Abstract
Subsequent vertebral fracture (SVF) is a common and refractory complication after percutaneous vertebral augmentation (PVA) for osteoporotic vertebral compression fracture (OVCF). Computed tomography (CT)-based Hounsfeld units (HU) and magnetic resonance imaging (MRI)-based vertebral bone quality (VBQ) score can evaluate osteoporosis quantitatively, hyperlipidemia(HLP) might affect measurement result of VBQ score. The primary objective of this study is to compare the predictive capabilities of HU and VBQ for SVF, and to clarify the impact of hyperlipidemia on the predictive abilities. This study included consecutive 341 patients with OVCF who were treated with PVA from March 1, 2020, to December 31, 2022. A multivariate logistic regression analysis was used to determine the relationship between HU and VBQ and SVF. ROC curves were plotted to calculate area under curve (AUC), and hoc analysis and Youden index was used to determine cut-off values of HU and VBQ. Compared with the non-SVF group, VBQ (4.69 ± 0.35 vs. 4.14 ± 0.41, P < 0.001) was higher and HU (58.2 ± 13.81 vs. 81.2 ± 16.68, P < 0.001) was lower in the SVF group. On multivariate logistic regression analysis, higher VBQ (odds ratio (OR) = 23.47,P < 0.001) and lower HU (OR = 0.93,P < 0.001) are independent predictors for SVF. The AUC using VBQ for predicting SVF was 0.84, the cut-off was 4.28. The AUC using HU for predicting SVF was 0.85, the cut-off was 64.40. In the HLP group, the AUC of VBQ was comparable with that of HU for SVF prediction, however, the sensitivity was lower in the HLP group (0.50 vs. 0.83). Furthermore, the AUC value of VBQ with HLP was lower than that of VBQ without HLP (0.78 vs. 0.90, P = 0.017). These findings demonstrated that both VBQ and HU can accurately predict the occurrence of SVF after PVA. HLP might cause a false increase of VBQ value, using HU could better assess bone quality and predict SVF occurrence when HLP is present.
Similar content being viewed by others
Introduction
Subsequent vertebral fracture (SVF) is a common and challenging complication that can occur after percutaneous vertebral augmentation (PVA) for osteoporotic vertebral compression fractures (OVCF), with reported incidence rates ranging from 6.2–51.8%1,2. SVF leads to recurrent pain, aggravated kyphosis, reduced quality of life, and even pose life-threatening risks, often requiring repeat procedures1,2,3. Osteoporosis is recognized as a significant risk factor for SVF. Dual-energy X-ray absorptiometry (DEXA) is the “gold standard” for diagnosing osteoporosis as recommended by the WHO. However, DEXA’s reliance on planar projection techniques to assess localized bone mineral density (BMD) can yield falsely elevated values due to factors like obesity, lumbar degeneration, and vascular calcification4,5. Additionally, DEXA is not universally accessible, as studies have shown it is completed in less than 10% of cases of fragility fractures6,7.
In 2011, Pickhardt et al. assessed vertebral bone quality by measuring the Hounsfield Units (HU) of L1 trabecular attenuation in chest CT scans. They found that HU demonstrates high sensitivity and specificity for diagnosing osteoporosis, achieving 90% specificity with a threshold of 110 HU8. Furthermore, Elena et al. reported that HU detected osteoporosis in 86% of patients as opposed to 59% with DEXA, with a sensitivity of 90% using a threshold of 1609. Zhang et al. found that lower L1 HU values were significantly associated with a higher risk of SVF, with a marked increase in risk when L1 HU fell below 9510. Additionally, studies have demonstrated a negative correlation between vertebral bone marrow signal intensity on MRI T1 images and vertebral BMD11,12,13. In 2019, Ehresman et al.14 developed the MRI-based vertebral bone quality (VBQ) score, and showed that VBQ score could effectively predict OVCF in patients with spinal metastatic tumors. Unlike HU, which reflects bone mass, VBQ score assesses bone quality indirectly through fat content in the vertebrae, indicating that both organic and inorganic components can affect its accuracy14,15,16. Notably, the direct correlation of VBQ with intravertebral fat content may be influenced by hyperlipidemia, thereby affecting the accuracy of its assessment of vertebral bone quality17.
