Abstract
Endoscopic retrograde cholangiopancreatography (ERCP) training remains challenging. This study used 3D printing techniques to develop and optimize a portable ERCP training simulator and to implement basic and advanced practical techniques. Subsequently, we aimed to determine whether endoscopy trainees acquired proficiency in ERCP techniques and assess any improvements in their skill levels from using this model. An ERCP training model was generated using 3D printing techniques, including five distinct interchangeable and transparent ampullar–common bile duct (CBD) modules. A prospective study using this model was conducted with ten trainees. The technical success rate and examination times for duodenoscope insertion and biliary cannulation were evaluated. In addition, the successful plastic-stent insertion rate and trainee satisfaction were measured. The success rates for duodenoscopy, cannulation, and plastic stent insertion were 94, 100, and 92%, respectively. The mean satisfaction scores for duodenoscope insertion, cannulation, and plastic stent insertion were 4.4, 4.7, and 4.6 on a 5-point scale, respectively. Five attempts decreased the insertion time (R = − 0.591, P < 0.001) and cannulation time (R = − 0.424, P = 0.002). This ERCP-training silicon model is durable, simulates ERCP techniques easily, and helps trainees improve their ERCP techniques.
Similar content being viewed by others
Introduction
Endoscopic retrograde cholangiopancreatography (ERCP) is a crucial diagnostic and therapeutic procedure for managing biliary and pancreatic diseases1. A duodenoscope cannulates the bile and pancreatic ducts while navigating through the esophagus, stomach, and duodenum. Performing ERCP requires theoretical knowledge, technical skills, and extensive training. However, the complex anatomical structures and potential complications associated with ERCP pose several challenges to training physicians in this field2,3.
Traditionally, ERCP training has relied mainly on real-world patient cases, which limits the number of cases available for training purposes, raises ethical concerns, and risks patient safety4,5. The learning curve for trainees is steep, and mistakes made during the initial stages of training can have serious consequences6. Thus, there is an increased need for effective training models that simulate the complexities of ERCP procedures, providing a safe and controlled environment for trainees to develop their skills7,8.
ERCP training models have been developed using live animal models, ex vivo porcine models, computer simulators, and mechanical methods9. Because each method is very different in terms of anatomical differences, ethical issues, storage problems, realistic tactile sensation, durability, and portability, there is no easy way to select an optimized model for ERCP training10. Three-dimensional (3D) printing technology has become a promising tool in the medical education field8. By utilizing computer-aided design software and additive manufacturing techniques, 3D printing allows the creation of anatomically accurate and patient-specific models11,12. These models replicate the complexities of human anatomy, providing an invaluable tool for procedural training.
Using 3D printing techniques in ERCP training simulators has limitations. For instance, these models cannot replicate realistic examinations and structures similar to the human body, which is an advantage of in vivo models. In addition, they cannot simulate the convenience of examinations without fluoroscopic guidance, which is advantageous for in vitro portability. Moreover, these models cannot encompass all stages of ERCP training; they often focus on creating only the common bile duct (CBD) and duodenal papilla, limiting practice to specific stages of the procedure. Furthermore, replicating dedicated techniques in 3D printing simulators has challenges, including shortening the duodenoscope during duodenal insertion.
This study used 3D-printing techniques to develop an optimized ERCP training simulator for simulating basic and advanced practical techniques. We also aimed to determine whether endoscopy trainees could acquire proficiency in ERCP techniques and assess any improvements in their skill levels using this model.
Materials and methods
3D modeling and fabrication of ERCP training simulator
To develop the fundamental component of the ERCP training simulator we built upon our previous experience with 3D simulators, utilizing the same computed tomography (CT) gastrography data for accurate replication of the interior space and pathways of the esophagus, stomach, and duodenum8,13,14.
Digital 3D models of the stomach and duodenum were generated by importing CT image data into proprietary software using the stereolithography file format. A hepatobiliary system comprising the ampulla of Vater, CBD, gallbladder, liver, and intrahepatic bile ducts was integrated and appropriately modified in 3D using the open-source programs MeshLab and MeshMixer (Fig. 1). In this model, emphasis was placed on endoscopic training involving various CBDs. The removable modules representing the ampulla and CBD were affixed to the phantom base to facilitate this. Five distinct ampullary CBD modules were designed individually for interchangeable use on the phantom base to accommodate different therapeutic procedures (Fig. 2). The modules were fabricated using transparent and highly pliable silicone to ensure usability regardless of whether fluoroscopic guidance was employed. This material allows observation of the inner lumen from the module’s external surface.
