Abstract
Nocturia is the most prevalent lower urinary tract symptom (LUTS) and is closely associated with various diseases and social burdens. Research on the relationship between physical activity and various diseases has progressed significantly. However, the association between nocturia and physical activity has received limited attention in prior studies. Thus, this study aimed to investigate the relationship between different domains of physical activity (e.g., occupation-related physical activity [OPA], transportation-related physical activity [TPA], and leisure-time physical activity [LTPA]) and nocturia. We included 5516 participants from the cross-sectional National Health and Nutrition Examination Survey (NHANES) conducted between 2007 and 2012, employing survey-weighted logistic regression, restricted cubic splines (RCS), subgroup analysis, and sensitivity analysis to assess the relationship between different domains of physical activity and nocturia. Multivariable logistic regression analysis revealed a significant negative correlation between PA, LTPA, and nocturia. Specifically, PA (OR 0.7523, 95% CI 0.6307–0.8974, P = 0.002) and LTPA (OR 0.7664, 95% CI 0.6314–0.9304, P = 0.007) were negatively associated with nocturia. The RCS curve demonstrated non-linear relationships between PA, LTPA, and nocturia. Additionally, subgroup analyses and sensitivity analyses further validated this association. Based on this cross-sectional study, we suggested that PA and LTPA are associated with a reduced risk of nocturia in adults aged 20 years and older in the United States. This underscores the importance of physical activity in preventing and managing nocturia may provide valuable guidance for clinical practice.
Similar content being viewed by others
Introduction
Nocturia is the most common and bothersome lower urinary tract symptom (LUTS), accounting for 33% of all LUTS-related complaints1. This symptom may be associated with multiple factors, including excessive urine production, increased nocturnal urine output, bladder storage issues, and reduced bladder capacity2. The International Continence Society defined nocturia as "the need to routinely void one or more times during the night, with each void preceded and followed by sleep, with two or more voids per night considered an average threshold for bothersomeness3." Typically, two or more voids per night are associated with decreased quality of life and increased mortality4,5,6. Previously, nocturia was not defined as an independent symptom; instead, it was considered a manifestation of overactive bladder (OAB) and benign prostatic hyperplasia (BPH)7,8. With continued research, nocturia has been identified as an independent symptom strongly associated with various chronic diseases and contributes to significant societal burdens, garnering widespread research interest8,9,10. Nocturia is not only highly prevalent—for example, among individuals aged 0–40 years, nocturia prevalence ranges from 11.0% to 35.2% in men and 20.4% to 43.9% in women, and in individuals aged 40–65 years, prevalence can reach 50%—but is also often challenging to treat in men11,12,13. Furthermore, studies have shown that nocturia is associated with several adverse health outcomes, such as depression, sleep disturbances, diabetes, and elevated mortality14,15,16.
Physical activity (PA) is a complex behavior that generates energy expenditure and is typically classified by light, moderate, or vigorous intensity. The Global Physical Activity Questionnaire (GPAQ), developed by the World Health Organization (WHO) as a tool for monitoring PA, has not only been validated for assessing risk factors for non-communicable diseases and chronic diseases but has also been widely used in various countries17. It assesses physical activity at work, during leisure time, and for transportation purposes and distinguishes between moderate and vigorous physical activity. Additionally, it includes questions on sedentary behaviour in daily life.
Previous studies have suggested that PA can reduce the risk of various diseases, such as diabetes, depression, and colorectal cancer, through multiple mechanisms18,19,20. Specifically, several studies have indicated that physical activity and moderate exercise can significantly reduce nocturnal urine output21,22,23. This effect may occur through mechanisms such as reducing sympathetic nervous system activity and lowering systemic inflammation levels1,24. However, a study has also shown that increased physical activity significantly increases nighttime and 24-h urine output, conflicting with the previously mentioned findings25. In summary, whether all domains of PA (e.g., occupation-related physical activity [OPA], transportation-related physical activity [TPA], and leisure-time physical activity [LTPA]) exert equally beneficial effects on nocturia remains unclear and controversial. Few studies have investigated the relationship between PA and nocturia, with most previous research focusing on total PA and little attention given to the correlation between different domains of PA and nocturia.