While previous studies have compared the predictive capabilities of HU and VBQ score for cage subsidence after OLIF surgery18, no research has yet analyzed their predictive performance for SVF or established the influence of hyperlipidemia on their effectiveness. We hypothesize that HU and VBQ score are independent risk factors for the occurrence of SVF. The primary objective of this study is to compare the predictive abilities of HU and VBQ score for SVF occurrence and to clarify the impact of hyperlipidemia on these predictive abilities.
Methods
This study was conducted in accordance with the ethical principles outlined in the World Medical Association Declaration of Helsinki and was approved by the Ethics Committee of Xuzhou Central Hospital. This study is a retrospective cross-sectional study, therefore the requirement for patient informed consent was waived by the Ethics Committee of Xuzhou Central Hospital. The study report follows the STROBE guidelines.
This study included consecutive patients with OVCF who were treated with PVA at our hospital from March 1, 2020, to December 31, 2022. Inclusion criteria were (1) patients aged 60 years and older; (2) Single-segment OVCF confirmed by medical history, physical examination, and MRI, treated with PVA; (3) Preoperative chest CT examination that could fully display the L1 vertebra. Exclusion criteria were (1) Patients with a history of lumbar surgery, trauma, or infection; (2) Two or more consecutive vertebral compression fractures; (3) Pathological fractures caused by tumors, tuberculosis, etc.
Identification of SVF
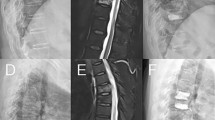
SVF was identified by Genant semiquantitative grading scale(radiographic SVF) and/or MRI plus new onset back pain(clinical SVF)19,20. Radiographic SVF was defined as loss of at least 20% of vertebral body height from T4 to L4 or an increase of at least one grade in vertebral body compression from baseline according to the Genant semiquantitative grading scale19. Clinical SVF was defined as MRI performed on patients who presented with a new onset of low back pain during follow-up showed T2 and STIR fat-suppression phase hyperintensities and T1 hypointensities suggestive of fresh vertebral compression fractures, excluding recollapse of the cemented vertebra.
L1 HU measurement
All included patients underwent a preoperative chest CT scan (by General Electric Company, Light Speed VCT) with the following scanning conditions: tube voltage of 120 kV, tube current of 355 mA, slice thickness of 5 mm, inter-slice gap of 5 mm, bone window width of 2000 HU, and window level of 350 HU. Following the method introduced by Pickhardt et al.8, the L1 vertebra (the first vertebra without ribs) is identified on the chest CT. The CT value of the vertebra is measured in HU using the Picture Archiving and Communication System (PACS). A mid-level axial cross-section of the vertebra is chosen, avoiding the cortical bone, posterior venous plexus, bone islands, and other heterogeneous structures. An elliptical region of interest (ROI) as large as possible is placed, and the PACS software automatically measures the HU value within the ROI, which represents the CT value of the vertebral trabecular bone(Fig. 1).
VBQ measurement
In this study, lumbar MRI was performed using a 3.0T GE Healthcare MRI scanner (Signa Premier, GE Healthcare, USA) with the following detailed imaging parameters: Patients were positioned supine, with the spine in a neutral position, to avoid any additional motion artifacts during imaging. Field of view for lumbar spine imaging was set between 24 and 30 cm to cover the L1 to L5 vertebral levels. Sequence type include T1-Weighted Imaging (T1WI), T2-Weighted Imaging (T2WI) and short tau inversion recovery (STIR). Axial and sagittal slices were acquired with a slice thickness of 4 mm, using a 0.4 mm slice space to ensure adequate anatomical coverage without excessive partial volume averaging. The matrix size was 320 × 320 or 256 × 256 depending on the sequence, ensuring high spatial resolution to visualize detailed vertebral and intervertebral structures.The repetition time (TR) and echo time (TE) for T1WI is as follow: TR: 500–700 ms, and TE: 12–15 ms, for T2WI: TR: 3000–4000 ms and TE: 80–120 ms; for STIR: TR: 3000–4000 ms and TE: 60–80 ms. A 16-channel lumbar spine coil was employed to enhance signal-to-noise ratio and improve image quality. VBQ score was calculated on non-contrast T1-weighted MRI of the lumbar spine according to the method introduced by Ehresman et al.14. The location of measurement is preferred to the mid-sagittal plane of the lumbar spine. The medullary portions of the L1-4 vertebral bodies and CSF space at the L3 level were used as ROIs to measure SI of the L1-4 vertebral body and CSF, respectively (Fig. 2). The VBQ score was calculated by dividing the median SI of the L1-L4 vertebral bodies by the SI of the CSF at the L3 level, using the following formula:
When scoliotic changes, venous plexus, or hemangiomas hinder accurate ROI assessments on midsagittal slices, measurements can be taken on parasagittal planes. If any abnormality extended across all sagittal sections, the vertebral body was excluded. In some cases, the ROI for the CSF space at L2 or L4 was utilized to accurately represent CSF signal intensity, as the L3 space was occupied by descending nerve roots. In this study, HU and VBQ were independently measured by two trained authors, and the mean value of the measured results was used as the required value for the study.
