Abstract
Lean body mass (LBM) serves as an indicator of muscle mass in body composition. Recently, studies have revealed increased mortality rates in dialysis patients with low LBM. However, studies analyzing the impact of age on LBM and mortality in dialysis patients remain limited. This study analyzed data from 26,625 adult hemodialysis patients registered in the Korean Society of Nephrology (KSN) registry from 2001 to 2020. We used the lean body mass index (LBMI), standardized by the square of height, to normalize lean body mass. Patients were first categorized by age group (20–49, 50–69, and ≥ 70 years). Subsequently, patients were categorized into quartile groups based on their LBMI (≤ 15.55, 15.55 < LBMI ≤ 16.52, 16.52 < LBMI ≤ 17.59, and > 17.59). This study revealed that the lowest LBMI group (≤ 15.55) was associated with decreased survival across all age groups, with this trend being particularly prominent in the older age groups. Within the 20–49 age group, factors such as diabetes exhibited a notable influence on mortality rates among hemodialysis patients. In the age group over 50, a low LBMI (≤ 15.55) had a greater impact on mortality rates in hemodialysis patients than diabetes. Understanding the importance of LBM in older patients undergoing dialysis is crucial for improving their prognosis.
Similar content being viewed by others
Introduction
During the initial 2 years following dialysis initiation, notable changes occur in body composition, such as a significant increase in fat mass and decrease in muscle mass1,2. Moreover, the prevalence of sarcopenia is remarkably high in older patients with end-stage renal disease (ESRD), with rates of 37% in men and 29.3% in women3. Therefore, understanding and managing body composition is crucial in patients with ESRD.
BMI is an indicator of the relationship between weight and height. A higher BMI may be associated with lower mortality rates in hemodialysis patients, a phenomenon known as the obesity paradox. This may occur because fat mass can serve as an energy reserve and help neutralize endotoxins in cases of chronic malnutrition4. However, recent studies have shown that overweight ESRD patients commonly exhibit a body composition characterized by a combination of excess weight and reduced muscle mass, referred to as sarcopenic obesity (SO)5,6,7,8,9. This condition has shown a progressive increasing trend, particularly among elderly individuals with ESRD10,11,12. The poor prognosis in these patients appears to be due to the sarcopenia component rather than the increase in fat mass13. Furthermore, it remains unclear which type of body component, fat or muscle, is more protective14. Considering BMI’s limitation in distinguishing between muscle and fat, these complex factors may obscure the obesity paradox in its relationship with mortality.
A recent study indicated that lower lean body mass (LBM), which reflects muscle mass, is a more reliable predictor of outcomes in patients undergoing dialysis compared to BMI, showing an association with reduced survival rates15,16,17,18. However, to date, the impact of LBM on mortality in different age groups has not been analyzed in patients undergoing dialysis. Therefore, focusing on age when considering muscle mass in patients undergoing hemodialysis is crucial. Kim et al. reported that aging induced significant muscle mass transitions in patients undergoing dialysis over time19. Additionally, Han et al. presented a recent study on the impact of LBM in chronic kidney disease, revealing a significant difference in trends according to age group20.
This study examined the relationship between LBM and survival in patients undergoing dialysis based on age. We aimed to provide more detailed information regarding the impact of muscle mass on mortality, with a focus on age groups, and to highlight the importance of body composition in patients undergoing dialysis.
Methods
Data source
The ESRD registry committee of the Korean Society of Nephrology (KSN) launched an official ESRD patient registry in 1985. Registry data were collected through an internet program that was opened in 2001 and revised in 2013 (http://www.ksn.or.kr). All participants were enrolled in the study voluntarily, and informed consent was obtained from all patients. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Inha University Hospital (IRB No. 2023-11-031).
Study design and outcome
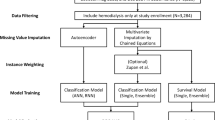
Patients aged ≥ 20 years undergoing hemodialysis were analyzed using data from the KSN registry. We excluded cases with inaccurate dates of birth, death, or hemodialysis initiation. Additionally, individuals lacking sufficient lean body mass index (LBMI) data were omitted. To enhance the robustness of our survival analysis, we excluded patients who died or were lost to follow-up within the first year. The index year was designated as the first year with data containing patient information, such as dialysis and blood tests. Subsequently, the survival period was examined from this point onward.
Demographic and clinical data were collected at the time of study enrollment, including age, sex, BMI, dialysis vintage, normalized protein catabolic rate (NPCR), single-pool Kt/V, blood pressure, functional status, cause of ESRD, and comorbidities such as diabetes, hypertension, coronary artery disease, congestive heart failure, cerebrovascular disease, and malignancy. All laboratory data were based on the initial values entered into the KSN registry.
