Abstract
Diffuse coronary artery disease (CAD) impacts the immediate hemodynamic and clinical outcomes of percutaneous coronary intervention (PCI). We evaluated whether the diffuse pattern of CAD derived from angiographic Quantitative flow ratio (QFR) impacts the immediate hemodynamic outcome post-PCI and the medium term predicted vessel-oriented composite endpoint (VOCE). Paired pre-procedure QFRs were assessed in 503 patients and 1022 vessels in the Multivessel TALENT (MVT) trial. The pathophysiological pattern of CAD was defined as “predominantly diffuse” or “focal” according to a virtual QFR pullback pressure gradient (PPG) index < 0.78 and ≥ 0.78, respectively. Physiological “focal severity” was assessed using the QFR gradient per mm (dQFR/ds), with a value ≥ 0.025/mm the threshold for a “major gradient”. A post-PCI QFR ≥ 0.91 was considered optimal. Median pre-PCI PPG index was 0.70 (IQR 0.59–0.80). The prevalence of “predominantly diffuse” CAD and “major gradient” were 68.6% and 85.8%, respectively. A “Predominantly diffuse” pattern with a major gradient had a higher risk of a post-PCI QFR < 0.91 (OR 1.52,95%CI 1.47–1.58). In multivariable analysis, low QFR PPG index (diffuse disease) was an independent determinant of a post-PCI QFR < 0.91 (per 0.1 decrease of QFR PPG index, OR:9.8, 95% CI 3.0–32.2, p < 0.001). Based on post-PCI QFR the predicted 2-year VOCE, a powered endpoint in the MVT trial, was 6.1% and 4.2% in diffuse and focal lesions, respectively. A pre-procedure physiological pattern of diffuse CAD is an independent determinant of an unfavourable immediate hemodynamic outcome post-PCI, and detrimentally affects the predicted 2-year VOCE.
Clinical Trial Registration URL: https://www.clinicaltrials.gov/ct2/show/NCT04390672
Unique Identifier: NCT04390672 (registration date 15/05/2020)
Similar content being viewed by others
Introduction
Percutaneous coronary intervention (PCI) is an effective treatment of ischemic anginal symptoms, however, up to a quarter of patients are left with residual symptoms1 Two fundamental mechanisms have been implicated in this: the first relates to the presence of diffuse epicardial coronary artery disease (CAD) as documented prior to PCI by the pullback pressure gradient (PPG) and the second, to insufficient dilatation of a major epicardial vessel stenosis in conjunction with pre-existing, or newly occurring coronary microvascular dysfunction (CMD). Currently, the interaction and interplay of these mechanistic factors are of great interest to the interventional community.
Despite the established benefits of physiological assessment post-PCI, which facilitates optimizing the hemodynamic result2, and identifying those patients likely to have favourable/unfavourable outcomes3, the ERIS (Evolving Routine Standards of FFR Use) study reported that invasive post-PCI FFR was used in less than 10% of lesions assessed with physiology pre-PCI4. The impediments of FFR, such as cost, invasiveness, prolonged procedure time, and the need for intravenous hyperaemic agents, may act as deterrents2 however, alternatives, such as Quantitative Flow Ratio (QFR), an angiography-derived FFR measurement which doesn’t require a pressure wire or hyperaemia3, are now available. Notably, in patients with de novo 3-vessel disease enrolled in the SYNTAX II trial the incidence of the 2-year vessel-oriented composite endpoint (VOCE) was significantly higher in vessels with a post-PCI QFR < 0.91 versus ≥ 0.91 (12.0% vs. 3.7%; hazard ratio [HR] 3.37; 95% confidence interval [CI] 1.91–5.97; p < 0.001)5.
Vessel-level FFR unravels the cumulative hemodynamic impact of atherosclerosis along the whole vessel, whereas a trans-stent delta in post-PCI FFR reflects residual flow-limiting factors specifically related to the stented area6. Indeed a sub-optimal FFR post-PCI can occur even after optimal stenting due to residual focal or diffuse disease in non-stented segments proximal or distal to the target lesion7. In the DEFINE PCI (Physiologic Assessment of Coronary Stenosis Following PCI) trial residual ischemia after angiographically successful PCI, defined as an instantaneous wave-free ratio (iFR) < 0.90, occurred in 24.0% of patients8 with 61.6% of residual stenoses located outside the stented segment. Therefore, pre-PCI assessment of the pathophysiological pattern of coronary atherosclerosis (diffuse or focal) could help predict the physiological response to PCI.