Given the above discussion, it is necessary to study the relationship between different domains of PA and nocturia through a large population study. Thus, in this paper, we explored the relationship between different domains of physical activity (including OPA, TPA, LTPA, and total PA) and nocturia risk in adults of all ages by analyzing data from the National Health and Nutrition Examination Survey (NHANES), suggested that physical activity is associated with a reduced risk of nocturia, thereby filling the existing knowledge gap.
Participants and methods
Data source
The study population was derived from the NHANES, a nationally representative cross-sectional survey of the civilian, noninstitutionalized population residing in the United States. This survey collected comprehensive data on the health and nutritional status of U.S. citizens to assess the population’s overall well-being. The data were classified into five categories: "Demographics Data," "Dietary Data," "Examination Data," "Laboratory Data," and "Questionnaire Data." Relevant data for this study were extracted from these categories. Specifically, we obtained data on nocturia and physical activity from the "Kidney Conditions—Urology" and “Physical Activity” files within the “Questionnaire Data” category. These data are available on the NHANES website (https://wwwn.cdc.gov/nchs/nhanes/default.aspx).
Study population
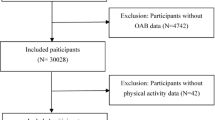
We selected cross-sectional data from three NHANES cycles: 2007–2008, 2009–2010, and 2011–2012. A total of 30,442 participants were included in the study, from which those meeting the following exclusion criteria were removed: (1) under the age of 20; (2) participants with missing nocturia data (3) participants with missing physical activity data; (4) Participants with missing covariate data. The missing variables included education status, PIR status, marital status, cotinine, drinking status, hypertension history, diabetes history, and hyperlipidemia history; (5) those diagnosed with prostate cancer; (6) those who had undergone prostate surgery; (7) those with prostate infection or inflammation; (8) those who had undergone a digital rectal exam within the past seven days; (9) those who had undergone a prostate biopsy within the past four weeks; (10) those who had undergone cystoscopy within the past four weeks; (11) those who had used diuretics within the past month; (12) pregnant or breastfeeding women. Following this rigorous screening process, the final sample consisted of 5516 participants. The participant selection process was illustrated in Fig. 1.
Exposure and outcome
Nocturia screening was conducted using a questionnaire that asked, "During the past 30 days, how many times per night did you most typically get up to urinate from the time you went to bed until waking up in the morning?" Responses were categorized into six groups: 0 times/night, 1 time/night, 2 times/night, 3 times/night, 4 times/night, and 5 or more times/night. Participants reporting urinating ≥ 2 times per night were defined as having nocturia.
The Physical Activity Questionnaire (PAQ), based on the GPAQ, assesses the physical activity levels of all participants (Figure S1). This questionnaire categorized physical activity into OPA, (work-related PA, including paid or unpaid work, household chores, and yard work) TPA (transport-related physical activity, including walking or riding a bicycle), and LTPA (any physical activity for recreation like sports, fitness, and leisure activities)26. and recorded the frequency (number of times per week) and duration (time per session) of these activities. OPA and LTPA were further subdivided into vigorous and moderate activities based on intensity. The formula for calculating the weekly duration of OPA and LTPA (in minutes) is as follows: Duration of OPA and LTPA per week (min) = (number of vigorous physical activity sessions per week × duration of each vigorous session × 2) + (number of moderate-intensity sessions per week × duration of each moderate-intensity session). Duration of TPA per week (min) = number of moderate-intensity sessions per week × duration of each moderate-intensity session27. Total physical activity is the sum of OPA, TPA, and LTPA.
Participants were classified as having diabetes if they met any of the following criteria: (1) previously diagnosed with diabetes, (2) taking prescription diabetes medications, (3) fasting blood glucose level ≥ 12.6 mmol/L, or (4) hemoglobin A1c level ≥ 6.5%28.
Hypertension was diagnosed in participants who met any of the following criteria: (1) previously diagnosed with hypertension, (2) taking prescription hypertension medications, (3) systolic blood pressure ≥ 130 mmHg, or diastolic blood pressure ≥ 80 mmHg during physical examination29.