The total patient cohort was categorized based on age groups (20–49, 50–69, and ≥ 70) and further stratified into four groups based on LBMI (LBMI ≤ 15.55, 15.55 < LBMI ≤ 16.52, 16.52 < LBMI ≤ 17.59, LBMI > 17.59). In this study, we designated LBMI ≤ 15.55 as group 1, 15.55 < LBMI ≤ 16.52 as group 2, 16.52 < LBMI ≤ 17.59 as group 3, and LBMI > 17.59 as group 4.
The primary outcome measure was the all-cause mortality. Notably, there was a possibility of overestimating patient survival owing to the voluntary nature of registration in the ESRD registry.
Data preprocessing
For continuous data spanning 2001–2020, the annual means were computed to serve as representative values for each year. The data from multiple tables were consolidated into a single entity. Duplicate data with identical dates and values were considered redundant and were removed before calculating the average.
The LBMI was calculated by first determining the estimated LBM using the following formula:
In previous studies, this LBMI formula has demonstrated a strong correlation with actual measurements, with a Pearson correlation coefficient of 0.93 (p < 0.001) between the derived LBM and the NIR interactance-measured LBM21.
Scr: Serum Creatinine. URR: Urea Reduction Ratio = (pre-HD BUN – post HD BUN) / pre-HD BUN x 100 (%). HD: Hemodialysis; BUN: blood urea nitrogen.
To standardize LBMI, we divided this value by the square of the patient’s height. The standardization method used in this study followed approaches employed in previous studies22,23.
Survival period was defined as the time interval between the index date of initial data collection for each patient to the date of death or last follow-up, expressed in years. Patients lost to follow-up or surviving were censored on the date of their last examination.
Statistical analysis
All statistical analyses were performed using R software 4.1.3 (http://www.R-project.org). Prior to statistical analysis, normality was assessed using the Kolmogorov–Smirnov test. Comparisons between groups were performed using analysis of variance or Student’s t-tests for continuous variables with normal distributions. The chi-square test or Fisher’s exact test was used for categorical variables. Data that did not show a normal distribution are presented as medians with interquartile ranges and were compared using the Mann–Whitney U test or the Kruskal–Wallis test. Survival rates were estimated using the Kaplan–Meier analysis and the log-rank test. Although the Cox proportional hazards models are widely used in mortality studies, they rely on the assumption of proportional hazards, which was not met for LBMI in our study. Consequently, we selected the Weibull model for the analysis. This approach, suitable for accelerated failure time analysis, allowed us to estimate the impact of LBMI on survival more accurately by providing time ratios (TRs) and 95% confidence intervals (CIs). The TR reflects how a covariate change affects survival duration. It is calculated as the exponential of the model coefficient for a given covariate. A TR > 1 signifies extended survival duration, whereas a TR < 1 denotes reduced survival time. To illustrate, when TR equals 1.2, it implies survival time lengthens by 20%. Conversely, a TR of 0.8 indicates survival time shortens by 20%. Additional analyses were conducted to further explore our findings. These included: (1) incorporation of pre-enrollment dialysis vintage as a covariate in the Weibull model, (2) a more granular analysis of the over-70 age group in 5-year intervals, and (3) an analysis using sex-adjusted LBMI quartiles, where quartiles were calculated separately for males and females and then combined to create overall sex-adjusted quartiles. Details of these analyses are presented in the Supplementary Materials. In addition to survival analysis, we used a linear mixed-effects model to examine the trajectories of LBMI across the LBMI quartile groups. The focus was to analyze how LBMI changed over time within each group, incorporating random intercepts and effects on individual variability.
Results
Characteristics of the study population
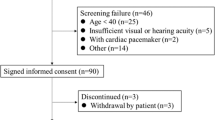
A total of 175,314 patients undergoing hemodialysis were initially screened in the KSN registry, and 26,625 patients aged ≥ 20 years were eventually analyzed, excluding cases with errors in date of birth, date of death, start date of hemodialysis, and with missing data required for the calculation of LBMI. The adjusted model further narrowed the sample to 8636 patients because of the absence of additional covariates. Patient enrollment is shown in Fig. 1.
The baseline characteristics of the patient groups, classified according to LBMI quartiles, are presented in Table 1. Among the total cohort, group 1 exhibited a higher prevalence of males and tended toward older demographic age. Additionally, this group showed comparatively lower phosphorus levels, total cholesterol, uric acid, intact parathyroid hormone, and BMI. The baseline characteristics of 8636 patients for adjusted model are presented in Supplementary Table 1. The trends observed in this subgroup were generally consistent with those seen in the full cohort.