Collet et al. proposed the PPG index, derived from a motorized coronary pressure pullback during continuous hyperemia to quantify the “physiological distribution” of coronary atherosclerosis9. Shin et al. reported the “physiological distribution” and “local severity” of CAD using virtual angiographic QFR pullbacks, showing that 2-year target vessel failure after PCI was determined mainly by the pre-procedural physiological distribution of coronary atherosclerosis7.
Outcomes from the SYNTAX II and TALENT trials influenced the design of the Multivessel TALENT trial, an ongoing randomized controlled trial comparing two drug-eluting stents (DES) in patients with de novo three-vessel disease (3VD) without left main (LM) disease, such that QFR was mandated pre-PCI to guide which lesions should be treated or deferred, and post-PCI to help predict medium term clinical outcomes10.
Therefore, this study aimed to ascertain the impact of the pre-procedural pathophysiological pattern of CAD on the immediate hemodynamic outcomes post-PCI, and tried to predict 2-year VOCE in the ongoing Multivessel TALENT trial. The probabilistic model used to predict VOCE at 2 years was developed using post-PCI QFRs from the SYNTAX II trial, and was incorporated into the design of the Multivessel TALENT trial, however it has not previously been tested prospectively. Importantly whilst this prospective ascertainment of the VOCE will indicate in advance of the actual outcome whether the trial assumptions, sample size and power calculation are seemingly correct, regardless of the outcome, it is not the intention of the steering committee to modify the sample size.
Methods
Study design
The Multivessel TALENT study (ClinicalTrials.gov, NCT04390672,registration date 15/05/2020) is a prospective, randomized controlled, equal allocation, multi-centre, open-label study comparing clinical outcomes between the SUPRAFLEX Cruz sirolimus-eluting stent (SMT, Gujarat, India) and the SYNERGY everolimus-eluting stent (Boston Scientific, Natick, MA) in 60 sites from 8 European countries. The details of the trial design have been previously published10.
Briefly, patients with de novo 3VD and without left main disease had QFR of their invasive coronary angiograms analysed by an independent Core lab (CORRIB Lab, Galway) before PCI. State-of-the-art PCI was then performed based on the following concepts: 1) legitimization of PCI treatment in multi-vessel disease based on SYNTAX Score II treatment recommendations11; 2) functional evaluation with QFR (central Core lab) of each stenotic lesion prior to treatment [ESC guidelines (GL) Class IB]12; 3) intravascular ultrasonography (IVUS)/optical coherence tomography (OCT) optimization (ESC GL, Class IIa, B)13; 4) use of contemporary chronic total occlusion (TO) techniques14 performed by an on-site accredited expert and 5) optimal medical therapy15 including P2Y12 monotherapy after one month of dual antiplatelet therapy16.
The primary endpoint of the Multi-Vessel Talent trial is a non-inferiority comparison of the patient-oriented composite endpoint (POCE) of the Supraflex Cruz cohort versus the SYNERGY cohort at 12 months post-procedure. The powered secondary endpoint is a superiority comparison in the per-protocol analysis—at a vessel level—of VOCE, a composite of vessel-related cardiovascular death, vessel-related myocardial infarction, or clinically and physiologically indicated target vessel revascularisation, at 24 months post-procedure10.
All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by local institutional committee. Written informed consent was obtained from all participants.
QFR analysis and pathophysiological pattern acquisition in Core lab
QFR was analysed off-line by either QAngio XA 3D/QFR® imaging software (Version 2.0.60.6 Medis medical imaging system, Leiden, The Netherlands) or AngioPlus Core software with Murray-law based QFR (μFR) analysis (version V2, Pulse Medical, Shanghai, China) depending on the availability of one or two angiographic views17,18 (Fig. 1). Both systems have equivalent diagnostic performance against invasive FFR/iFR as reported by our group19. In this paper, the term QFR is used to indicate the angiography-derived physiology metric for the enhanced readability of this paper.
Following informed consent, and before randomization, the central core lab identified functionally significant lesions. These QFR results, as well as the recommended length and diameter of stents to be used, were then forwarded to investigators through a deck of approximately 10 PowerPoint slides. The anatomical SYNTAX score was calculated on-site and by the independent Core lab. Post-PCI QFR analysis was performed retrospectively by the Core lab.