According to the Adult Treatment Panel III (ATP III) of the National Cholesterol Education Program (NCEP), hyperlipidemia was defined by any of the following criteria: total cholesterol level ≥ 200 mg/dL, triglyceride level ≥ 150 mg/dL, high-density lipoprotein (HDL) level ≤ 40 mg/dL in men or ≤ 50 mg/dL in women, or low-density lipoprotein (LDL) level ≥ 130 mg/dL30.
Covariates
This study included three types of covariates: sociodemographic factors (age, gender, race/ethnicity, education level, PIR, marital status), lifestyle behaviors (serum cotinine concentration, alcohol consumption), and chronic diseases (hypertension, diabetes, hyperlipidemia). Participants were divided into two age groups (20–50 years, ≥ 50 years), and race was categorized into five groups (non-Hispanic White, non-Hispanic Black, Mexican American, other Hispanic, and other races). Educational attainment was classified as less than high school, high school graduate, and more than high school. PIR (< 1, 1–4, > 4) and serum cotinine concentration (< 0.015 ng/mL, 0.015–3 ng/mL, > 3 ng/mL) were divided into three categories. Gender (male, female), marital status (married/living with partner, widowed/divorced/separated/never married), diabetes history (yes, no), hypertension history (yes, no), alcohol consumption history (yes, no), and hyperlipidemia history (yes, no) were also categorized into two groups.
Statistical analysis
Given the complex stratified and cluster sampling design of NHANES, we performed weighted analyses in this study. Since data from three cycles were included, the combined weight was expressed as WEIGHT07-12 = (1/3) × WTMEC2YR07-08 + (1/3) × WTMEC2YR09-10 + (1/3) × WTMEC2YR11-12. Initially, we utilized t-tests or ANOVA to compare differences between groups and assess the characteristics of nocturia and non-nocturia participants, with continuous variables expressed as mean ± standard deviation and categorical variables presented as counts and percentages. Subsequently, we employed survey-weighted logistic regression models to evaluate the relationship between different domains of physical activity and nocturia risk. Model 1 was unadjusted; Model 2 adjusted for age, gender, and race; and Model 3 further adjusted for marital status, education level, serum cotinine concentration, alcohol consumption, PIR, diabetes, hypertension, and hyperlipidemia, based on Model 2. Additionally, weighted restricted cubic splines (RCS) were applied to analyze the non-linear relationship between various domains of physical activity and nocturia, adjusting for all covariates. Finally, interaction and stratified analyses were conducted considering the listed covariates, and propensity score matching (PSM) was performed to verify the stability of the relationship between physical activity domains and nocturia risk. All statistical analyses were conducted using R software (version 4.2.2) and STATA v16.0, with a significance threshold set at P < 0.05.
Results
Prevalence analysis
We first analyzed the prevalence of nocturia from 2007 to 2012 and created a bar-line chart to present the results intuitively (Fig. 2). The findings indicated a high prevalence of nocturia in the overall population, with a notable increase in prevalence as age increased.
Baseline characteristics
The research included 5516 participants from the NHANES cycles between 2007 and 2012 (Table 1). In this study, 48.15% of the participants were male, 51.85% were female, and the mean age was 46.26 years (± 0.6 years). Participants were further divided into two groups based on nocturia status: the non-nocturia group comprised 3923 participants (71.12%), while 1593 participants (28.88%) reported nocturia. Significant differences were observed between the two groups in most characteristics, particularly in PA, LTPA, and OPA, which were significantly lower in the nocturia group. However, no significant difference was found in TPA or cotinine levels.
Linear association between physical activity and nocturia
We utilized a survey-weighted logistic regression model to assess the associations between different domains of physical activity and the risk of nocturia. As shown in Table 2, total PA (OR: 0.5176, 95% CI: 0.4418–0.6064, P < 0.0001), OPA (OR: 0.8056, 95% CI: 0.6832–0.9500, P = 0.01), and LTPA (OR: 0.5227, 95% CI: 0.4382–0.6235, P < 0.0001) were negatively associated with nocturia risk. After adjusting for all confounders, the association between OPA and nocturia was no longer significant, while total PA (OR: 0.7523, 95% CI: 0.6307–0.8974, P = 0.002) and LTPA (OR: 0.7664, 95% CI: 0.6314–0.9304, P = 0.007) remained negatively associated with nocturia risk. We further examined the dose–response relationship between PA and nocturia by categorizing PA into four groups (0 min/week, 1–149 min/week, 150–299 min/week, and ≥ 300 min/week) (Fig. 3). Even in fully adjusted models, total PA in the 150–299 min/week (OR: 0.7121, 95% CI: 0.5296–0.9575, P = 0.025) and ≥ 300 min/week (OR: 0.7279, 95% CI: 0.5937–0.8925, P = 0.002) groups showed a significant negative association with nocturia risk. LTPA ≥ 300 min/week (OR: 0.7368, 95% CI: 0.5796–0.9365, P = 0.013) also demonstrated a significant negative correlation.