Survival analysis and differences in time ratios based on LBMI
Total age groups
Among 26,625 patients aged 20 and older, 7644 deaths were observed during the study period. Patients in group 4 demonstrated the most favorable survival outcomes, whereas those in group 1 had the lowest survival. Statistically significant differences in mortality rates across the LBMI categories were noted (p < 0.001) (Fig. 2A). In the Weibull model, the TR was represented as 0.84 (95% CI: 0.80–0.87, p < 0.001) in group 1, 1.07 (95% CI: 1.02–1.12, p = 0.004) in group 3, and 1.13 (95% CI: 1.08–1.18, p < 0.001) in group 4. When considering multiple variables, the adjusted TR was statistically significant in groups 1 and 4, with values of 0.82 (95% CI: 0.76–0.90, p < 0.001) and 1.22 (95% CI: 1.10–1.36, p < 0.001), respectively. Additionally, diabetes and albumin had an impact on the mortality rate, with adjusted TR of 0.86 (95% CI: 0.81–0.92, p < 0.001) and 1.66 (95% CI: 1.56–1.78, p < 0.001), respectively (Table 2; Fig. 3).
Survival graph of age group. Each figure presents a survival graph of the following age groups: (A) all age groups, (B) 20–49 years, (C) 50–69 years, and (D) > 70 years. The total patient cohort was categorized into four groups based on LBMI (LBMI ≤ 15.55, 15.55 < LBMI ≤ 16.52, 16.52 < LBMI ≤ 17.59, LBMI > 17.59). Each LBMI group is represented by a dashed line, a dotted line, a dash-dot line, and a solid line, respectively. Abbreviation: LBMI, lean body mass index.
Adjusted Time Ratios of age for Mortality. Age group-specific adjusted time ratios for mortality across various factors. Each figure presents the adjusted time ratios for mortality within specific age groups: (A) all age groups, (B) 20–49 years, (C) 50–69 years, and (D) > 70 years. These ratios were adjusted for a range of factors, including LBMI categories, sex, diabetes, hypertension, and albumin. The squares indicate the point estimates of adjusted time ratios, with horizontal lines depicting 95% Confidence Intervals. An adjusted time ratio > 1 signified longer survival time (lower risk of mortality) associated with that factor, whereas a value < 1 indicated a shorter survival time (higher risk). Abbreviation: LBMI, lean body mass index; DM, diabetes mellitus; HTN, hypertension.
Age group of 20–49 years
In the younger cohort of 7165 patients, 1241 died during the study period. Survival analysis indicated no significant difference in the risk of mortality across LBMI categories (p = 0.41) (Fig. 2B). Notably, diabetes status was a significant predictor of mortality, with an adjusted TR of 0.55 (95% CI: 0.43–0.70, p < 0.001), highlighting the substantial impact of this comorbidity on survival outcomes within this age group. Higher albumin levels were significantly associated with better survival, with a TR of 1.73 (95% CI: 1.38–2.16, p < 0.001) (Table 2; Fig. 3).
Age group of 50–69 years
Of the 13,264 patients in this age group, 4111 died during the study period. Group 4 demonstrated the highest survival outcome, whereas group 1 showed the lowest survival rate, with this distinction being statistically significant (p < 0.001) (Fig. 2C). In the Weibull model, the TR for group 1 was 0.82 (95% CI: 0.78–0.87, p < 0.001), and that for group 4 was 1.10 (95% CI: 1.04–1.16, p = 0.001). The adjusted TR for individuals in group 1 was 0.81 (95% CI: 0.71–0.93, p = 0.003), and that for those in group 4 was 1.20 (95% CI:1.02–1.41, p = 0.03). Diabetes was associated with a higher mortality risk, with a TR of 0.84 (95% CI: 0.76–0.94, p = 0.002). Additionally, higher albumin was associated with decreased mortality risk, with a TR of 1.48 (95% CI: 1.33–1.64, p < 0.001) (Table 2; Fig. 3).
Age group of 70 years and older
In our cohort, 6196 patients were 70 years of age or older, with 2292 deaths occurring during the study period. The differences in survival outcomes among the LBMI groups were statistically significant (p < 0.001), and the older cohort generally exhibited lower survival rates than the other age groups (Fig. 2D). The Weibull model analysis demonstrated that group 1 had a TR of 0.86 (95% CI: 0.80–0.91, p < 0.001), while for group 4, the TR was 1.11 (95% CI: 1.03–1.20, p = 0.006). The adjusted analysis for this age group revealed a higher mortality risk for patients in group 1, exhibiting a TR of 0.87 (95% CI: 0.78–0.97, p = 0.016). However, diabetes did not remain a notable factor, with a TR of 0.94 (95% CI: 0.86–1.02, p = 0.157). Additionally, albumin showed a significant positive effect on survival, showing a TR of 1.50 (95% CI: 1.37–1.65, p < 0.001) (Table 2; Fig. 3).