For reproducibility, pre-procedural pathophysiological patterns were extracted from the automated calculations by QAngio XA 3D/QFR® imaging software (Version 2.1 research edition). For vessels with only one optimal projection, μFR values per 1 mm were obtained by μFR pullback curves using WebPlotDigitizer. The pre-procedural physiological distribution (“diffuseness or focality”) of CAD was assessed using the QFR PPG index calculated as follows:9
Maximal PPG represents the greatest change in QFR within a 20 mm segment of interest and accounts for partial value of the vessel QFR itself. It reflects the drop of pressure and flow and the increase in resistance over a specific segment of the coronary epicardial conductance vessel due to either a focal or a diffuse narrowing. As PPG varies depending on a specific 20 mm segment of interest within the vessel, mechanical assistance (pullback of the pressure wire in the original description of the invasive method )or software assistance is required for seamless calculation.
The maximal PPG is the highest at the site of the stenosis with the greatest degree of flow limitation. Moreover, as it is a partial value of the vessel QFR, it is expressed in decimal terms ranging between 0 and 1.
Since the QFR ranges from approximately 0 to 1, the value of ΔQFR vessel is calculated by subtracting from the nominal value of 1 the overall QFR of the vessel, and represents the reduction in QFR attributable to the entire lesional disease (focal or diffuse).
Therefore, dividing the maximal PPG by ΔQFR vessel results in a value closer to 1 for the most focal lesions.
Conversely, subtracting (length with “functional disease”/total diseased vessel length) from 1 indicates the proportion of the vessel with “unfunctional disease”, ranging from 0 to 1. This value also approaches 1 for the most focal lesions.
Since the two components of the formula can sum up to 2, the result must be divided by 2 to align with existing indices like FFR and QFR which have a maximum value of 1.
The physiological distribution of CAD was defined as predominantly diffuse or focal according to a QFR PPG index < 0.78 or ≥ 0.78, respectively7. The pre-PCI physiological local severity of a lesion was assessed using the QFR gradient per mm (dQFR/ds), with a value ≥ 0.025/mm defining the presence of a “major gradient.”7.
The vessel PPG index was not evaluated in TO, whilst the dQFR/ds value was unreliable in ostial lesions; hence these vessels (n = 62) were excluded from the analysis. The QFR PPG index and dQFR/ds were used to categorize the pathophysiological pattern of CAD into four groups7: predominantly focal (QFR PPG index ≥ 0.78) with (Group 1) or without (Group 2) a major gradient (dQFR/ds ≥ 0.025/mm) and predominantly diffuse (QFR PPG index < 0.78) with (Group 3) or without (Group 4) a major gradient.
A Core lab driven assessment comparing the QFR software using either one or two angiographic views against a benchmark of 325 iFR and FFR measurements has been recently reported19.
Lesion QFR was defined as the QFR of a stenotic lesion in the target vessel that was automatically detected by the software on the baseline angiogram (Fig. 1s). The cut-off value of QFR for physiological significance was ≤ 0.80.
Vessel QFR was analysed from the ostia of the main vessels (right coronary artery [RCA], left anterior descending [LAD], and left circumflex coronary arteries [LCX]) to an anatomic landmark (e.g., side branch) located distal to the lesion. If an anatomical landmark was not identifiable, the distal point was set 10 mm distal to the stent edge. Delta QFR across the stent was measured and regarded as “in-stent QFR” drop (Fig. 1s).
Information about the anatomical SYNTAX score20 (including lesion characterization such as bifurcation, heavy calcification, and TO, etc.…) and implanted stents (number of stents, stent diameter and length) was collected in an electronic case report form (eCRF).
Based on previous reports describing the impact of post-PCI QFR values on 2-year VOCE, a favourable VOCE was expected when the post-PCI vessel QFR was ≥ 0.915.
Statistical analysis
Categorical variables were summarized as frequencies and proportions. Continuous variables are presented as mean (SD) or median (interquartile range). For comparison of the two groups, a 95% confidence interval (CI) for the difference of the two proportions for categorical variables and the difference of the mean for continuous variables were provided.
To predict 2-year VOCE in the current study based on the QFR measurements available in this interim sample, we first fitted a logistic regression model in the SYNTAX II trial data, with the 2-year VOCE as the binary response variable, and post-procedure QFR as the continuous predictor variable. A restricted cubic spline was used to model the change in the log odds of 2-year VOCE with changes in QFR to flexibly model any non-linearity. Once the model was fitted, it was possible to predict a probability for 2-year VOCE for each treated vessel in the interim sample, based on their measured post-procedure QFR. The 95% prediction interval (PI) for the rate of 2-year VOCE was obtained using the predicted probabilities in the interim sample and bootstrap resampling (see Text 1 s)21.
Once the full trial is completed, the relation between post-PCI QFR results and 2-year VOCE will be examined to confirm the correctness of the prediction and prediction model.
All data analyses were performed using R version 4.1.1.