Non-Linear relationship between physical activity and nocturia
In Model 3, we employed the RCS curve to describe the non-linear relationships between various physical activity domains and nocturia, adjusting for all covariates (Fig. 4). In the RCS model, total PA (P for non-linear < 0.0001) and LTPA (P for non-linear = 0.0486) exhibited significant non-linear relationships with nocturia. Total PA initially decreased to a nadir at 7.143 h/week, then slightly increased and peaked at 47.619 h/week before gradually decreasing again. LTPA decreased to a low point at 5.364 h/week, followed by a slight rise and stabilization. No significant non-linear relationship was identified between TPA, OPA, and nocturia.
Propensity score matching and subgroup analyses
To further assess the robustness of the association between PA and nocturia, we performed sensitivity analyses using PSM. The characteristics of the study population regarding PSM analysis were displayed in Table S1. After applying the same survey-weighted logistic regression model, the results still indicated a negative association between total PA (OR: 0.8393, 95% CI: 0.6901–1.020, P = 0.039) and nocturia (Table 3). Furthermore, we conducted subgroup analyses stratified by all confounders, revealing that PA was significantly associated with nocturia across most strata, including age ≥ 50, female, Non-Hispanic White, education level (more than high school), marital status (married/living with partner), PIR between 1 and 4, cotinine concentration between 0.015 ng/mL and 3 ng/mL, alcohol consumption, hypertension, diabetes, and hyperlipidemia (Fig. 5). However, no significant interaction effects were observed. In the subgroup analysis of the association between total PA and nocturia frequency, we identified a significant interaction effect in the hyperlipidemia (P = 0.006) (Figure S2).
Discussion
Although some studies have explored the association between physical activity and LUTS, nocturia—recognized as the most prevalent and bothersome LUTS in men—has received limited attention1. In this nationally representative cross-sectional study, we refined the classification of PA domains and found that total PA and LTPA were associated with a reduced risk of nocturia. These findings align with PA guidelines, which recommend that adults engage in 150–300 min of moderate-intensity PA or 75–150 min of vigorous-intensity PA per week, or a combination of both. However, no significant associations were observed for OPA or TPA.
There is increasing recognition that nocturia is a common chronic condition closely associated to a significant decline in quality of life31,32. Previous studies have identified several factors associated with nocturia, including reduced bladder capacity, bladder storage issues, and excessive urine production. As research on nocturia advances, some studies have focused on the impact of PA and demonstrated that appropriate PA serves as a protective factor in preventing nocturia13,21,22. However, few studies have investigated whether all PA domains (such as OPA, TPA, and LTPA) affect nocturia equally. For this reason, we conducted a study using the NHANES database to explore the relationship between PA, its various domains, and nocturia. Our findings were consistent with previous reports, suggesting that PA may be an effective strategy for preventing nocturia. A study from the PLCO screening trial cohort indicated a significant negative correlation between PA and severe nocturia in men, with this correlation being more pronounced in individuals with nocturia15. Additionally, Sugaya et al. conducted an 8-week walking exercise program involving 30 older men, significantly reducing both nighttime and daytime urination compared to pre-exercise levels23. Furthermore, two studies investigated the potential of PA interventions to reduce nocturia in older women found similar results. Yuko et al. recruited 35 older women for a 52-week exercise program, observing significant improvements in overactive bladder symptoms, particularly in frequency and nocturia15. Surveys from community-dwelling older women also indicated that lower levels of PA were associated with more frequent nocturia episodes and more severe urinary incontinence symptoms21. Meanwhile, our study identified a correlation between the duration of PA and LTPA with nocturia, and the RCS analysis provided an optimal weekly PA duration. Based on our results and previous studies, we proposed that PA may influence nocturia and guide clinical treatment.