Additional analyses
To further examine our findings, we conducted three additional analyses. First, to account for the potential impact of dialysis duration, an additional analysis was performed incorporating pre-enrollment dialysis vintage as a covariate. This analysis did not substantially alter our primary findings regarding the association between LBMI and mortality risk across different age groups. The results showed lower TRs in groups with the lowest LBMI, particularly in individuals aged 50 and older. Specifically, for those aged 50–69 years, the adjusted TR for Group 1 (lowest LBMI) was 0.83 (95% CI: 0.73–0.95, p = 0.006), and for those aged 70 and older, the adjusted TR for Group 1 was 0.88 (95% CI: 0.79–0.99, p = 0.029) (Supplementary Table 2). Second, in order to gain a more detailed insight into the effects of LBMI on mortality in older patients, a more granular analysis was performed on the over-70 age group in 5-year intervals, using LBMI quartiles specific to this age group. This analysis generally reinforced our primary findings, showing that lower LBMI was associated with lower TR and higher LBMI with higher TR across all subgroups. However, we observed some subtle variations in the strength of these associations. In the 70–74 and 75–79 age groups, only Group 1 (lowest LBMI) exhibited significantly lower TRs (TR = 0.83, 95% CI: 0.74–0.93, p = 0.001 for 70–74 years; TR = 0.82, 95% CI: 0.73–0.92, p = 0.001 for 75–79 years), while in the ≥ 80 subgroup, only Group 4 (highest LBMI) demonstrated significantly higher TR (TR = 1.30, 95% CI: 1.12–1.51, p < 0.001) (Supplementary Fig. 1, Supplementary Table 3). Third, to address the disproportionate distribution of males and females across LBMI groups in the primary analysis (with more females in lower LBMI groups and more males in higher LBMI groups), a further analysis was conducted using sex-adjusted LBMI quartiles. This analysis showed some differences from our primary analysis. In the 50–69 and ≥ 70 age groups, both Q3 (50–75%) and Q4 (> 75%) showed significantly higher TRs, whereas in the primary analysis, only the highest LBMI group (Q4) showed a significant benefit. For individuals aged 50–69 years, TR for Q3 was 1.13 (95% CI: 1.07–1.20, p < 0.001), and for Q4 it was 1.13 (95% CI: 1.07–1.19, p < 0.001). For those aged 70 and older, the TR for Q3 was 1.11 (95% CI: 1.03–1.19, p = 0.004), and for Q4 it was 1.09 (95% CI: 1.01–1.18, p = 0.022). The overall pattern of results remained similar to our primary findings, with higher LBMI quartiles associated with higher TRs. (Supplementary Fig. 2, Supplementary Table 4).
Changes in LBMI during the follow-up period
We examined the trajectory of average LBMI during the follow-up period in the total cohort group, with a focus on the differences over time across various LBMI quartiles.
The analysis revealed that each LBMI group exhibited distinct patterns of change in LBMI over the follow-up period. The group 4 and the group 1 demonstrated notably different trends in LBMI changes compared with group 2 as the reference. Specifically, the group 4 showed a decreasing trend over time, as indicated by the interaction term with a yearly change estimate of -0.1. In contrast, the group 1 exhibited an increasing LBMI trend, with a yearly change estimate of 0.07 (Fig. 4; Table 3).
Discussion
Among individuals in all age groups, those in group 1 (LBMI < 15.55) demonstrated diminished survival rates, with this tendency being particularly notable among older participants. Diabetes has also been identified as a significant risk factor for mortality. However, the reversal of this trend was observed only in the older group aged over 50. In all age groups, a distinct correlation was observed between albumin levels and mortality rates. Moreover, over the observation period, the decline in survival rates was more pronounced in patients with higher LBMI compared to those with lower LBMI.
Sarcopenia is a change resulting in reduced skeletal muscle mass, leading to diminished strength and functional ability24. Recently, sarcopenia has emerged as a considerable concern in older patients, as it is associated with adverse clinical outcomes such as hospitalization and an increased risk of mortality25. Furthermore, it is notably prevalent among older individuals diagnosed with ESRD, with a prevalence ranging from 28.5–40.3%26. Several studies have reported that sarcopenia is strongly associated with long-term mortality in patients undergoing hemodialysis24. According to recent research, the association between muscle mass and mortality rate in the general older population is controversial. Mortality shows a stronger correlation with muscle function and physical performance than with muscle mass27,28,29. However, LBM, representing muscle mass, is more strongly associated with the survival of patients undergoing hemodialysis, providing evidence in recent research15,16,17,18,23,30. Our study revealed that lower LBM was associated with survival in the older group.
One possible explanation is altered metabolism in ESRD, which is associated with characteristics such as metabolic acidosis, chronic inflammation, insulin resistance, endocrine disorders, and the presence of uremic toxins31,32. Metabolic acidosis affects insulin-like growth factor 1 and insulin signaling in skeletal muscle, leading to the upregulation of proteolytic systems such as the ubiquitin-proteasome system33. Furthermore, elevated cytokine activity contributes to sarcopenia in patients undergoing dialysis19. Patients undergoing dialysis are in a state of chronic inflammation with tumor necrosis factor and interleukin-6 acting as a key factor among the related cytokines34,35. This is associated with CRP and promotes muscle breakdown. Additionally, resistance to insulin and a decrease in anabolic hormones such as Growth hormone(GH) enhance protein catabolism in dialysis patients19. In renal impairment, changes in hormones such as ghrelin, leptin, and uremic toxins are associated with appetite loss and muscle loss32. Moreover, the uremic toxins in patients undergoing dialysis contribute to myocyte apoptosis involving inflammatory cytokines and reactive oxygen species36. Additionally, indoxyl sulfate induces the expression of genes related to muscle atrophy, including myostatin37,38. This decline in muscle mass, in addition to specific factors related to hemodialysis, contributes to increased mortality19,36,39.