Results
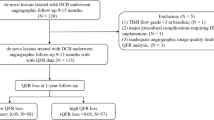
This exploratory analysis included the first 503 consecutive, randomised patients enrolled in the Multivessel TALENT trial who had 1182 diseased vessels, and were treated between September 2020 and August 2022. The mean number of vessels treated per patient was 2.35. Those vessels with TO (n = 62), ostial lesions (n = 78), and where angiography did not permit QFR analysis (e.g. lack of optimal projection, vessel overlap, isocenter issue, incomplete contrast filling, n = 20) were excluded, leaving 1022 in the final analysis. The QAngio XA 3D/QFR® research edition could not calculate the QFR or pathophysiological disease pattern in 159 patients pre-PCI and 236 patients post-PCI due to the absence of two optimal angiographic projections, and in these cases, the μFR AngioPlus Core software was used. (Fig. 1).
Baseline patient characteristics are presented in Table 1. The mean age was 68.2 (9.1) years and 77% were male. Acute coronary syndrome accounted for 41% of the population, whilst the prevalence of medically treated diabetes was 30%. Mean left ventricle ejection fraction was 54.3 (10.4)%. The mean SYNTAX scores calculated on-site and by the core laboratory were 18.8 (8.0) and 22.1 (8.3), respectively.
Baseline lesion and procedural characteristics are shown in Table 2; 90% of vessels were assessed by intracoronary imaging (IVUS 61%, OCT 29%). The average number of stents per treated vessel and per patient was 1.6 ± 0.8 and 3.8 ± 1.5, respectively, with a mean nominal stent diameter of 3.0 ± 0.4 mm, and an average total stent length of 39.6 ± 22.9 mm. As per protocol, ostial lesions and TOs were excluded. There were some differences in lesion characteristics between the analysable (n = 1022) and non-analysable (n = 160) vessels.
The distribution of the pathophysiological pattern of disease pre-PCI and the post-PCI QFR in the 1022 analysed vessels are displayed in Fig. 2. Over half the vessels (n = 609, 59.6%) had predominantly diffuse disease (PPG index < 0.78) with a major gradient (dQFR/ds ≥ 0.025). Predominantly focal disease was seen in 31.4%, and this was associated with a higher rate of a post-PCI QFR ≥ 0.91 compared to diffuse disease (83.8% vs 72.9%).
Figure 3 shows the cumulative distribution curve of pre- and post-PCI QFRs for the predominantly diffuse and focal groups. A post-PCI QFR ≥ 0.91 was achieved in 83.8% and 72.9% of the treated vessels, respectively.
Cumulative frequency of pre- and post-PCI QFR in 2 groups. Left panel showed cumulative curves of both pre- and post-PCI QFR in predominantly focal disease defined by QFR PPG index at pre-PCI (≥ 0.78) and right panel showed those in predominantly diffuse disease (QFR PPG index < 0.78). Abbreviations: PCI, percutaneous coronary intervention; QFR, quantitative flow ratio; PPG, pressure pullback curve gradient.
Figure 4 shows the correlation between the pre-PCI PPG index and the odds ratio of a sub-optimal QFR post-PCI. The restricted cubic spline curve shows the exponential increase in the odds ratio for an optimal QFR post-PCI when the pre-PCI PPG index is over 0.78.
Relation between pre-PCI PPG index and odds ratio of optimal post-PCI QFR. Cubic spline curve showing the odds ratio of optimal post-PCI QFR (vertical axis) according to the pre-PCI QFR PPG index (horizontal axis). Abbreviations: PCI, percutaneous coronary intervention; QFR, quantitative flow ratio; PPG, pullback pressure curve gradient.
In the univariate analysis, the pre-procedure QFR (HR 3.75, 95%CI 1.69–8.29, p = 0.001) and PPG index (HR 7.49, 95%CI 2.56–21.9, p < 0.001) were predictors of a suboptimal QFR post-PCI, while dQFR/ds was not (HR 1.86, 95%CI 0.212–16.3, p = 0.58). However, after adjustment for confounding factors (QFR, dQFR/ds, and the pre-procedure index of microvascular resistance), only the pre-procedure PPG index remained as an independent predictor of a post-PCI QFR < 0.91 (per 0.1 decrease of QFR PPG index, OR 14.6, 95% CI 3.0–72.1, p < 0.001). The sensitivity analysis was done for the population with analysable QFR both pre- and post-procedure and the results were concordant to what we found in the whole population.
Based on the predictions from the fitted SYNTAX II model, and the available post-procedure QFR in the interim Multivessel TALENT data, the predicted rate of 2-year VOCE in the trial was 6.1% with a bootstrap 95% PI of 4.9% to 7.3% for diffuse disease, and 4.2% with a 95% PI of 2.5%-6.3% in focal lesions (Fig. 2s and 3s).