We reviewed the existing literature to further investigate the mechanisms by which PA may prevent nocturia. One study hypothesized that sustained physical fitness reduces systemic sympathetic nervous system activity at rest and decreases prostatic smooth muscle tension, alleviating lower urinary tract symptoms33. Interestingly, studies have found that patients with nocturia significantly reduce nighttime urination frequency and daytime serum catecholamine levels after exercise23. The findings further supported previous hypotheses. Additionally, other studies have indicated that elderly individuals with nocturia have significantly lower serum melatonin levels compared to those without nocturia34. Melatonin, one of the most potent natural antioxidants, has been reported to treat nocturia effectively when administered before sleep35. It has also been found that adjusting PA can enhance melatonin secretion36. Based on these findings, we suggested that PA might regulate nocturia by promoting melatonin secretion. Furthermore, PA and exercise were well-established for their roles in weight loss, reducing body fat, preventing type 2 diabetes, and maintaining reduced body weight after weight loss37. Previous studies have shown that obesity influences the development of nocturia through various mechanisms, such as obesity-induced atherosclerosis in the pelvic arteries, which may result in ischemia-driven collagen deposition and bladder fibrosis38,39. Obesity can also increase intra-abdominal pressure and promote prostate enlargement40. Moreover, obesity-related insulin resistance and hyperinsulinemia may activate hypothalamic centers that regulate sympathetic activity, leading to elevated catecholamine levels and promoting prostate enlargement41,42. Therefore, we suggested that PA might alleviate nocturia by reducing obesity and regulating it through the abovementioned mechanisms. Based on these findings, the influence of PA on the risk of nocturia is plausible and may serve as a potential indicator for assessing nocturia risk. PA interventions may also help prevent or slow the progression of nocturia. However, in attempting to explore the mechanisms through which different domains of physical activity (OPA, TPA, LTPA) affect nocturia, we found minimal relevant literature, and previous studies have not provided confirmed or precise mechanisms, which has hindered further analysis.
Our study had several strengths. First, since it was unclear whether all PA domains were equally beneficial for nocturia, we evaluated the associations of total PA, OPA, TPA, and LTPA with nocturia, addressing a gap in the research. Second, NHANES was a nationally representative cross-sectional survey targeting the civilian, noninstitutionalized population residing in the United States. Our results may have had practical implications for guiding nocturia patients with various occupational backgrounds to engage in appropriate PA. Lastly, this was the first attempt to explore the relationship between nocturia and PA using the NHANES database.
However, our study had several limitations. First, because NHANES data were cross-sectional, our study only explored associations and could not infer causality. Second, the exact mechanisms by which PA affected nocturia remained unclear. More prospective and experimental studies were needed to evaluate the potential effects of PA on nocturia, verify our conclusion, and further investigate the underlying mechanisms. Additionally, the domains of physical activity and nocturia were assessed through self-reported questionnaires rather than objective measurements. PA data were limited to one week, making it challenging to capture PA’s long-term stability or trends. Moreover, due to the limited research on this topic, we cannot further investigate the specific mechanisms through which different domains of PA affect nocturia and explain why nocturia is associated with total PA and LTPA but not with OPA and TPA. Finally, despite incorporating various potential confounding factors and applying exclusion criteria, there may still been unaddressed confounders that could have impacted the precision of our findings.
Conclusion
This study established a direct association between physical activity and the risk of nocturia, suggesting that total PA and LTPA—but not OPA or TPA—were linked to a reduced risk of nocturia. These findings may guide preventive strategies for nocturia; however, the underlying mechanisms require further exploration. Future studies should expand the study population and further differentiate physical activity by intensity (vigorous, moderate, or combined) and domain (LTPA, TPA, and OPA) to provide clinical insights for nocturia patients across different age groups and severity levels. Additionally, more longitudinal studies and objective measurement data are essential to confirm our conclusion.
Data availability
All original data can be accessed publicly through the NHANES database at the following link: https://www.cdc.gov/nchs/nhanes/index.htm. Additionally, the data and code used in this study are freely and publicly available in the Figshare repository (https://doi.org/https://doi.org/10.6084/m9.figshare.28082405.v4).