Endoscopic retrograde cholangiopancreatography (ERCP) training simulator 3D modeling and fabrication. The ampulla-common bile duct (CBD) module represents the removable ampullary and CBD component. The ERCP phantom is fixed in the prone position using a supporting stand (saddle part). (A) 3D modeling. (B) Overhead view of the 3D modeling. (C) Disassembled view of the 3D modeling. (D) Fabrication of the ERCP training simulator.
All organ components in this model were created by combining silicone molding techniques and 3D printing; the saddle was produced exclusively by a 3D printer (3DM Tough-3.6, 3DMaterials, Zeron-2500; Zeron, Korea). Negative 3D-printed molds were designed to manufacture each organ part with silicone (3DM DW-06, 3DMaterials, Zeron-2500, Zeron; Korea). Injection molding was subsequently employed to produce the organ parts using a silicone material (Ecoflex 00–50, Smooth-on, USA; shore A (hardness) = 00–50(0.5), elongation = 980%).
Training outcome measurement
This study was conducted with ten novice ERCP trainees registered at the CHA Bundang Medical Center and Dongtan Sacred Heart Hospital. Trainees were defined as having participated in over 200 upper endoscopic procedures with experience in ERCP observation only and not performance. The trainees underwent three examinations: duodenoscope insertion, biliary cannulation, and plastic stent insertion. Each trainee performed duodenoscope insertion five times; the success rate and times taken were recorded. Successful duodenoscope insertion was defined as reaching the ampulla of Vater from the oral cavity, including the shortening procedure. Failure was defined as insertion not completed within 5 min. Each trainee also performed biliary cannulation five times, recording the success rate and times taken. A Tandem™ XL Triple-Lumen ERCP cannula (Boston Scientific Co. Natick, MA.) was used for biliary cannulation. Successful cannulation was determined when the catheter was inserted into the endoscopic channel, and deep cannulation was achieved, and the time taken was recorded. Failure was defined as the inability to complete cannulation within 5 min. Each trainee tried plastic stent insertion five times, with success defined as accurately deploying the plastic stent within the CBD. We used 0.025-inch guidewires (VisiGlide 2™; Olympus Co. Tokyo, Japan) and 7-Fr, 7-cm straight Cotton–Leung plastic stents (Cook Medical Inc., Winston-Salem, NC). After completing the examinations, the trainees evaluated their satisfaction with duodenoscope insertion, biliary cannulation, and plastic stent insertion on a standardized 5-point Likert scale. The scale ranged from 1 (very dissatisfied) to 5 (very satisfied), allowing trainees to provide a comprehensive assessment of their experience with each procedure.
The study protocol was conducted in accordance with the principles of the Declaration of Helsinki. The acquisition of human data for this study was approved by the CHA Bundang Medical Center Institutional Review Board (no. CHAMC 2019-08-025) and Hallym Dongtan Sacred Hospital Institutional Review Board (no. 2023-05-003). Informed consent was obtained from all participants.
Statistical analysis
Categorical variables are presented as frequencies and percentages. Continuous variables are presented as the mean (± standard deviation) or median with range. The Spearman rank method was used to evaluate the relationship between the procedure time and frequency. Statistical significance was set at P < 0.05. All statistical analyses were performed using SPSS version 21.0 for Windows (SPSS Inc., Chicago, IL).
Results
Implementation of ERCP procedures
Once the initial model was completed, its shape was modified by inserting a duodenoscope and performing fluoroscopic examinations (Fig. 3). The larynx, esophagus, stomach, and duodenum were adjusted accordingly, considering the smoothness and directionality of the duodenoscope insertion. The most challenging step involves positioning the duodenoscope correctly in front of the ampulla by inserting it into the duodenum and implementing a shortening procedure. This difficulty is due to the silicone material needed to maintain compatibility with the surrounding organs. As a solution, the position and extent of the outer hard saddle were adjusted to provide support, thereby enabling the shortening procedure. After using endoscopic observations to determine the optimal positioning of the ampulla-CBD module, it was integrated seamlessly with the liver, allowing external visualization and closely resembling the arrangement found in the human body during fluoroscopic examination. The model’s shape was modified continuously and iteratively.
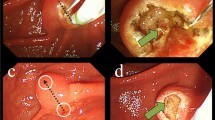
Actual presentation of the endoscopic retrograde cholangiopancreatography (ERCP) training simulator. (A) The ERCP phantom is positioned on the fluoroscopy table, with fluoroscopic and endoscopic imaging displayed on the monitors. (B,C) Fluoroscopic images captured before (B) and after (C) the shortening procedure. (D–F) Endoscopic images are captured at the antrum (D) and the duodenum before (E) and after (F) the shortening procedure.