Furthermore, it’s important to consider the age-related changes in body composition. In the normal aging process, fat mass typically increases while lean mass decreases, with body fat percentage continuing to rise until age 8040,41. In older individuals, factors such as reduced protein intake, a sedentary lifestyle, neurological changes such as the transition from fast motor units to slow motor units can contribute to muscle mass decline19. However, there is strong evidence that combined aerobic and resistance exercise can improve muscle mass and strength in elderly patients by enhancing nitric oxide production and increased insulin sensitivity, improving muscle protein synthesis7,42,43. Recent studies indicate that a relatively smaller decrease in skeletal muscle mass, despite age-related changes, reflects better overall health and resilience, which is associated with improved prognosis44,45. Therefore, organized exercise can serve as modifiable factors that contribute to better outcomes. These observations further highlight the importance of age-specific considerations in assessing and managing LBMI in dialysis patients, particularly in the very elderly.
Our analysis of the over-70 age group in 5-year intervals revealed nuanced differences while generally aligning with our primary findings. Across all subgroups, lower LBMI was generally associated with lower TR and higher LBMI with higher TR. However, the strength and significance of these associations varied among age groups. Notably, in the 80 and above age group, we observed a particularly intriguing pattern. In this oldest cohort, only the highest LBMI group showed a statistically significant association, demonstrating a markedly higher TR (TR = 1.30, 95% CI: 1.12–1.51, p < 0.001). This finding suggests that the protective effect of higher lean body mass may be especially pronounced in the very elderly dialysis population. The variations observed across age groups might be partially explained by the reduced sample size in these subgroup analyses, which could have affected statistical power. Additionally, survival bias might play a role in the oldest age group, where those reaching these ages, especially while on dialysis, may represent a select group with exceptional physiological reserve.
According to recent studies, diabetes has been identified as an independent predictor of sarcopenia in patients undergoing dialysis, contributing to mortality regardless of age, as reflected in the findings of our study46. This appears to be associated with insulin deficiency. Although the exact mechanism remains unknown, the progression of sarcopenia is alleviated when insulin treatment is administered to patients with diabetes47. Additionally, the correlation between albumin levels and mortality rates was evident across all age groups. Another study suggested that serum albumin could bind to endotoxins, thereby attenuating cytokine cascade activation. This anti-inflammatory effect may explain the beneficial impact of albumin on survival48,49.
This study has several strengths. Muscle mass was measured using an equation that provides values that can be easily obtained from blood tests, height, and weight. This facilitates their practical application in clinical settings. Bioelectrical impedance analysis and dual-energy X-ray absorptiometry are frequently used to assess muscle mass50. However, these approaches present challenges owing to their associated costs and the complexity of measurements. In our study, we adopted a formula that utilizes easily obtainable blood tests, such as BUN and creatinine levels, along with height and weight, allowing for the tracking of muscle mass trends over several years. This approach enables easier detection of conditions change that may affect muscle mass, such as catabolic illness and acidemia commonly observed in dialysis patients51,52,53. In addition, this study was conducted using Korean multicenter cohort data spanning approximately 20 years. This can be considered sufficiently large to assess the overall trends in patients undergoing dialysis and may help alleviate the impact of different dialysis protocols across centers. Furthermore, we analyzed the annual change in LBMI. Considering the steeply declining tendency of high LBMI over time, recognizing the significance of muscle mass at an early stage of dialysis can significantly enhance the long-term prognosis of patients undergoing dialysis.
This study had certain limitations. First, as a retrospective study, it was not possible to evenly distribute the patient groups across the LBMI categories, and sex bias occurred owing to a higher enrollment of males in group 1 (≤ 15.55). To account for this bias, we conducted additional analysis using sex-adjusted quantiles. The results, which demonstrated a greater impact of LBMI in individuals over 50 and a more pronounced effect of DM in younger individuals, were consistent with the primary analysis. This finding suggests that the conclusion remains applicable regardless of the presence of gender bias.
Additionally, since the study relied on registry-based data, CRP, which is an important factor affecting body component metabolism in hemodialysis, was not included as an input variable. Also, the diagnosis of sarcopenia typically requires screening tests such as the SARC-F scale, muscle strength assessments like hand grip, and the 2-Minute Seated Step Test. However, since our study was based on existing retrospective data, it was not possible to include these specific assessments. Furthermore, patients not recorded in the registry were excluded, which may introduce potential selection bias. Further controlled and comprehensive studies are required to overcome these limitations. Nevertheless, given that our study spans approximately 20 years of longitudinal data, it reflects the overall characteristics of patients undergoing dialysis. Therefore, our study results enhance our understanding of the importance of muscle mass in patients with ERSD and serve as valuable reference material for the future treatment and management of patients undergoing dialysis.
