious about potential AEs in elderly patients to prevent life-threatening complications. The study, based on the FAERS database, includes data from multiple countries (such as the United States, China, France, and Japan), highlighting the global diversity of drug-related adverse events. Understanding the frequency of AEs and their management approaches in different regions may provide valuable data to support international treatment standards and clinical guidelines.
Our disproportionality analysis indicates that the most commonly reported SOCs associated with pembrolizumab include gastrointestinal disorders, hepatobiliary diseases, investigations, benign, malignant, and unspecified tumors (including cystic and polypoid), metabolic and nutritional disorders, respiratory conditions, chest and mediastinal diseases, endocrine disorders, and cardiac diseases. These results align with the drug labeling and safety data from clinical trials32. The reported gastrointestinal disorders showed a consistent pattern of the most common PTs with pembrolizumab monotherapy and plus lenvatinib. The highest incidence rates were seen for diarrhea, gastrointestinal bleeding, ascites, and immune-mediated colitis. Additionally, we noted that intestinal perforation was observed as severe AEs. Previous clinical trials have reported cases of intestinal perforation leading to discontinuation of pembrolizumab treatment in patients with lung cancer or other tumors33,34. However, an individual report suggests that reintroducing pembrolizumab after an episode of intestinal perforation may be safe35.
Hepatobiliary disorders are also among the most common SOCs associated with pembrolizumab in the treatment of HCC. The most frequently reported AEs in this category include hepatic function abnormal, immune-mediated hepatitis, cholangitis, and hepatic failure. Of these, immune-mediated hepatitis is the AE most strongly associated with hepatobiliary disorders. When analyzing the AEs related to pembrolizumab monotherapy and its combination with lenvatinib, we found that the combination therapy was more strongly associated with immune-mediated hepatitis than pembrolizumab alone (ROR 370.77, 95% CI (175.67-782.56) vs. ROR 250.18, 95% CI (118.67–527.4)). Previous findings from a real-world study suggested that immune-mediated hepatitis can occur during lenvatinib treatment for HCC36. Based on this, we suspect that the combination of pembrolizumab and lenvatinib may have an additive effect, potentially exacerbating immune-mediated liver damage.
Our findings indicate that hepatic encephalopathy was the most common AE among neuropsychiatric disorders in patients receiving pembrolizumab monotherapy and its combination with lenvatinib. However, the combination therapy seems to have a stronger association with hepatic encephalopathy than pembrolizumab alone. In terms of etiology, the development of hepatic encephalopathy is linked to abnormal liver metabolism and portosystemic shunting37,38. Studies have shown that the hepatotoxicity of TKI drugs is related to the metabolism of their active metabolites, which can interfere with cellular components, disrupting cell function and inducing cell death39. Furthermore, Mukozu et al. proposed that lenvatinib not only inhibits tumor blood supply by suppressing arterial formation but also reduces portal vein blood flow, which accelerates the development of portosystemic shunts. This process can lead to the accumulation of toxic substances, such as ammonia, in the bloodstream, resulting in neurotoxic effects40. Therefore, Kuwahara et al. recommended performing portal vein intervention to block shunting before initiating lenvatinib treatment in order to prevent hepatic encephalopathy and hyperammonemia41. Interestingly, we observed that reactive capillary endothelial proliferation was the AE most strongly associated with pembrolizumab monotherapy. However, it was not detected when pembrolizumab was used in combination with lenvatinib. This may be attributed to lenvatinib’s intrinsic ability to inhibit endothelial cell proliferation, and further prospective clinical trials are needed to validate this hypothesis.
Adrenal insufficiency and hypothyroidism were the primary endocrine-related adverse events observed in patients receiving pembrolizumab monotherapy and its combination with lenvatinib, with no significant difference in incidence between the two treatment groups. Both of these AEs are prominently listed in the prescribing information for lenvatinib and pembrolizumab. Kurokawa et al. found that 59 of 186 patients with advanced non-small cell lung cancer treated with pembrolizumab had low adrenocorticotropic hormone levels, but all patients recovered completely after corticosteroid replacement therapy42. Pembrolizumab-induced hypothyroidism is typically associated with systemic symptoms like reduced appetite, edema, nausea, vomiting, and fatigue43. However, a study by Finsterer indicates that it can also present with myopathy and psychiatric symptoms, with most patients needing thyroid hormone replacement therapy44. As a result, clinicians should remain especially vigilant in monitoring adrenal and thyroid function when administering lenvatinib and pembrolizumab. In addition, the study identified some unexpected ADRs, such as dehydration, skin ulcers, and intestinal perforation. These findings provide new points of focus for future clinical practice and drug monitoring, urging healthcare professionals to remain vigilant regarding these less common AEs.