Discussion
The main findings of this study are;
-
1.
The majority of vessels treated in the ongoing Multivessel TALENT trial have predominantly diffuse CAD as based on the QFR-derived PPG index at baseline.
-
2.
Compared to predominantly focal disease, those vessels with predominantly diffuse CAD have a lower likelihood of achieving an optimal physiological result after PCI.
-
3.
The pre-procedural PPG index is an independent predictor of a sub-optimal QFR post-PCI.
-
4.
The predicted 2-year VOCE was higher in the predominantly diffuse group compared to the predominantly focal group.
The cut-off value of the PPG index to predict acute surrogate hemodynamic outcomes (post-procedure QFR)
Recently new data have raised the questions of whether the current cut-off criteria for defining the pathophysiological patterns of CAD need to be redefined1. QFR-PPG in an attempt to replicate the original PPG derived from mechanical wire pull-back could be at variance with the pressure-derived parameter due to the following factors. First, QFR cannot specifically quantify the roughness in the lesion encroaching the lumen or the in-stent surface apposed or not apposed causing micro-turbulence which generates pressure loss and a decrease in FFR value. Also, it has been established that in diffuse lesions, pressure loss mainly stems from laminar shear stress in the lesion, which cannot be captured by the angiography-derived FFR technology while in focal lesions, the loss in kinetic energy is mainly due to edges and diverging layers of flow at the exit of the lesion. Shin et al. differentiated between predominantly focal and diffuse disease using a QFR PPG index cut-off of 0.78, which was the median value in their study of 341 patients with functionally significant coronary stenoses (pre-PCI FFR ≤ 0.80), who underwent angiographically successful PCI with residual stenoses by visual estimation of < 20%7. The most suitable PPG cut-off (PPG 0.73) to predict optimal post-PCI FFR suggested by the PPG Global trial supports the threshold we set in this report (QFR-PPG 0.78) and a prospective trial would be needed to set the optimal cut-off for QFR-PPG22. For clarity, it has to be stressed that the recent original analysis revealed a moderate agreement between the PPG derived from invasive FFR and the PPG derived from virtual FFR (r = 0.64)23.
Collet et al. argued that the PPG index should be interpreted as a continuous metric rather than a binary one when defining the pattern of CAD, and showed in their study of 158 vessels in 117 patients undergoing clinically indicated coronary angiography due to stable angina that the mean PPG index that differentiated pathophysiologically focal narrowings from diffuse disease was 0.58 ± 0.18 (p < 0.001)9. In the PANDA III study which included 1444 vessels in 1003 patients with a QFR ≤ 0.80, the mean PPG index was 0.73 ± 0.1424. In addition, based on ROC analysis the PANDAIII study showed that the best QFR-PPG index cut-off value for predicting 2-year VOCE was 0.77.
In the Multivessel TALENT trial, the median pre-PCI QFR-derived PPG index was 0.70, with more than 70% of vessels having the predominantly diffuse pattern using a PPG index threshold of 0.78. As presented in Fig. 2, a PPG index between 0.70 and 0.78 had the same rate of optimal QFR post-PCI; the Youden index to detect optimal post-PCI QFR from the continuous pre-PCI PPG index was 0.79, which indicates that the previously determined cut-off of 0.78 is quite reasonable in this population.
Prediction model of 2-year VOCE from post-PCI QFR value and its impact on considering PCI procedure for diffuse and focal lesions in clinical practice
Retrospective analysis of QFR data from the SYNTAX II study has established that a post-PCI QFR ≥ 0.91 is associated with improved 2-year VOCE5. Similar to SYNTAX II, the current study promoted the so-called “best practice PCI”, with a comparable mean number of vessels treated per patient (2.4 vs 2.35). Given that 76% of vessels achieved the physiological threshold of 0.91, we can reasonably assume that the 2-year VOCE (at least the TLR component) in the 503 Multi-vessel TALENT population could be 6.3%, with an acceptable PI (4.8%-7.9%), which is similar to that used in trial’s power calculation10.
QFR, as confirmed in the FAVOR Pilot study25, identifies stenotic vessels with a pressure-derived FFR ≤ 0.80 with good diagnostic accuracy, without needing a pressure wire or induction of pharmacologic hyperaemia. In a systematic review and Bayesian meta-analysis, our group previously confirmed the high sensitivity and specificity of QFR against pressure wire-derived physiological assessment26. Recently, FAVOR III China demonstrated significantly fewer myocardial infarctions and ischaemia-driven revascularisations at 1-year and major adverse cardiac events at 2-years in the QFR versus angiography-guided group27,28.