Statistical analysis
Data were statistically analyzed using R-Studio version 2023.12.0 (Posit Software, Boston, Massachusetts, USA). The Shapiro-Wilk test was used to assess the normality of continuous variables. For comparisons between groups, the two independent samples t-test (for normally distributed variables) or the Mann–Whitney U test (for non-normally distributed variables) was used. Categorical variables were represented by numbers and frequencies, and analyzed using the chi-square test.
Confounding factors (age, sex, body mass index, HLP, chronic steroid use, fracture site, anti-osteoporosis drugs, intervertebral cement leakage) were determined according to the literature review and clinical judgment, and a multivariable logistic regression model was used to adjust for confounding factors, and to determine the relationship between HU and VBQ score and SVF. ROC curves were plotted to calculate area under curve (AUC). Hoc analysis and Youden index was used to determine cut-off values of HU and VBQ. AUCs were compared using the Delong test. In the SVF group, subgroup analyses were performed according to the presence or not of HLP. Pearson correlation was used to analyze the relationship between HU and VBQ. A P-value of < 0.05 with two sided was considered statistically significant.
Results
From March 1, 2020, to December 31, 2022, a total of 650 patients with OVCF treated with PVA were admitted to the hospital. According to the inclusion and exclusion criteria, 341 patients (mean age, 75.6 ± 7.39years, 77.7% femals)were included in this study. After followed up for an average of 33.5 months, 97 (28.4%) patients developed SVF and were included in the SVF group.The baseline data and surgical related data of the two groups are shown in Table 1.
A total of 341 patients were included in this study, 97(28.4%) patients developed SVF. Compared with the non-SVF group, VBQ(4.69 ± 0.35 vs. 4.14 ± 0.41, P < 0.001) was higher and HU (58.2 ± 13.81 vs. 81.2 ± 16.68, P < 0.001) was lower in the SVF group. On multivariate logistic regression analysis, higher VBQ(odds ratio(OR) = 23.465, 95% confidence interval(CI) = 7.585–72.589, P < 0.001) and lower HU (OR = 0.927, 95% CI = 0.902–0.953, P < 0.001) (Table 2).
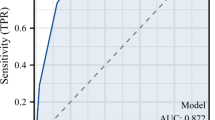
The area under curve(AUC) using VBQ for predicting SVF was 0.843(95% CI:0.810–0.889), the cut-off was 4.275. The AUC using HU for predicting SVF was0.852(95% CI:0.810–0.894), the cut-off was 64.400. There was no statistical difference between the two AUC values(P = 0.733, Delong test) (Table 3; Fig. 3).
In the SVF group, 39 (40.2%) patients had HLP, the VBQ in the HLP group was higher (4.75 ± 0.30 vs. 4.58 ± 0.34, P = 011), and HU was comparable when compared with the non-HLP group (56.27 ± 14.99 vs. 61.16 ± 11.39, P = 0.087). In the HLP group, the AUC of VBQ was comparable with that of HU for SVF prediction (0.778 vs. 0.820 of HU, P = 0.403), however, the sensitivity was lower in the HLP group (0.5000 vs. 0.827)(Table 3; Fig. 3).