We also examined the time to onset of AEs and found that the median onset was slightly earlier with combination lenvatinib therapy compared to single-agent pembrolizumab (77.5 days vs. 80.5 days), while the difference was not statistically significant. Previous studies have shown that 44% of AEs associated with single-agent lenvatinib occur within the first month of treatment36. In our study, 38.6% of patients on single-agent pembrolizumab experienced AEs within the same period. Thus, we hypothesize that the earlier onset of AEs with lenvatinib is the main reason for the observed difference in median onset times between the two groups. Ultimately, early and accurate identification of drug-induced adverse reactions is crucial for minimizing the treatment window.
It is undeniable that this study has certain limitations, some inherent to the FAERS database. First, while disproportionality analysis provides statistically significant signal strength, it cannot establish a direct causal relationship between drug exposure and AEs. Second, the FAERS database cannot determine the true incidence of AEs, as the total number of exposures (denominator) is unclear45. Additionally, duplicate reports from multiple sources and missing data may bias results. In this study, we analyzed AEs associated with pembrolizumab monotherapy and its combination with lenvatinib in treating HCC, but we cannot exclude the influence of concomitant medications, comorbidities, or other patient-specific factors. Indeed, no interaction analysis between pembrolizumab and lenvatinib was conducted. It is worth noting that our results showed a relatively small number of AEs associated with the pembrolizumab and lenvatinib combination therapy group, which is unrelated to the period during which adverse event reporting data was collected. Therefore, this result may not accurately reflect the drug’s safety in the real world and could be influenced by reporting bias. This is a limitation of our study. Therefore, further prospective studies and clinical trials are needed to confirm the causal relationship between pembrolizumab and these AEs and to improve methods for incorporating such data into the FAERS database. Despite these limitations, the FAERS database remains essential in identifying oncology drug-related AEs and can help determine risk patterns and duration, supporting clinical treatment decisions and updating drug labeling.
Conclusion
We performed a pharmacovigilance analysis using the FAERS database to systematically identify patient characteristics and the timing of adverse event signals associated with pembrolizumab monotherapy and its combination with lenvatinib in the treatment of HCC. The most common adverse reactions were gastrointestinal disorders and hepatobiliary diseases, with hepatic encephalopathy being the most frequent AE in both treatment groups. In addition, we identified new and unexpected serious AEs, such as dehydration, skin ulcers, and intestinal perforation, which warrant close attention. We recommend enhanced monitoring and risk assessment of these AEs, particularly in high-risk populations, to better evaluate the risk-benefit profile of pembrolizumab. Moreover, further cohort studies and clinical trials are needed to validate these findings and gain a deeper understanding of pembrolizumab’s safety profile.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
References
Toh, M. R. et al. Global epidemiology and genetics of hepatocellular carcinoma. Gastroenterology 164, 766–782. https://doi.org/10.1053/j.gastro.2023.01.033 (2023).
Rizzo, A., Ricci, A. D. & Brandi, G. Trans-arterial chemoembolization plus systemic treatments for hepatocellular carcinoma: an update. J. Pers. Med. 12, 788. https://doi.org/10.3390/jpm12111788 (2022).
Finn, R. S. et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 38, 2960–2970. https://doi.org/10.1200/JCO.20.00808 (2020).
Torrens, L. et al. Immunomodulatory effects of lenvatinib plus anti-programmed cell death protein 1 in mice and rationale for patient enrichment in hepatocellular carcinoma. Hepatology 74, 2652–2669. https://doi.org/10.1002/hep.32023 (2021).
Yi, C. et al. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology 74, 2544–2560. https://doi.org/10.1002/hep.31921 (2021).
Wang, Y. et al. The safety and efficacy of lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma. Biomed. Pharmacother. 132, 110797. https://doi.org/10.1016/j.biopha.2020.110797 (2020).
Guven, D. C. et al. Immune checkpoint inhibitor-related hearing loss: a systematic review and analysis of individual patient data. Support Care Cancer 31, 624. https://doi.org/10.1007/s00520-023-08083-w (2023).
Rizzo, A. & Ricci, A. D. Challenges and future trends of hepatocellular carcinoma immunotherapy. Int. J. Mol. Sci. 23, 363. https://doi.org/10.3390/ijms231911363 (2022).
Liu, Q. et al. Efficacy and safety of anti-PD-1 monotherapy versus anti-PD-1 antibodies plus lenvatinib in patients with advanced hepatocellular carcinoma: a real-world experience. Ther. Adv. Med. Oncol. 15, 17588359231206274. https://doi.org/10.1177/17588359231206274 (2023).