Several ERCP procedures were performed successfully using the simulator, including biliary cannulation, plastic stent insertion, metal stent insertion, stone extraction, and biliary sphincterotomy (Fig. 4 and Supplementary video 1). Biliary sphincterotomy was performed on a specialized ampulla-CBD module using a Vienna sausage connected to an electrical plate for electric current flow. These procedures were performed using endoscopic, fluoroscopic, and direct external views. Throughout the procedures, the ampulla remained intact without any damage or tears. Injecting a dye into the CBD section was unnecessary because all parts were visualized clearly under fluoroscopy. The entire procedure was observed directly in the ampulla-CBD module outside the simulator. For repeated training procedures, plastic and metal stents were retrieved after separating the ampulla-CBD module from the main body. The metal stents were easily reinserted into the deployed catheters using a 3D-printed assistance cap (Fig. 5 and Supplementary video 2).
Several procedures are performed using the endoscopic retrograde cholangiopancreatography (ERCP) training simulator, including (A) biliary cannulation, (B) plastic stent insertion, (C) metal stent insertion, (D) stone extraction, and (E) biliary sphincterotomy using Vienna sausage. These procedures are observed through endoscopic, fluoroscopic, and direct external views.
Training outcome measurement
The study enrolled 10 trainees with a mean age of 35.1 years; 50% were men (Table 1). The insertion success rate was 94%, with 47 successful insertions in 50 attempts. The total insertion time was 71.87 ± 67.93 s. The cannulation success rate was 100%; all 50 attempts succeeded. The total cannulation time was 74.16 ± 67.36 s. The success rate for plastic stent insertion was 92%, with 46 successful insertions in 50 attempts. The satisfaction levels with duodenoscope insertion, biliary cannulation, and plastic stent insertion were 4.4, 4.7, and 4.6 on a 5-point scale. After five attempts, the insertion time (R = − 0.591, P < 0.001) and cannulation time (R = − 0.424, P = 0.002) showed decreasing trends (Table 2).
Discussion
In this study, an innovative ERCP simulator that overcomes the problems associated with existing 3D-printed ERCP training simulators was created. This ERCP simulator provide a more realistic examination compared to other artificial simulators and has a structure similar to the human body, an advantage of using in vivo models. This model can also simulate the convenience of examinations without fluoroscopic guidance, an advantage of portable in vitro models. Moreover, this model encompasses all stages of ERCP training, including a shortening procedure during duodenoscope insertion. When practicing with trainees, a significant decreasing trend was observed in duodenoscope insertion and biliary cannulation times.
The shortcomings of the previous ERCP training model generated using 3D printing technique are two-fold. First, the procedure is possible only under fluoroscopic guidance, limiting its applicability. Second, only certain parts of the target organs were implemented because of the high friction force and uncontrolled expansion of silicone used as a raw material. An ideal ERCP training model should encompass all steps of the procedure and enable smooth insertion of the endoscope, mimicking the actual process in the human body during endoscopic and fluoroscopic examinations. In addition, the model should allow ERCP training in the absence of fluoroscopic guidance.
The most challenging step during our development process was performing a shortening procedure when the duodenoscope was inserted into the second portion of the duodenum. To overcome this hurdle, we applied a hard saddle that wraps around the inner silicone organs (including the stomach and duodenum) to hold and support it. The application range was adjusted gradually to determine the most appropriate fit for the shortening process. Therefore, as depicted in Fig. 1, the saddle covered the entire stomach and duodenum during the 3D modeling. However, some parts of the saddle were missing in the final fabricated model (Fig. 1D).
After completing this step successfully, we determined the position of the ampulla-CBD module. The liver and gallbladder portions were designed to be integrated with the saddle part without affecting duodenoscope insertion and shorten the steps. The final challenge, enabling ERCP training without fluoroscopic guidance, was achieved using a special silicone material to enhance the transparency of the CBD part of the ampulla-CBD module (Fig. 2). Ensuring transparency was crucial; locating the mounting area on the saddle, observed easily from the outside, posed an additional difficulty. After the successful fabrication of the basic ampulla-CBD module, variants of the ampulla-CBD module were created in different shapes to enable the practice of several ERCP procedures.
The need to reuse ERCP accessories cannot be overlooked in ERCP training. The most challenging aspect of reusing ERCP accessories is reinserting a metal stent into the deployed catheter after deployment. This issue was addressed using a mounting assistance kit developed with a 3D printing technique; the simplified process is illustrated in Fig. 5. This innovation allows metal stents and deployed catheters to be reused many times.
The trainees were provided practical experience by performing duodenoscope insertion, biliary cannulation, and plastic stent insertion five times each. Before practice, a lubricant was generously applied within the phantom to facilitate smooth duodenoscope insertion. An expert demonstrated the duodenoscope insertion once during the examination. Trainees with no ERCP experience encountered initial difficulties but demonstrated an increasing success rate and shorter practice time up to the fifth attempt. Although attempting ERCP on actual patients increases the risk of adverse events, utilizing the ERCP 3D phantom for practice reduces trainees’ anxiety and results in greater satisfaction levels. Although the training was conducted using the simplified CBD module, it offers the advantage of allowing practice in different scenarios. Additionally, the ERCP 3D phantom can be cleaned and stored for repeated practice at any time.