According to Pearson correlation analysis, there is a significant negative correlation between VBQ and HU in all patients (r=−0.60, P < 0.001), patients with HLP (r=−0.51, P < 0.001), and patients withou HLP (r=−0.68, P < 0.001). There is significantly difference on correlation coefficient of the patients with HLP vs. without HLP (P = 0.022,Fisher’s Z test) (Table 4; Fig. 4).
Discussion
This study found that after an average of 33.5 months follow-up duration for patients with OVCF treated with PVA, 97 (28.4%) patients developed SVF. Both HU and VBQ were good predictors for SVF. The predictive threshold for HU was 64.4, with an AUC of 0.852, and the predictive threshold for VBQ was 4.275, with an AUC of 0.843. HLP did not affect the evaluation of vertebral bone quality by HU or its predictive ability for SVF. However, patients with HLP had a higher VBQ than those without HLP, and the predictive ability for SVF was inferior than that of VBQ in patients without HLP. Asymptomatic SVF is not uncommon. In order to obtain information on as many patients as possible, both clinical and radiological SVF was included in the study. The incidence rate of SVF was consistent with the previous study10, both higher than the study included only patients of clinical SVF24.
Predictive power of HU and VBQ for SVF development
Most studies suggest that SVF is associated with osteoporosis and altered local mechanical environment after cement implantation into the vertebral body, with osteoporosis being the most important risk factor21,22. Scott J Lee et al.23 followed patients over 65 years of age who completed thoracoabdominal CT for other reasons for 5.8 years and found that L1 HU was continuously associated with fracture-free survival after adjusting for confounding factors such as age, sex, and prior fracture, and significantly reduced fracture-free survival when L1 HU was ≤ 90. Jiang et al.24 found that 47 of 105 patients (44.8%) who underwent PKP had adjacent vertebral fractures after 14-month follow-up, and when the HU threshold was 66.9 (with an AUC of 0.901, the prediction accuracy = 90.4%) could accurately predict the occurrence of adjacent vertebral fractures. In this study, after 33.5 months followed up, 97 (28.4%) patients developed SVF. The threshold of L1 HU at 64.4 could predict the occurrence of SVF, which is consistent with previous studies24,25.
Ehresman et al.14 developed VBQ predicted new vertebral compression fractures in spinal metastasis, and 56 (53.3%) patients were found to have vertebral fractures after 33.6 months of follow-up in 105 patients, and multivariate analysis and ROC analysis revealed that VBQ could more accurately predict the occurrence of vertebral fractures. Wenshuai Li et al.26 measured the VBQ score (L1-4) and single-level VBQ score (L1) in 109 OVCF patients, and found that the VBQ score (L1-4) of 3.22 and single level VBQ of 3.16 were optimal thresholds to predict fragility fractures in high-risk patients. The results of our study show that the cut-off value of the VBQ score for predicting the occurrence of spinal vertebral fractures (SVFs) is 4.275, and the OR value from the multivariable logistic regression analysis for the VBQ score is 23.465. This indicates that with every one-unit increase in the VBQ score, the risk of developing SVFs may increase by over 23 times. The VBQ score is negatively correlated with bone quality, meaning a higher VBQ score indicates more severe osteoporosis and, consequently, a greater risk of SVFs following percutaneous vertebroplasty (PVA).
Effect of HLP on VBQ and HU Measurement Results and predictive performance
There is no consensus on the effect of HLP on bone mineral density. Saoji et al.27 found a significant correlation between lipid metabolism and osteoporosis, and high-density lipoprotein and triglycerides were important predictors of osteoporosis and osteopenia. On the contrary, Samelson et al.28 found no significant correlation between total cholesterol and bone mineral density after 34 years of follow-up. Aynaszyan et al.17 performed a retrospective analysis of 156 patients (64% with HLP) who completed lumbar MRI and DEXA scans within 2 years, subgroup analysis revealed that HLP may cause falsely elevated VBQ, so it may be impossible to accurately distinguish osteoporotic bone from healthy bone. Our study also found that HLP affected VBQ measurements in osteoporotic patients. The vertebral body was filled with blood vessels, and patients with HLP had increased adipose tissue content in vertebral vessels, which may be responsible for falsely elevated VBQ. In this study, 136 patients (39.9%) had HLP, which is lower than reported by Aynaszyan Stephan et al.17. All the patients in our study were Han Chinese, from central China, regional and ethnic difference may be a reasonable explanation for the different incidence of HLP. HLP may also affect the measurement accuracy of HU values, but our findings found that the values were comparable with or without HLP. Further studies are warranted to explore the effect of hyperlipidemia on HU values.