In conclusion, among older age groups, regardless of the underlying disease, LBMI is a crucial factor for mortality in patients undergoing hemodialysis, and its preservation is important for improving outcomes when the LBM is relatively preserved. However, further prospective studies are necessary for a more comprehensive understanding of LBMI and mortality in patients undergoing hemodialysis.
Data availability
The raw dataset, obtained under the authorization of the Korean Society of Nephrology, remains restricted from public access. However, the data derived from this investigation have been integrated into the manuscript. The datasets utilized and analyzed in this study are available from the corresponding author upon a reasonable request.
References
Marcelli, D. et al. Longitudinal changes in body composition in patients after initiation of Hemodialysis Therapy: Results from an International Cohort. J. Ren. Nutr. 26, 72–80. https://doi.org/10.1053/j.jrn.2015.10.001 (2016).
Broers, N. J., van der Cuijpers, A. C., Leunissen, K. M. & Kooman, J. P. The first year on haemodialysis: A critical transition. Clin. Kidney J. 8, 271–277. https://doi.org/10.1093/ckj/sfv021 (2015).
Kim, J. K. et al. Prevalence of and factors associated with Sarcopenia in elderly patients with end-stage renal disease. Clin. Nutr. 33, 64–68. https://doi.org/10.1016/j.clnu.2013.04.002 (2014).
Kalantar-Zadeh, K. et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin. Proc. 85, 991–1001. https://doi.org/10.4065/mcp.2010.0336 (2010).
Zamboni, M., Mazzali, G., Fantin, F., Rossi, A. & Di Francesco, V. Sarcopenic obesity: A new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis. 18, 388–395. https://doi.org/10.1016/j.numecd.2007.10.002 (2008).
Newman, A. B. et al. Weight change and the conservation of lean mass in old age: The Health, Aging and Body Composition Study. Am. J. Clin. Nutr. 82, 872–878. https://doi.org/10.1093/ajcn/82.4.872 (2005). quiz 915 – 876.
Prado, C. M., Batsis, J. A., Donini, L. M., Gonzalez, M. C. & Siervo, M. Sarcopenic obesity in older adults: A clinical overview. Nat. Rev. Endocrinol. 20, 261–277. https://doi.org/10.1038/s41574-023-00943-z (2024).
Friedman, A. N. Obesity in patients undergoing dialysis and kidney transplantation. Adv. Chronic Kidney Dis. 20, 128–134. https://doi.org/10.1053/j.ackd.2012.10.009 (2013).
Saitoh, M. et al. Sarcopenic obesity and its association with frailty and protein-energy wasting in hemodialysis patients: Preliminary data from a single center in Japan. Ren. Replace. Therapy. 5 https://doi.org/10.1186/s41100-019-0240-9 (2019).
Dahl, H., Rosendahl-Riise, H., Marti, H. P. & Dierkes, J. The Association of Sarcopenia and Central Obesity with mortality risk in patients with chronic kidney disease - a 2-Year observational study. Curr. Dev. Nutr. 7, 100014. https://doi.org/10.1016/j.cdnut.2022.100014 (2023).
Yang, Y., Zeng, Y., Lv, W., Fu, P. & Yuan, H. Prevalence and severity of Sarcopenia in patients on maintenance hemodialysis: A cross-sectional study. BMC Nephrol. 25, 385. https://doi.org/10.1186/s12882-024-03836-z (2024).
Bellafronte, N. T., de Queiros Mattoso Ono, A. & Chiarello, P. G. Sarcopenic obesity in chronic kidney disease: cChallenges in diagnosis using different diagnostic criteria. Med. Princ Pract. 30, 477–486. https://doi.org/10.1159/000517597 (2021).
Honda, H. et al. Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am. J. Clin. Nutr. 86, 633–638. https://doi.org/10.1093/ajcn/86.3.633 (2007).
Vashistha, T. et al. Effect of age and dialysis vintage on obesity paradox in long-term hemodialysis patients. Am. J. Kidney Dis. 63, 612–622. https://doi.org/10.1053/j.ajkd.2013.07.021 (2014).
Kalantar-Zadeh, K., Abbott, K. C., Salahudeen, A. K., Kilpatrick, R. D. & Horwich, T. B. Survival advantages of obesity in dialysis patients. Am. J. Clin. Nutr. 81, 543–554. https://doi.org/10.1093/ajcn/81.3.543 (2005).