Furthermore, the latest meta-analysis comparing angiography-derived FFR and FFR derived from IVUS and OCT shows an area under the curve of each receiver operator characteristic curve of more than 0.9529. Therefore, the systematic physiological assessment of all diseased vessels by imaging-based QFR, pre- and post-PCI is attractive in terms of patient comfort, cost, time, and safety.
If ascertainment of the accuracy of our statistical assumption based on the outcome of the SYNTAX II trial is reassuring, then prediction of the estimated rate of VOCE in the current study according to our Syntax II probabilistic model will only be convincing if made prospectively and blindly to the actual event rate. Previously we made a similar attempt to predict outcomes, when we reported all-cause mortality at 4-years in the EXCEL trial—immediately at the end of recruitment30,31.
This approach—a form of ongoing monitoring for the “search of futility” of the secondary endpoint—may potentially identify the moment where the secondary objective of the present study (VOCE at 2 years) could be considered achievable or unachievable, even if the study completes its planned enrolment32.
The prediction model in this study confirms that predominantly diffuse disease represents a major hurdle in achieving an optimal QFR post-PCI, which consequently leads to poorer outcomes at 2-years5 It follows that operators must consider lesion characteristics when selecting the best strategy for an individual patient. As both the SYNTAX II trial and the ongoing Multivessel TALENT trial follow the same state-of-the-art PCI procedure, little additional procedural care can be recommended to manage this diffuse CAD, except to emphasise the importance of very aggressive pharmacological treatment combining,-for instance-, high-intensity statins, ezetimibe, and inhibition of PCSK-9 (CLASS IA)33. Therefore, the assessment of the diffuseness of disease based on the PPG index has the potential to be an effective modifier of our revascularization strategy34.
Limitation
There is no clear consensus on the threshold value of the PPG index impacting clinical outcomes, and the search for an optimal binary threshold is ongoing. Indeed, we need more evidence of the impact of the pre-procedural PPG index on clinical outcomes after mechanical revascularisation and aggressive pharmacotherapy.
Pre-PCI QFR, as mandated by the protocol was available in 93% of all the lesions suspected to be visually more than 50% in diameter stenosis. Provision was also made to use alternative but equally accurate QFR software—necessitating only a single angiographic view—and that was used in 17% of vessels.
The stated assumptions that (1) the available QFR data of these initial patients will be representative of the eventual full trial sample and (2) the relationship between QFR and VOCE in SYNTAX II is similar to that in Multivessel TALENT seem plausible although they will only be verified on completion of the study. In addition, VOCE rates based on SYNTAX II and Multivessel TALENT with the same proportion of multivessel disease and comparable baseline characteristics can only be generalized to a similar population. After the main trial is completed, the relation between 2-year VOCE and post-PCI QFR results should be examined whether the prediction is reasonable.
Conclusions
A pre-procedure physiological pattern of diffuse CAD is an independent determinant of an unfavourable immediate hemodynamic outcome post-PCI, and detrimentally affects the predicted 2-year VOCE compared to focal disease.
Impact on daily practice
This study shows that pre-procedure QFR contains not only physiological information about the flow-limiting nature of coronary stenoses but also characterizes, with the PPG index, its diffuseness/focality and defines, with dQFR/ds, the magnitude of the QFR change. Predominantly diffuse disease tends to result in a lower achievement of optimal QFR results post-procedure, which ultimately results in worse outcomes compared to focal lesions. In a population with multivessel disease, target vessels need to be judged by integrating these factors and discussions at the Heart Team for an individualized optimal treatment strategy.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Abbreviations
- CAD:
-
Coronary artery disease
- dQFR/ds:
-
Instantaneous QFR ratio gradient per unit length
- FFR:
-
Fractional flow reserve
- PCI:
-
Percutaneous coronary intervention
- PPG:
-
Pullback pressure gradient
- QFR®:
-
Quantitative flow ratio
- µFR:
-
Murray law-based quantitative flow ratio
- VOCE:
-
Vessel-oriented composite endpoint
- PCI:
-
Percutaneous coronary intervention
- QFR:
-
quantitative flow ratio
- PPGI:
-
Pullback pressure gradient index
- PI:
-
Prediction interval
References
Serruys, P. W., Kageyama, S., Garg, S. & Onuma, Y. In the beginning there was angina pectoris, at the end there was still angina pectoris. JACC Cardiovasc. Interv. 15, 2519–2522 (2022).
