Abstract
Acute internal carotid artery occlusion (AICAO) can result in malignant cerebral edema and unfavorable patient outcomes. This study evaluated the utility of transcranial Doppler (TCD) in assessing contralateral flow compensation and predicting outcomes in patients with AICAO. We enrolled 51 patients within 6 h of symptom onset and conducted TCD examinations to evaluate collateral circulation. Among the 51 patients, 40 (78.4%) had collateral flow. TCD showed excellent agreement with magnetic resonance angiography (MRA)/CT angiography (CTA) in assessing anterior communicating artery (ACoA) status (kappa = 0.873, p < 0.001). Our findings indicated that the absence of collaterals (OR = 7.649, p = 0.032), younger age (OR = 0.907, p = 0.048), and lower Alberta Stroke Program Early CT Score 24 h after onset (ASPECTs1) (OR = 0.276, p = 0.025) were independent predictors of malignant cerebral edema. Additionally, advanced age, elevated National Institutes of Health Stroke Scale Score (NIHSSs) in the Emergency Department, sole extracranial-to-intracranial collateral circulation (EICC), and absence ACoA were independently associated with worse outcomes (all p < 0.05). In conclusion, TCD evaluation of collateral circulation in AICAO patients can effectively predict the risk of malignant cerebral edema, with ACoA presence correlating with favorable outcomes and sole EICC linked to poorer prognosis. While age, NIHSSs and ASPECTs also contribute, TCD’s assessment of collaterals provides key insights for patient management.
Similar content being viewed by others
Introduction
Acute internal carotid artery occlusion (AICAO) accounts for approximately 15–20% of all ischemic strokes and frequently progresses to malignant cerebral edema in 10–30% of cases1. Without adequate collateral circulation, malignant edema develops within 2–5 days after stroke onset, carrying mortality rates of up to 80% if left untreated2. In China, with only 1.45% of acute cerebral infarction patients receiving endovascular treatment3, collateral circulation becomes the critical determinant of outcomes4. Well-developed collaterals can maintain cerebral perfusion pressure and reduce malignant edema risk, while poor collateral status predicts early neurological deterioration5. Therefore, rapid assessment of collateral circulation is essential for risk stratification and timely intervention decisions.
While CTA and digital subtraction angiography (DSA) directly visualize collateral pathways6,7,8, they require patient transport, involve radiation, and may not be readily available. Patients without thrombectomy often cannot access emergency vascular imaging. TCD offers a non-invasive, bedside alternative for evaluating intracranial blood flow dynamics. Previous studies have demonstrated TCD’s utility in assessing collateral flow in acute stroke9,10,11, but its correlation with malignant cerebral edema risk and neurological outcomes in AICAO remains unclear.
This study aimed to evaluate TCD’s feasibility in assessing collateral flow patterns within 6 h of AICAO symptom onset. This approach could enhance outcomes by facilitating timely decompression before irreversible damage occurs. Additionally, we investigated early TCD assessment of collateral circulation for predicting 90-day neurological functional prognosis.
Methods
Study population
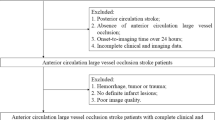
We enrolled 51 acute ischemic stroke (AIS) patients admitted to the neurointensive care unit (NCU) of the People’s Hospital of Inner Mongolia Autonomous Region between October 2018 and March 2024. The Inner Mongolia Hospital Ethics Committee approved the study (approval number 202204212L). All patients’ family members provided informed consent. Treatment followed standardized protocols based on the 2018 and 2019 AHA/ASA guidelines for early management of AIS12.
Initial evaluation in the emergency department included head CT and NIHSS assessment (NIHSSe). TCD examination for collateral circulation assessment was performed upon NCU admission following the 2015 Chinese Guidelines for Vascular Ultrasound for Stroke13,14. Follow-up CT and clinical assessment were performed at 24 h (ASPECTs1 and NIHSS1). ICA occlusion was confirmed by MRA or CTA within 72 h of admission.