Rymarz, A., Gibinska, J., Zajbt, M., Piechota, W. & Niemczyk, S. Low lean tissue mass can be a predictor of one-year survival in hemodialysis patients. Ren. Fail. 40, 231–237. https://doi.org/10.1080/0886022X.2018.1456451 (2018).
Caetano, C., Valente, A., Oliveira, T. & Garagarza, C. Body composition and mortality predictors in Hemodialysis patients. J. Ren. Nutr. 26, 81–86. https://doi.org/10.1053/j.jrn.2015.10.005 (2016).
Koefoed, M. et al. Nutritional status of maintenance Dialysis patients: Low lean body Mass Index and obesity are Common, protein-energy wasting is uncommon. PLoS One. 11, e0150012. https://doi.org/10.1371/journal.pone.0150012 (2016).
Kim, J. C., Kalantar-Zadeh, K. & Kopple, J. D. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J. Am. Soc. Nephrol. 24, 337–351. https://doi.org/10.1681/ASN.2012010047 (2013).
Han, S. S. et al. Age- and gender-dependent correlations between body composition and chronic kidney disease. Am. J. Nephrol. 31, 83–89. https://doi.org/10.1159/000258660 (2010).
Noori, N. et al. Novel equations to estimate lean body mass in maintenance hemodialysis patients. Am. J. Kidney Dis. 57, 130–139. https://doi.org/10.1053/j.ajkd.2010.10.003 (2011).
HATTORI, K. Body composition and lean body mass index for Japanese college students. J. Anthropol. Soc. Nippon. 99, 141–148 (1991).
Kakiya, R. et al. Body fat mass and lean mass as predictors of survival in hemodialysis patients. Kidney Int. 70, 549–556. https://doi.org/10.1038/sj.ki.5000331 (2006).
Macedo, C., Amaral, T. F., Rodrigues, J., Santin, F. & Avesani, C. M. Malnutrition and Sarcopenia Combined increases the risk for mortality in older adults on Hemodialysis. Front. Nutr. 8, 721941. https://doi.org/10.3389/fnut.2021.721941 (2021).
Beaudart, C., Zaaria, M., Pasleau, F., Reginster, J. Y. & Bruyere, O. Health outcomes of Sarcopenia: A systematic review and Meta-analysis. PLoS One. 12, e0169548. https://doi.org/10.1371/journal.pone.0169548 (2017).
Fu, C. et al. High prevalence of Sarcopenia and myosteatosis in patients undergoing hemodialysis. Front. Endocrinol. (Lausanne). 14, 1117438. https://doi.org/10.3389/fendo.2023.1117438 (2023).
Cesari, M. et al. Skeletal muscle and mortality results from the InCHIANTI Study. J. Gerontol. Biol. Sci. Med. Sci. 64, 377–384. https://doi.org/10.1093/gerona/gln031 (2009).
Gale, C. R., Martyn, C. N., Cooper, C. & Sayer, A. A. Grip strength, body composition, and mortality. Int. J. Epidemiol. 36, 228–235. https://doi.org/10.1093/ije/dyl224 (2007).
Legrand, D. et al. Muscle strength and physical performance as predictors of mortality, hospitalization, and disability in the oldest old. J. Am. Geriatr. Soc. 62, 1030–1038. https://doi.org/10.1111/jgs.12840 (2014).
Marcelli, D. et al. Body composition and survival in dialysis patients: Results from an international cohort study. Clin. J. Am. Soc. Nephrol. 10, 1192–1200. https://doi.org/10.2215/CJN.08550814 (2015).
Price, S. R., Mitch, W. E. & Garibotto, G. Muscle atrophy in CKD: A historical perspective of advancements in its understanding. J. Ren. Nutr. 33, S88–S92. https://doi.org/10.1053/j.jrn.2022.09.009 (2023).
Wang, X. H., Mitch, W. E. & Price, S. R. Pathophysiological mechanisms leading to muscle loss in chronic kidney disease. Nat. Rev. Nephrol. 18, 138–152. https://doi.org/10.1038/s41581-021-00498-0 (2022).
Wesson, D. E., Mathur, V. & Tangri, N. Metabolic basis and Pathogenesis of Skeletal Muscle Dysfunction as cause of Frailty in chronic kidney disease. Am. J. Nephrol. 53, 740–752. https://doi.org/10.1159/000526679 (2022).
Barreto, D. V. et al. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 77, 550–556. https://doi.org/10.1038/ki.2009.503 (2010).
Collins, R. A. & Grounds, M. D. The role of tumor necrosis factor-alpha (TNF-α) in skeletal muscle regeneration: Studies in TNF-α (-/-) and TNF-α (-/-)/LT-α (-/-) mice. J. Histochem. Cytochem.. 49, 989–1001 (2001).
Bauer, J. et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 14, 542–559. https://doi.org/10.1016/j.jamda.2013.05.021 (2013).
Enoki, Y. et al. Indoxyl sulfate potentiates skeletal muscle atrophy by inducing the oxidative stress-mediated expression of myostatin and atrogin-1. Sci. Rep. 6, 32084. https://doi.org/10.1038/srep32084 (2016).