Kogame, N. et al. The impact of coronary physiology on contemporary clinical decision making. Jacc-Cardiovasc. Interv. 13, 1617–1638 (2020).
Biscaglia, S. et al. Invasive coronary physiology after stent implantation. Jacc-Cardiovasc. Interven. 14, 237–246 (2021).
Tebaldi, M. et al. Evolving routine standards in invasive hemodynamic assessment of coronary stenosis The Nationwide Italian SICI-GISE cross-sectional ERIS study. Jacc-Cardiovasc. Interven. 11, 1482–1491 (2018).
Kogame, N. et al. Clinical implication of quantitative flow ratio after percutaneous coronary intervention for 3-vessel disease. Jacc-Cardiovasc. Interven. 12, 2064–2075 (2019).
Lee, J. M. et al. Prognostic implications of relative increase and final fractional flow reserve in patients with stent implantation. Jacc-Cardiovasc. Interven. 11, 2099–2109 (2018).
Shin, D. et al. Physiological distribution and local severity of coronary artery disease and outcomes after percutaneous coronary intervention. Jacc-Cardiovasc. Interven. 14, 1771–1785 (2021).
Jeremias, A. et al. Blinded physiological assessment of residual ischemia after successful angiographic percutaneous coronary intervention The DEFINE PCI Study. Jacc-Cardiovasc. Interven. 12, 1991–2001 (2019).
Collet, C. et al. Measurement of hyperemic pullback pressure gradients to characterize patterns of coronary atherosclerosis. J. Am. Coll. Cardiol. 74, 1772–1784 (2019).
Hara, H., Gao, C., Kogame, N. et al. A randomised controlled trial of the sirolimus-eluting biodegradable polymer ultra-thin Supraflex stent versus the everolimus-eluting biodegradable polymer SYNERGY stent for three-vessel coronary artery disease: Rationale and design of the Multivessel TALENT trial. Eurointervention. 16 E997-+. (2020).
Serruys, P. W. et al. The SYNTAX score on its way out or … towards artificial intelligence: part I. Eurointervention 16, 44–59 (2020).
Vrints, C. et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 45, 3415–3537 (2024).
Raber, L., Mintz, G. S., Koskinas, K. C. et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. 39, 3281 (2018). European Heart Journal (2019). 40, 308–308.
Neumann, F. J., Chettibi, M., Sisakia, H. et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 40 87-+. (2019).
Kawashima, H., Serruys, P., Ono, M., McEvoy, J. & Onuma, Y. Impact of optimal medical therapy on 10-year mortality after coronary revascularization. J. Am. Coll. Cardiol. 78, B49–B49 (2021).
Serruys, P. W., Takahashi, K., Chichareon, P. et al. Impact of long-term ticagrelor monotherapy following 1-month dual antiplatelet therapy in patients who underwent complex percutaneous coronary intervention: insights from the Global Leaders trial. Eur. Heart J. 40 2595-+. (2019).
Koltowski, L. et al. Quantitative flow ratio derived from diagnostic coronary angiography in assessment of patients with intermediate coronary stenosis: A wire-free fractional flow reserve study. Clin. Res. Cardiol. 107, 858–867 (2018).
Tu, S. et al. Diagnostic accuracy of quantitative flow ratio for assessment of coronary stenosis significance from a single angiographic view: A novel method based on bifurcation fractal law. Catheter. Cardiovasc. Interv. 97(Suppl 2), 1040–1047 (2021).
Ninomiya, K., Serruys, P. W., Kotoku, N. et al. Anonymous comparison of various angiography-derived fractional flow reserve software with pressure-derived physiological assessment. JACC Cardiovasc. Interv. (2023).
Serruys, P. W. et al. Assessment of the SYNTAX score in the syntax study. Eurointervention 5, 50–56 (2009).
Kageyama, S. et al. Quantitative flow ratio for the prediction of coronary events after percutaneous coronary intervention. EuroIntervention 20, 104–106 (2024).
Collet, C. et al. Influence of pathophysiologic patterns of coronary artery disease on immediate percutaneous coronary intervention outcomes. Circulation 150, 586–597 (2024).
Seki, R. et al. Validation of virtual fractional flow reserve pullback curves. Catheter. Cardiovasc. Interv. 104, 1178–1188 (2024).
Dai, N. et al. Integrated coronary disease burden and patterns to discriminate vessels benefiting from percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 99, E12–E21 (2022).
Tu, S. et al. Diagnostic accuracy of fast computational approaches to derive fractional flow reserve from diagnostic coronary X-ray angiography in the international multicenter FAVOR (Functional Assessment by Various FlOw Reconstructions) Pilot Study. J. Am. Coll. Cardiol. 68, B5–B5 (2016).