Cunningham, M., Gupta, R. & Butler, M. Checkpoint inhibitor hepatotoxicity: pathogenesis and management. Hepatology 79, 198–212. https://doi.org/10.1097/HEP.0000000000000045 (2024).
Ramos-Casals, M. et al. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 6. https://doi.org/10.1038/s41572-020-0160-6 (2020).
Michot, J. M. et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur. J. Cancer 54, 139–148. https://doi.org/10.1016/j.ejca.2015.11.016 (2016).
Ishihara, K. Liver function and bleeding complications associated with lenvatinib. J. Gastrointest. Liver Dis. 30, 185–187. https://doi.org/10.15403/jgld-3579 (2021).
Kim, B. H. et al. Expert consensus on the management of adverse events in patients receiving lenvatinib for hepatocellular carcinoma. J. Gastroenterol. Hepatol. 37, 428–439. https://doi.org/10.1111/jgh.15727 (2022).
Rimassa, L., Danesi, R., Pressiani, T. & Merle, P. Management of adverse events associated with tyrosine kinase inhibitors: improving outcomes for patients with hepatocellular carcinoma. Cancer Treat. Rev. 77, 20–28. https://doi.org/10.1016/j.ctrv.2019.05.004 (2019).
Rothman, K. J., Lanes, S. & Sacks, S. T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 13, 519–523. https://doi.org/10.1002/pds.1001 (2004).
Evans, S. J., Waller, P. C. & Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 10, 483–486. https://doi.org/10.1002/pds.677 (2001).
Bate, A. et al. A bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54, 315–321. https://doi.org/10.1007/s002280050466 (1998).
Almenoff, J. S., LaCroix, K. K., Yuen, N. A., Fram, D. & DuMouchel, W. Comparative performance of two quantitative safety signalling methods: implications for use in a pharmacovigilance department. Drug Saf. 29, 875–887. https://doi.org/10.2165/00002018-200629100-00005 (2006).
Wu, J., Pan, H., Shen, L. & Zhao, M. Assessing the safety of bedaquiline: insight from adverse event reporting system analysis. Front. Pharmacol. 15, 1382441. https://doi.org/10.3389/fphar.2024.1382441 (2024).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092. https://doi.org/10.1056/NEJMoa1801005 (2018).
Weber, J. S. et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): a randomised, phase 2b study. Lancet 403, 632–644. https://doi.org/10.1016/S0140-6736(23)02268-7 (2024).
Shitara, K. et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 6, 1571–1580. https://doi.org/10.1001/jamaoncol.2020.3370 (2020).
Burtness, B. et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394, 1915–1928. https://doi.org/10.1016/S0140-6736(19)32591-7 (2019).
Yarchoan, M. et al. Personalized neoantigen vaccine and pembrolizumab in advanced hepatocellular carcinoma: a phase 1/2 trial. Nat. Med. 30, 1044–1053. https://doi.org/10.1038/s41591-024-02894-y (2024).
Qin, S. et al. Pembrolizumab versus placebo as second-line therapy in patients from Asia with advanced hepatocellular carcinoma: a randomized, double-blind, phase III trial. J. Clin. Oncol. 41, 1434–1443. https://doi.org/10.1200/JCO.22.00620 (2023).
Llovet, J. M. et al. Lenvatinib plus Pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): a randomised, double-blind, phase 3 trial. Lancet Oncol. 24, 1399–1410. https://doi.org/10.1016/S1470-2045(23)00469-2 (2023).
Xu, M. H. et al. Effectiveness and safety of lenvatinib plus anti-programmed death-1 antibodies in patients with hepatocellular carcinoma: a real-world cohort study. Cancer Med. 12, 9202–9212. https://doi.org/10.1002/cam4.5661 (2023).
Llovet, J. M. et al. Randomized phase 3 LEAP-012 study: Transarterial chemoembolization with or without lenvatinib plus pembrolizumab for intermediate-stage hepatocellular carcinoma not amenable to curative treatment. Cardiovasc. Intervent. Radiol. 45, 405–412. https://doi.org/10.1007/s00270-021-03031-9 (2022).
Tran, S. et al. Updates in characteristics and survival rates of hepatocellular carcinoma in a nationwide cohort of real-world US patients, 2003–2021. J. Hepatocell. Carcinoma 10, 2147–2158. https://doi.org/10.2147/JHC.S420603 (2023).