Verzola, D., Barisione, C., Picciotto, D., Garibotto, G. & Koppe, L. Emerging role of myostatin and its inhibition in the setting of chronic kidney disease. Kidney Int. 95, 506–517. https://doi.org/10.1016/j.kint.2018.10.010 (2019).
Rousset, S., Patureau Mirand, P., Brandolini, M., Martin, J. F. & Boirie, Y. Daily protein intakes and eating patterns in young and elderly French. Br. J. Nutr. 90, 1107–1115. https://doi.org/10.1079/bjn20031004 (2003).
Schaap, L. A., Koster, A. & Visser, M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol. Rev. 35, 51–65. https://doi.org/10.1093/epirev/mxs006 (2013).
Ohkawa, S. et al. Association of age with muscle mass, fat mass and fat distribution in non-diabetic haemodialysis patients. Nephrol. Dial Transpl. 20, 945–951. https://doi.org/10.1093/ndt/gfh643 (2005).
Pender, D., McGowan, E., McVeigh, J. G. & McCullagh, R. The effects of Intradialytic Exercise on Key indices of Sarcopenia in patients with end-stage renal disease: A systematic review of Randomized controlled trials. Arch. Rehabil. Res. Clin. Transl. 5, 100252. https://doi.org/10.1016/j.arrct.2022.100252 (2023).
Eglseer, D. et al. Nutritional and exercise interventions in individuals with sarcopenic obesity around retirement age: A systematic review and meta-analysis. Nutr. Rev. 81, 1077–1090. https://doi.org/10.1093/nutrit/nuad007 (2023).
Srikanthan, P. & Karlamangla, A. S. Muscle mass index as a predictor of longevity in older adults. Am. J. Med. 127, 547–553. https://doi.org/10.1016/j.amjmed.2014.02.007 (2014).
Monereo-Munoz, M. et al. Prognostic value of muscle mass assessed by DEXA in elderly hospitalized patients. Clin. Nutr. ESPEN. 32, 118–124. https://doi.org/10.1016/j.clnesp.2019.04.001 (2019).
Mori, K. et al. Impact of diabetes on Sarcopenia and mortality in patients undergoing hemodialysis. BMC Nephrol. 20, 105. https://doi.org/10.1186/s12882-019-1271-8 (2019).
Bouchi, R. et al. Insulin treatment attenuates decline of muscle Mass in Japanese patients with type 2 diabetes. Calcif Tissue Int. 101, 1–8. https://doi.org/10.1007/s00223-017-0251-x (2017).
Kalantar-Zadeh, K. et al. Revisiting mortality predictability of serum albumin in the dialysis population: Time dependency, longitudinal changes and population-attributable fraction. Nephrol. Dial Transpl. 20, 1880–1888. https://doi.org/10.1093/ndt/gfh941 (2005).
de Mutsert, R. et al. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J. Ren. Nutr. 19, 127–135. https://doi.org/10.1053/j.jrn.2008.08.003 (2009).
Ballesteros-Pomar, M. D. et al. Bioelectrical impedance analysis as an alternative to dual-energy x-ray absorptiometry in the assessment of fat mass and appendicular lean mass in patients with obesity. Nutrition 93, 111442. https://doi.org/10.1016/j.nut.2021.111442 (2022).
Wang, J. et al. Lean body mass and survival in hemodialysis patients and the roles of race and ethnicity. J. Ren. Nutr. 26, 26–37. https://doi.org/10.1053/j.jrn.2015.07.003 (2016).
Vodicar, J., Pajek, J., Hadzic, V. & Bucar Pajek, M. Relation of lean body Mass and muscle performance to serum creatinine concentration in Hemodialysis patients. Biomed. Res. Int. 2018, 4816536. https://doi.org/10.1155/2018/4816536 (2018).
Sabatino, A. et al. Estimation of muscle mass in the integrated assessment of patients on hemodialysis. Front. Nutr. 8, 697523. https://doi.org/10.3389/fnut.2021.697523 (2021).
Acknowledgements
This study utilized data from the Korean Society of Nephrology (KSN). KSN data includes information on hemodialysis patients in Korea. The responsibility of interpretation for the results derived from this data entirely rests with the authors. We express our gratitude to all individuals who contributed to English editing and participation in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
J-E.K. and J.Y. contributed to the original draft of the manuscript, with J.Y. developing the methodology. J.H.K., K.K., and J.H.S. participated in the investigation. J-E.K. handled the review and editing. S.W.L. and S.D.H. provided conceptualization and supervision. The manuscript’s final version has received approval from all authors for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, JE., Yi, J., Kim, J.H. et al. The role of lean body mass in predicting mortality in hemodialysis patients across different age groups. Sci Rep 15, 2150 (2025). https://doi.org/10.1038/s41598-025-85994-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-85994-0