Limitations
The limitations of this study are as follows: (1) This single-center retrospective study focuses exclusively on Han Chinese participants, limiting generalizability; (2) We used L1 HU to predict SVF occurrence rather than assessing the HU of the fractured vertebra, which may not accurately reflect its bone quality. The cases were collected during the COVID-19 pandemic, and all hospitalized patients underwent chest CT, allowing for a broader inclusion of cases; (3) Although DXA measurements are the gold standard for osteoporosis screening, we did not include DXA results for comparison with VBQ and HU in predicting SVF occurrence. Similar to previous studies6, only 40 out of 341 patients (11.7%) completed DXA scans, so we could not include these results; (4) There are significant signal-to-noise ratio differences among MRI systems. While the VBQ score standardizes SI using CSF, varying cut-off values may still affect clinical applicability; (5) HLP is divided into hypertriglyceridemia and hypercholesterolemia, but we did not conduct subgroup analyses. Future research could investigate how these types of HLP affect VBQ score measurements.
Conclusion
Both VBQ and HU can accurately predict the occurrence of SVF after PVA. HLP might cause a false increase of VBQ value, using HU could better assess bone quality and predict SVF when HLP is present.
Data availability
Data is provided as the supplementary information file.
References
Zhan, Y., Jiang, J., Liao, H., Tan, H. & Yang, K. Risk factors for cement leakage after vertebroplasty or kyphoplasty: a meta-analysis of published evidence. World Neurosurg. 101, 633–642 (2017).
Lee, D. G., Park, C. K., Park, C. J., Lee, D. C. & Hwang, J. H. Analysis of risk factors causing new symptomatic vertebral compression fractures after percutaneous vertebroplasty for painful osteoporotic vertebral compression fractures: a 4-year follow-up. J. Spinal Disord. Tech. 28 (10), E578–E583 (2015).
Shi-Cheng, L. W. G. H., Gang, D., Jin-He, G. & Wen, F. Zhu Guang-Yu, Teng Gao-Jun. Risk factors for new vertebral fractures after percutaneous vertebroplasty in patients with osteoporosis: a prospective study. J. Vasc. Interv. Radiol. 23 (9), 1143–1149 (2012).
Shepherd, J. A., Schousboe, J. T., Broy, S. B., Engelke, K. & Leslie, W. D. Executive summary of the 2015 ISCD position development conference on advanced measures from DXA and QCT: fracture prediction beyond BMD. J. Clin. Densitom. 18 274–86. (2015).
ZouD, L. W. & DengC, DuG, X. N. The use of CT Hounsfield unit values to identify the undiagnosed spinal osteoporosis in patients with lumbar degenerative diseases. Eur. Spine J. 28, 1758–1766 (2019).
Leslie, W. D. et al. A population-based analysis of the post-fracture care gap 1996–2008: the situation is not improving. Osteoporos. Int. 23 (5), 1623–1629 (2012).
Muraki, S. et al. Impact of degenerative spinal diseases on bone mineral density of the lumbar spine in elderly women. Osteoporos. Int. 15, 724–728 (2004).
Pickhardt, P. J. et al. Simultaneous screening for osteoporosis at CT colonography: bone mineral density assessment using MDCT attenuation techniques compared with the DXA reference standard. J. Bone Min. Res. 26 (9), 2194–2203 (2011).
., Alacreu Elena & Estanislao, M. Opportunistic screening for osteoporosis by routine CT in Southern Europe. Osteoporos. Int. 28 (3), 983 – 90 (2017).
Zhang, S. B. et al. Evaluation of the use of CT attenuation for the prediction of subsequent vertebral fracture in patients with osteoporosis. Pain Phys. 24 (4), 493–500 (2021).