Inclusion criteria were:
-
(1)
admission within 6 h of symptom onset with AIS diagnosis on emergency CT;
-
(2)
Follow-up CT at 24 h showing cerebral infarction encompassing ≥ 2/3 of the ICA territory with lesion volumes > 100 ml;
-
(3)
ICA occlusion confirmed by MRA or CTA within 72 h of admission;
-
(4)
No history of previous stroke with residual deficits;
-
(5)
Some patients received intravenous rt-PA (0.90 mg/kg), but imaging indicated ineffectiveness.
Exclusion criteria were:
-
(1)
cerebral infarctions with other causes or unknown etiology;
-
(2)
poor temporal window penetration preventing TCD assessment;
-
(3)
hemorrhagic transformation on neuroimaging, defined according to ECASS classification as:
-
HI1: small petechiae along the margins of the infarct
-
HI2: confluent petechiae within the infarcted area
-
PH1: hematoma in ≤ 30% of the infarcted area with mild space-occupying effect
-
PH2: hematoma in > 30% of the infarcted area with significant space-occupying effect
-
Clinical assessment
Malignant large hemispheric infarction was defined as:
Imaging criteria:
-
Brain edema with midline shift > 5 mm on CT
-
Space-occupying cerebral edema causing ≥ 50% compression of lateral ventricle
-
Effacement of basal cisterns
Neurological deterioration was defined as:
-
NIHSSs increase > 2 points from baseline
-
Decrease in level of consciousness (decrease in NIHSS 1a score ≥ 1)
-
New or worsening weakness
-
Clinical signs of elevated intracranial pressure
Three-month functional outcome was assessed using modified Rankin Scale (mRS):
-
Good outcome: mRS 0–2
-
Poor outcome: mRS 3–6
Collateral circulation assessment
TCD methodology
The TCD examinations were performed by two certified neurosonologists using a Doppler-box DWL system following standardized protocols.
Technical Specifications:
-
Equipment: Doppler-box DWL ultrasound system
-
2 MHz probe for transcranial insonation
-
4 MHz probe for extracranial vessel examination
-
Sample volume: 10–15 mm
-
Filter setting: 100 Hz
Standardized Examination Protocol:
-
Patient positioned supine with head elevated 30°
-
Systematic bilateral examination through temporal windows:
-
MCA (M1 segment): depth 45–65 mm
-
ACA (A1 segment): depth 60–75 mm
-
PCA (P1/P2 segments): depth 55–75 mm
-
Ophthalmic artery examination through orbital windows: Ophthalmic artery: depth 40–50 mm
Assessment of collateral pathways:
-
ACoA collaterals were assessed by: (1) decreased mean flow velocity (MFV) and pulsatility in ipsilateral middle cerebral artery (MCA) with normal contralateral flow; (2) no significant MFV change in ipsilateral MCA and A1 ACA with ipsilateral common carotid artery (CCA) compression, but decreased MFV with contralateral CCA compression; (3) ipsilateral ACA flow direction consistent with MCA, and increased contralateral A1 ACA flow.
-
PCoA collaterals were evaluated by asymmetry in posterior cerebral artery velocities, with higher velocity ipsilateral to ICA occlusion.
-
EICC was assessed by detecting supratrochlear artery flow with a 4 Hz probe, significantly reduced by ipsilateral superficial temporal and mandibular artery compression. The ophthalmic artery flowing away from the probe can be detected through the orbital window simultaneously.
Statistical analysis
Continuous variables were tested for normality using the Kolmogorov–Smirnov test. Normally distributed data are presented as mean ± standard deviation, while categorical variables are expressed as percentages or absolute numbers. Between-group comparisons were performed using Fisher’s exact test for categorical variables, and Student’s t-test or Mann–Whitney U test for continuous variables based on their distribution. For identifying independent predictors of malignant cerebral edema, multivariate logistic regression analysis was performed. Bootstrap analysis was applied to evaluate functional outcomes using mRS. Agreement between TCD and CTA/MRA findings was assessed using kappa statistics. Statistical significance was defined as P < 0.05. All analyses were conducted using SPSS version 22.0. (IBM Corp, Armonk, NY, USA).
Results
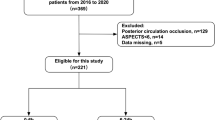
Analysis of All Patients (n = 82), among the 51 patients, 40 (78.4%) had collateral circulation (Fig. 1).