Ratnapradipa, K. L., Li, T., Hsieh, M. C., Tenner, L. & Peters, E. S. Most deprived Louisiana census tracts have higher hepatocellular carcinoma incidence and worse survival. Front. Oncol. 14, 1331049. https://doi.org/10.3389/fonc.2024.1331049 (2024).
Abulizi, A. et al. Cardiovascular adverse events and immune-related adverse events associated with PD-1/PD-L1 inhibitors for head and neck squamous cell carcinoma (HNSCC). Sci. Rep. 14, 25919. https://doi.org/10.1038/s41598-024-75099-5 (2024).
Sanz-Segura, P., Garcia-Camara, P., Fernandez-Bonilla, E. & Arbones-Mainar, J. M. Bernal Monterde, V. Gastrointestinal and liver immune-related adverse effects induced by immune checkpoint inhibitors: a descriptive observational study. Gastroenterol. Hepatol. 44, 261–268. https://doi.org/10.1016/j.gastrohep.2020.07.009 (2021).
Qian, Y. et al. Immune-related intestinal pseudo-obstruction caused by immune checkpoint inhibitors: case report. Front. Oncol. 14, 1415117. https://doi.org/10.3389/fonc.2024.1415117 (2024).
Beck, T. N., Kudinov, A. E., Dulaimi, E. & Boumber, Y. Case report: reinitiating pembrolizumab treatment after small bowel perforation. BMC Cancer 19, 379. https://doi.org/10.1186/s12885-019-5577-5 (2019).
Yang, Y., Wang, Y., Chen, B., Liu, Y. & Gu, K. A real-world drug safety surveillance study of lenvatinib from the FAERS database. Expert Opin. Drug Saf. 1, 1–13. https://doi.org/10.1080/14740338.2024.2393284 (2024).
European Association for the Study of the Liver. EASL Clinical Practice guidelines on the management of hepatic encephalopathy. J. Hepatol. 77, 807–824. https://doi.org/10.1016/j.jhep.2022.06.001 (2022).
Elsaid, M. I. & Rustgi, V. K. Epidemiology of hepatic encephalopathy. Clin. Liver Dis. 24, 157–174. https://doi.org/10.1016/j.cld.2020.01.001 (2020).
Chen, B. et al. Risk factors for hepatic encephalopathy in hepatocellular carcinoma after sorafenib or lenvatinib treatment: a real-world study. Drug Des. Dev. Ther. 16, 4429–4437. https://doi.org/10.2147/DDDT.S386829 (2022).
Mukozu, T. et al. Adaptation of lenvatinib treatment in patients with hepatocellular carcinoma and portal vein tumor thrombosis. Cancer Chemother. Pharmacol. 89, 11–20. https://doi.org/10.1007/s00280-021-04359-2 (2022).
Kuwahara, A. et al. Shunt occlusion prior to lenvatinib administration prevents hepatic encephalopathy and hyperammonemia. JGH Open 4, 775–776. https://doi.org/10.1002/jgh3.12351 (2020).
Kurokawa, K. et al. Clinical characteristics of adrenal insufficiency induced by pembrolizumab in non-small-cell lung cancer. Thorac. Cancer 14, 442–449. https://doi.org/10.1111/1759-7714.14761 (2023).
Hashinokuchi, A. et al. Hypothyroidism with ACTH deficiency during pembrolizumab therapy for lung cancer: case report and literature review. Cancer Diagn. Progn. 3, 498–503. https://doi.org/10.21873/cdp.10246 (2023).
Finsterer, J. Pembrolizumab-induced hypothyroidism manifesting as myopathy and psychosis. Melanoma Res. 31, 405–406. https://doi.org/10.1097/CMR.0000000000000759 (2021).
Ha, A., Langroudi, A. P. & Eisenberg, M. L. What is the validity of the federal adverse event reporting system in contemporary clinical research? J. Sex. Med. 21, 744–745. https://doi.org/10.1093/jsxmed/qdae072 (2024).
Author information
Authors and Affiliations
Contributions
HW: Data curation, Formal Analysis, Methodology, Software, Supervision, Visualization, Writing–original draft, Writing—review and editing. JL: Data curation, Formal Analysis, Resources, Software, Writing—review and editing. XZ: Data curation, Supervision, Validation, Writing—review and editing. RW: Data curation, Supervision, Validation, Writing—review and editing. YW: Data curation, Formal Analysis, Supervision, Visualization, Writing—original draft, Writing–review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, H., Li, J., Zhu, X. et al. A real-world drug safety surveillance study from the FAERS database of hepatocellular carcinoma patients receiving pembrolizumab alone and plus lenvatinib. Sci Rep 15, 1425 (2025). https://doi.org/10.1038/s41598-025-85831-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-85831-4