Shen, W. et al. Relationship between MRI-measured bone marrow adipose tissue and hip and spine bone mineral density in African-American and caucasian participants: the CARDIA study. J. Clin. Endocrinol. Metab. 97, 1337–1346 (2012).
Shah, L. M. & Hanrahan, C. J. MRI of spinal bone marrow: part 1, techniques and normal age-related appearances. Am. J. Roentgenol. 197 (6), 1298–1308 (2011).
Bandirali, M. et al. A new diagnostic score to detect osteoporosis in patients undergoing lumbar spine MRI. Eur. Radiol. 25 (10), 2951–2959 (2015).
Ehresman, J. et al. A novel MRI-based score assessing trabecular bone quality to predict vertebral compression fractures in patients with spinal metastasis. J. Neurosurg. Spine 32, 499–506 (2019).
Ehresman, J. et al. Vertebral bone quality score predicts fragility fractures independently of bone mineral density. Spine J. 21 (1), 20–27 (2021).
Rosen, C. J. & Bouxsein, M. L. Mechanisms of disease: is osteoporosis the obesity of bone? Nat. Clin. Pract. Rheumatol. 2 (1), 35–43 (2006).
Aynaszyan, S., Devia, L. G., Udoeyo, I. F., Badve, S. A. & DelSole, E. M. Patient physiology influences the MRI-based vertebral bone quality score. Spine J. 22, 1866–1874 (2022).
Pu, X. et al. Comparison of predictive performance for cage subsidence between CT-based Hounsfield units and MRI-based vertebral bone quality score following oblique lumbar interbody fusion. Eur. Radiol. 33 (12), 8637–8644 (2023).
Genant, H. K., Wu, C. Y., van Kuijk, C. & Nevitt, M. C. Vertebral fracture assessment using a semiquantitative technique. J. Bone Min. Res. 8 (9), 1137–1148 (1993).
Kendler, D. L. et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 391 (10117), 230–240 (2018).
Bae, J. S., Park, J. H., Kim, K. J., Kim, H. S. & Jang, I. T. Analysis of risk factors for secondary new vertebral compression fracture following percutaneous vertebroplasty in patients with osteoporosis. World Neurosurg. 99, 387–394 (2017).
Ji, C., Rong, Y., Wang, J., Yu, S. & Cai, W. Risk factors for refracture following primary osteoporotic vertebral compression fractures. Pain Phys. 24, 335–340 (2021).
Lee, S. J., Graffy, P. M., Zea, R. D., Ziemlewicz, T. J. & Pickhardt, P. J. Future osteoporotic fracture risk related to lumbar vertebral trabecular attenuation measured at routine body CT. J. Bone Min. Res. 33 (5), 860–867 (2018).
Jiang, L. M. et al. The vertebral hounsfield units can quantitatively predict the risk of adjacent vertebral fractures after percutaneous kyphoplasty. Quant. Imaging Med. Surg. 13 (2), 1036–1047 (2023).
Pitton, M. B. et al. CT-guided vertebroplasty in osteoprotic vertebral fractures: incidence of secondary fractures and impact of intradiscal cement leakages during follow-up. Eur. Radiol. 18 (1), 43–50 (2008).
Li, W. et al. Characteristics of MRIbased vertebral bone quality scores in elderly patients with vertebral fragility fractures. Eur. Spine J. 32 (7), 2588–2593 (2023).
Saoji, R. et al. Association of high-density lipoprotein, triglycerides, and homocysteine with bone mineral density in young Indian tribal women. Arch. Osteoporos. 13 (1), 108 (2018).
Samelson, E. J. et al. Long-term effects of serum cholesterol on bone mineral density in women and men: the Framingham osteoporosis study. Bone 34, 557–561 (2004).
Author information
Authors and Affiliations
Contributions
Youdi Xue and Kun Shi wrote the main manuscript text , Jie Li and Weixiang Dai prepared figures and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xue, Y., Shi, K., Dai, W. et al. Prediction of subsequent vertebral fracture after percutaneous vertebral augmentation using MRI-based vertebral bone quality and CT-based Hounsfield units: a retrospective cross-sectional study. Sci Rep 15, 3524 (2025). https://doi.org/10.1038/s41598-025-86721-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-86721-5