Factors associated with malignant cerebral edema
Patients who developed malignant cerebral edema were significantly younger, had lower ASPECTs1, and lacked collateral circulation and ACoA flow (all P < 0.05). Other factors did not differ significantly between groups (all P > 0.05) (Table 1).
Multivariate analysis showed independent predictors of malignant cerebral edema: lack of collateral circulation (OR = 7.649, p = 0.032), ASPECTs1 score (OR = 0.276, p = 0.025), age (OR = 0.907, p = 0.048).
(Table 2).
Factors associated with functional outcome
Eleven patients who lacked collateral circulation developed malignant cerebral edema. As the study’s focus was on examining how different patterns of collateral circulation affect outcomes in AICAO patients, these eleven patients were consequently excluded from the mRS analysis.
Poor outcome (mRS 3–6) group characteristics: advanced age (77.05 vs 67.38 years, p = 0.002), higher NIHSSe score (17.79 vs 13.81, p < 0.001), absence of anterior communicating artery (26.3% vs 66.7%, p = 0.014), presence of isolated EICC (42.1% vs 0%, p < 0.001) (Table 3).
Multivariate analysis showed independent predictors of poor outcome: advanced age, high NIHSSe score, presence of EICC, absence of anterior communicating artery (all p < 0.05) (Table 4).
Comparison of three-month mRS scores across various collateral circulation in AICAO patients (Fig. 2).
The agreement between TCD and CTA/MRA varied across different collateral pathways. For ACoA detection, the two methods demonstrated excellent concordance (κ = 0.873, p < 0.001). A substantial level of agreement was also observed in PCoA detection (κ = 0.635, p < 0.001). In contrast, the agreement between the two methods was poor in detecting EICC (κ = 0.194, p = 0.106) (Table 5).
Discussion
Our study demonstrates that TCD is a valuable tool for rapid bedside assessment of collateral circulation in AICAO patients. Consistent with previous research15,16, we found that younger age and lower ASPECTs1 at 24 h were associated with a higher risk of malignant cerebral edema. Additionally, the absence of collateral circulation on TCD independently predicted malignant cerebral edema development (OR = 7.649, p = 0.032), which aligns with findings by Hoon Kim et al., who reported that poor collateral status on CTA was strongly associated with malignant edema development in middle cerebral artery occlusion patients17.
In evaluating specific collateral patterns, we found excellent agreement between TCD and CTA/MRA in detecting ACoA (κ = 0.873, p < 0.001). The presence of ACoA collaterals correlated significantly with favorable neurological outcomes at 90 days (p = 0.003). This observation is supported by Chen et al.'s recent work demonstrating that anterior circulation collaterals assessed by DSA were crucial determinants of functional outcomes in AICAO patients undergoing endovascular treatment18. Conversely, EICC flow was associated with poor functional outcomes (P = 0.001), potentially indicating more severe hemodynamic compromise when secondary collateral pathways are recruited.
While advanced imaging techniques like multiphase CTA, SWI19, and ASL20 provide valuable information about collateral status, TCD offers unique advantages through its ability to provide continuous, real-time bedside monitoring of cerebral hemodynamics. As demonstrated by Bang et al., well-developed collaterals can maintain cerebral perfusion pressure and reduce malignant edema risk4, making early assessment crucial for patient management.
Although limited by its small sample size, our study provides initial evidence that TCD assessment of collateral circulation may guide management strategies and prognostication in AICAO. The ability of TCD to provide real-time, non-invasive assessment of cerebral hemodynamics makes it particularly valuable in settings where advanced imaging may not be immediately available or in patients too unstable for transport.
Future research should focus on validating these findings in larger cohorts and exploring the integration of TCD with advanced imaging modalities such as SWI and ASL for comprehensive collateral assessment. Additionally, investigating the temporal evolution of multiple collateral pathways and their dynamic interactions could provide valuable insights into optimizing patient care. While our pilot study demonstrates TCD’s potential utility in AICAO management, multicenter randomized controlled trials are needed to establish standardized protocols and translate these findings into clinical practice guidelines. Understanding the complex interplay between different collateral patterns and their impact on outcomes may ultimately help identify patients most likely to benefit from specific therapeutic interventions.
Data availability
All data generated or analyzed during this study are included in this published article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Wu, S. et al. Early prediction of malignant brain edema after ischemic stroke. Stroke 49, 2918–2927 (2018).