References
Wolin, K. Y. et al. Physical activity and benign prostatic hyperplasia-related outcomes and nocturia. Med. Sci. Sports Exerc. 47, 581–592 (2015).
Jin, M. H. & du Moon, G. Practical management of nocturia in urology. Indian J. Urol. 24, 289–294 (2008).
van Kerrebroeck, P. et al. The standardisation of terminology in nocturia: Report from the Standardisation Sub-committee of the International Continence Society. Neurourol. Urodyn. 21, 179–183 (2002).
Makarov, D. V. Editorial comment on: Nocturia frequency, bother, and quality of life: How often is too often? A population-based study in Finland. Eur. Urol. 57, 497–498 (2010).
Galizia, G. et al. Association between nocturia and falls-related long-term mortality risk in the elderly. J. Am. Med. Dir. Assoc. 13, 640–644 (2012).
Chen, M. et al. Association of nocturia with cardiovascular and all-cause mortality: A prospective cohort study with up to 31 years of follow-up. Front. Public Health 11, 1292362 (2023).
van Kerrebroeck, P. & Weiss, J. Standardization and terminology of nocturia. BJU Int. 84(Suppl 1), 1–4 (1999).
Miller, P. S., Hill, H. & Andersson, F. L. Nocturia work productivity and activity impairment compared with other common chronic diseases. Pharmacoeconomics 34, 1277–1297 (2016).
Coyne, K. S. et al. The prevalence of nocturia and its effect on health-related quality of life and sleep in a community sample in the USA. BJU Int. 92, 948–954 (2003).
Holm-Larsen, T. The economic impact of nocturia. Neurourol. Urodyn. 33(Suppl 1), S10–S14 (2014).
Bosch, J. L. & Weiss, J. P. The prevalence and causes of nocturia. J. Urol. 189, S86-92 (2013).
Irwin, D. E. et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur. Urol. 50, 1306–1314 (2006).
Rohrmann, S. et al. Association of cigarette smoking, alcohol consumption and physical activity with lower urinary tract symptoms in older American men: Findings from the third National Health and Nutrition Examination Survey. BJU Int. 96, 77–82 (2005).
Kupelian, V. et al. Nocturia and quality of life: Results from the Boston area community health survey. Eur. Urol. 61, 78–84 (2012).
Johnson, T. M., Vaughan, C. P., Goode, P. S., Bliwise, D. L., Markland, A. D. & Huisingh, C., et al. Pilot results from a randomized trial in men comparing alpha-adrenergic antagonist versus behavior and exercise for nocturia and sleep. Clin Ther (2016).
Fan, Y., Wei, F., Lang, Y. & Qi, W. Meta-analysis of nocturia and risk of all-cause mortality in adult population. Int. J. Cardiol. 195, 120–122 (2015).
Mumu, S. J., Ali, L., Barnett, A. & Merom, D. Validity of the global physical activity questionnaire (GPAQ) in Bangladesh. BMC Public Health 17, 650 (2017).
Divney, A. A. et al. Diabetes prevalence by leisure-, transportation-, and occupation-based physical activity among racially/ethnically diverse US adults. Diabetes Care 42, 1241–1247 (2019).
He, F., Li, Y., Hu, Z. & Zhang, H. Association of domain-specific physical activity with depressive symptoms: A population-based study. Eur. Psychiatry 66, e5 (2022).
An, S. & Park, S. Association of physical activity and sedentary behavior with the risk of colorectal cancer. J. Korean Med. Sci. 37, e158 (2022).
Chu, C. M. et al. Physical activity patterns and sedentary behavior in older women with urinary incontinence: An accelerometer-based study. Female Pelvic Med. Reconstr. Surg. 25, 318–322 (2019).
Ko, I. G., Lim, M. H., Choi, P. B., Kim, K. H. & Jee, Y. S. Effect of long-term exercise on voiding functions in obese elderly women. Int. Neurourol. J. 17, 130–138 (2013).
Sugaya, K. et al. Effects of walking exercise on nocturia in the elderly. Biomed. Res. 28, 101–105 (2007).
Abramson, J. L. & Vaccarino, V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch. Intern. Med. 162, 1286–1292 (2002).
Deger, M. et al. The impact of movement, physical activity and position on urine production: A pilot study. Int. J. Clin. Pract. 75, e14743 (2021).