Collet, C., Onuma, Y., Sonck, J. et al. Diagnostic performance of angiography-derived fractional flow reserve: A systematic review and Bayesian meta-analysis. Eur. Heart J. 39 3314-+. (2018).
Xu, B. et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): A multicentre, randomised, sham-controlled trial. Lancet 398, 2149–2159 (2021).
Song, L. et al. 2-Year outcomes of angiographic quantitative flow ratio-guided coronary interventions. J. Am. Coll. Cardiol. 80, 2089–2101 (2022).
Takahashi, T. et al. Diagnostic performance of fractional flow reserve derived from coronary angiography, intravascular ultrasound, and optical coherence tomography; a meta-analysis. J. Cardiol. 80, 1–8 (2022).
Campos, C. M., van Klaveren, D., Farooq, V. et al. Long-term forecasting and comparison of mortality in the evaluation of the xience everolimus eluting stent vs. coronary artery bypass surgery for effectiveness of left main revascularization (EXCEL) trial: Prospective validation of the SYNTAX Score II. Eur. Heart J. 36 1231–1241. (2015).
Stone, G. W. et al. Five-year outcomes after PCI or CABG for left main coronary disease. N. Engl. J. Med. 381, 1820–1830 (2019).
Ware, J. H., Muller, J. E. & Braunwald, E. The futility index - an approach to the cost-effective termination of randomized clinical-trials. Am. J. Med. 78, 635–643 (1985).
Mach, F. et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 41, 111–188 (2020).
Takahashi, K. et al. Redevelopment and validation of the SYNTAX score II to individualise decision making between percutaneous and surgical revascularisation in patients with complex coronary artery disease: Secondary analysis of the multicentre randomised controlled SYNTAXES trial with external cohort validation. Lancet 396, 1399–1412 (2020).
Acknowledgements
We thank all the investigators and study participants who are involved in The Multivessel TALENT trial interim population. (Julien Lemoine, Adrian Wlodarczak, Faisal Sharif, Marc Silvestri, Anirban Choudhury, Helge Moellmann, Joanna Wykrzykowska, Manel Sabate, Raul Moreno, Maciej Lesiak, Murugu Veerasamy, Abhishek Kumar, Ralph Tölg, Michael Magro, Nicolas Meneveau, Bruno Farah, Peter O’Kane, Sean Gallagher, Stephan Achenbach, Rohit Oemrawsingh, Eric Van Belle, Alper Oner, Jacek Legutko, Janusz Kochman, Alexander Lauten, Azfar Zaman, Milosz Dziarmaga, Simone Biscaglia, Piotr Wacinski, Benoit Lattuca, Angela Hoye, Piotr Kwiatkowski, Haitham Abu Sharar, James Cockburn, Stanislaw Bartus, Robert Jozwa, Andrzej Ochala, Mark Kennedy, Olivier Morel, Juan Sanchís, and Ignacio Amat Santos)
Funding
The Multivessel TALENT trial is an investigator-initiated trial sponsored by The National University of Ireland Galway which received an unrestricted grant from SMT (Sahajanand Medical Technologies, India) to investigate the value of QFR in multivessel disease.
Author information
Authors and Affiliations
Contributions
The contribution of each author is as follows: Conception and design, SK, PWS, and YO; planning and supervising statistics. NOL; analysis and interpretation of data, PCR, TTY, KM, AT, AZ, MS, HM, FS, JL, and AW; drafting of the manuscript, SK; critically revising for important intellectual content, PWS, SG, JHR, ST; final approval of the manuscript submitted, SK.
Corresponding author
Ethics declarations
Competing interests
Dr Serruys reports institutional grants from Philips/Volcano, SMT, Novartis, Xeltis, Merillife, outside the submitted work. Dr Tu reports research grants and consultancy from Pulse Medical. Dr Sabaté has received consultant fees from Abbott Vascular and Ivascular outside the submitted work. Dr Möllmann reports speaker honoraria from Abbott, Boston Scientific, and SMT. All other authors have no conflict of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kageyama, S., Revaiah, P.C., Tsung-Ying, T. et al. Diffuseness of coronary artery disease impacts on immediate hemodynamic and predicted clinical outcomes. Sci Rep 15, 2228 (2025). https://doi.org/10.1038/s41598-025-85872-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85872-9
Keywords
This article is cited by
-
Non-invasive assessment of plaque characteristics in diffuse and focal coronary artery disease; plaque vulnerability and high-risk features
The International Journal of Cardiovascular Imaging (2026)