Huang, X. et al. Predictors of malignant brain edema after mechanical thrombectomy for acute ischemic stroke. J. Neurointerv. Surg. 10, 994–998 (2019).
Wu, S. et al. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 18, 394–405 (2019).
Bang, O. Y. et al. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke 49, 2884–2890 (2018).
Sperti, M. et al. Determinants of cerebral collateral circulation in acute ischemic stroke due to large vessel occlusion. Front Neurol. 14, 1181001. https://doi.org/10.3389/fneur.2023.1181001 (2023).
Bang, O. Y., Goyal, M. & Liebeskind, D. S. Collateral circulation in ischemic stroke: Assessment tools and therapeutic strategies. Stroke. 46(11), 3302–3309 (2015).
Zhang, X., Huang, P. & Zhang, R. Evaluation and prediction of post-stroke cerebral edema based on neuroimaging. Front. Neurol. 12, 763018. https://doi.org/10.3389/fneur.2021.763018 (2022).
Yang, L. et al. Comparison of methods between CT perfusion source images and CT angiography in collateral flow assessment. Acta Radiol. 62(1), 73–79 (2021).
Hermes, M., Br, H. & Schimrigk, K. Transcranial Doppler ultrasound in the evaluation of collateral blood flow in patients with internal carotid artery occlusion: Correlation with cerebral angiography. Am. J. Neuroradiol. 16(1), 195–202 (1995).
Galego, O. et al. Collateral pial circulation relates to the degree of brain edema on CT 24 hours after ischemic stroke. Neuroradiol. J. 31(5), 456–463 (2018).
Connolly, F. et al. Pattern of activated pathways and quality of collateral status in patients with symptomatic internal carotid artery occlusion. Cerebrovasc. Dis. 48(3–6), 244–250 (2019).
Powers, W. J. et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50(12), e344–e418 (2019).
Yang, H., Pinjin, H. & Yingqi, X. Chinese guidelines for vascular ultrasound for stroke. Chin. J. Med. Ultrasound. (Electron. Ed.) 12, 159–176 (2015).
Liu, L. et al. Guidelines for evaluation and management of cerebral collateral circulation in ischaemic stroke 2017. Stroke Vasc. Neurol. 3(3), 117–130 (2018).
Shimoyama, T. et al. Early prediction of malignant brain edema after ischemic stroke. Stroke 49(12), 2918–2927 (2018).
Chen, J. et al. New insight in massive cerebral infarction predictions after anterior circulation occlusion. Sci. Rep. 13(1), 23021 (2023).
Kim, H. et al. Predictors of malignant brain edema in middle cerebral artery infarction observed on CT angiography. J Clin. Neurosci. 22(3), 554–560 (2015).
Chen, W. et al. Clinical efficacy of collateral circulation in the evaluation of endovascular treatment for acute internal carotid artery occlusion. Interv. Neuroradiol. 26(6), 774–782 (2020).
Lee, H. J. et al. Collateral estimation by susceptibility-weighted imaging and prediction of functional outcomes after acute anterior circulation ischemic stroke. Sci. Rep. 11(1), 21370 (2021).
Okell, T. W. et al. Measurement of collateral perfusion in acute stroke: a vessel-encoded arterial spin labeling study. Sci. Rep. 9(1), 8181 (2019).
Funding
Research was supported by Medical Health Science and technology Project of Inner Mongolia Health Commission 202201001.
Author information
Authors and Affiliations
Contributions
Jin Chen conceived and designed the experiments, collected and analyzed the data, wrote the manuscript. Yichen Wang and Hong Chang operated the TCD machine and collected data. Peng Bai drafted the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study has obtained approval from the Ethics Committee of Inner Mongolia Autonomous Region People’s Hospital (Approval No: 202204212L). All family members of the patients involved in this study were informed about the research purposes and signed written informed consent forms. Patient privacy was protected. This study complied with local and international ethical guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Chang, H., Bai, P. et al. Evaluation of contralateral arterial flow compensation using transcranial Doppler in acute internal carotid artery occlusion and implications for neurological outcome. Sci Rep 15, 2998 (2025). https://doi.org/10.1038/s41598-025-86640-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-86640-5