Pu, R., Fu, M., Yang, G. & Jiang, Z. The association of work physical activity and recreational physical activity with periodontitis in the NHANES (2009–2014). J. Periodontol. 94, 1220–1230 (2023).
Kim, D., Vazquez-Montesino, L. M., Li, A. A., Cholankeril, G. & Ahmed, A. Inadequate physical activity and sedentary behavior are independent predictors of nonalcoholic fatty liver disease. Hepatology 72, 1556–1568 (2020).
ElSayed, N. A. et al. 2. Classification and diagnosis of diabetes: Standards of care in diabetes-2023. Diabetes Care 46, S19–S40 (2023).
Whelton, P. K. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 138, e426–e483 (2018).
National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation, 106, 3143–421 (2002).
Vaughan, C. P. & Bliwise, D. L. Sleep and nocturia in older adults. Sleep Med. Clin. 13, 107–116 (2018).
Agarwal, A. et al. What is the most bothersome lower urinary tract symptom? Individual- and population-level perspectives for both men and women. Eur. Urol. 65, 1211–1217 (2014).
Hart, L. E. Physical activity and benign prostatic hyperplasia. Clin. J. Sports Med. 9, 106 (1999).
Sugaya, K. et al. Biochemical and body composition analysis of nocturia in the elderly. Neurourol. Urodyn. 27, 205–211 (2008).
Drake, M. J., Mills, I. W. & Noble, J. G. Melatonin pharmacotherapy for nocturia in men with benign prostatic enlargement. J. Urol. 171, 1199–1202 (2004).
Kruk, J., Aboul-Enein, B. H. & Duchnik, E. Exercise-induced oxidative stress and melatonin supplementation: Current evidence. J. Physiol. Sci. 71, 27 (2021).
Oppert, J. M., Bellicha, A. & Ciangura, C. Physical activity in management of persons with obesity. Eur. J. Intern. Med. 93, 8–12 (2021).
Laven, B. A. et al. Birth weight, abdominal obesity and the risk of lower urinary tract symptoms in a population based study of Swedish men. J. Urol. 179, 1891–1895 (2008).
Tarcan, T., Azadzoi, K. M., Siroky, M. B., Goldstein, I. & Krane, R. J. Age-related erectile and voiding dysfunction: The role of arterial insufficiency. Br. J. Urol. 82(Suppl 1), 26–33 (1998).
Lee, S. H. et al. Effects of obesity on lower urinary tract symptoms in Korean BPH patients. Asian J. Androl. 11, 663–668 (2009).
Hammarsten, J. & Hogstedt, B. Hyperinsulinaemia as a risk factor for developing benign prostatic hyperplasia. Eur. Urol. 39, 151–158 (2001).
Alvarez, G. E., Beske, S. D., Ballard, T. P. & Davy, K. P. Sympathetic neural activation in visceral obesity. Circulation 106, 2533–2536 (2002).
Funding
The financial support received for this article was provided by the Medicine and Health Project of Zhejiang Province (2025KY1284), Zhejiang Medical Association Clinical Medical Research Special Fund Project (2024ZYC-Z82), the Natural Science Foundation of Ningbo Municipality (2021J281), the Key Cultivating Discipline of LihHuiLi Hospital (2022-P09) and Ningbo Key Clinical Speciality Construction Project (2023-BZZ).
Author information
Authors and Affiliations
Contributions
JY: Data analysis; writing – original draft, writing – review and editing. SR: supervision or mentorship. DX and YF: Data analysis; validation. LM: Research idea; funding acquisition. ZL: Study design; writing – review and editing. All authors made contributions to the manuscript and have given their approval for the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All data in this study were derived from the National Health and Nutrition Examination Survey, the survey was approved by the Research Ethics Review Board of the National Center for Health Statistics (NCHS), and all participants provided written informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jia, Y., Shen, R., Dong, X. et al. Association of domain-specific physical activity with nocturia: a population-based study. Sci Rep 15, 2768 (2025). https://doi.org/10.1038/s41598-025-86182-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-86182-w
Keywords
This article is cited by
-
Association between physical activity intensity and nocturia risk: the mediating role of mental health
Journal of Health, Population and Nutrition (2026)