The ERCP 3D phantom has several limitations. First, even when a lubricant was applied generously, the friction varied because of the nature of silicone, making it difficult to measure the procedure time consistently. Second, it’s worth noting that this training is not applicable to all procedures, as only the normal CBD module was utilized. Third, ethical concerns prevented the evaluation of whether the training provided to the trainees using the ERCP 3D phantom was beneficial when performing ERCP on actual patients. Fourth, although experts participated in the simulator’s development and usage, an objective evaluation by independent experts was not conducted. Additionally, this study focused on the development and evaluation of the simulator, but comparisons with other simulators were not performed. Finally, another limitation is the design of the papilla in the 3D model, which remains gaping and led to an unrealistic 100% CBD cannulation success rate by trainees without prior ERCP experience. However, its strengths include the ability to conduct training without the need for fluoroscopy, allowing observation through an endoscope, and providing the capability to observe the inside of the CBD from the outside. Furthermore, the study implemented duodenoscopic insertion from the oral cavity to the duodenum, including a shortening procedure. By changing the ampulla-CBD module, various types of examinations can be performed; plastic or metal stents can be used.
In this pilot study, using advanced 3D printing techniques we developed an optimized ERCP training model. This model, distinguished by its durability and adaptability, has demonstrated efficacy in both fundamental and advanced ERCP procedures, leading to notable enhancements in trainees’ procedural skills. Future research should explore larger samples for a more comprehensive evaluation of this innovative training tool’s long-term efficacy.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Johnson, G. et al. Curriculum for ERCP and endoscopic ultrasound training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) position Statement. Endoscopy 53, 1071–1087 (2021).
Dumonceau, J. M. et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 52, 127–149 (2020).
Johnson, K. D. et al. Endoscopic retrograde cholangiopancreatography-related complications and their management strategies: a scoping literature review. Dig. Dis. Sci. 65, 361–375 (2020).
Jovanovic, I. & Monkemuller, K. Quality in endoscopy training-the endoscopic retrograde cholangiopancreatography case. Ann. Transl. Med. 6, 264 (2018).
Sharma, Z. D. & Puri, R. Quality indicators in endoscopic retrograde cholangiopancreatography: a brief review of established guidelines. Clin. Endosc. 56, 290–297 (2023).
Khan, U. et al. Learning curves in ERCP during advanced endoscopy training: a Canadian multicenter prospective study. Endosc. Int. Open. 10, E1174–E1180 (2022).
Frimberger, E. et al. A novel and practicable ERCP training system with simulated fluoroscopy. Endoscopy 40, 517–520 (2008).
Kwon, C. I. et al. Production of ERCP training model using a 3D printing technique (with video). BMC Gastroenterol. 20, 145 (2020).
Baillie, J. Endoscopic retrograde cholangiopancreatography simulation. Gastrointest. Endosc. Clin. N. Am. 16, 529–542 (2006).
Leung, J. W. et al. Development of a novel ERCP mechanical simulator. Gastrointest. Endosc. 65, 1056–1062 (2007).
Thomas, J. et al. 3D printed model of extrahepatic biliary ducts for biliary stent testing. Mater. (Basel). 13, 4788 (2020).
Yang, Y. et al. Application of 3D visualization and 3D printing technology on ERCP for patients with hilar cholangiocarcinoma. Exp. Ther. Med. 15, 3259–3264 (2018).
Kim, G. B. et al. 3D-printed phantom study for investigating stent abutment during gastroduodenal stent placement for gastric outlet obstruction. 3D Print. Med. 3, 10 (2017).
Kim, H. J. et al. Positioning during CT gastrography in patients with gastric cancer: the effect on gastric distension and lesion conspicuity. Korean J. Radiol. 10, 252–259 (2009).
Acknowledgements
We thank Mr. Kyu Seok Kim and Sehwan Park (Interventional Research Center, M.I.Tech, Co. Ltd.) for their cooperation and for providing the metal stent with the 3D-printed assistance cap.
Funding
This work was supported by the Technology Innovation Program (or Industrial Strategic Technology Development Program) (20017645, The development of manufacturing technology for interventional medical devices based on biocompatible polymer nanofiber) funded By the Ministry of Trade, Industry & Energy (MOTIE, Korea), and a grant from the Korean Gastrointestinal Endoscopy Research Foundation (2023 Investigation Grant).
Author information
Authors and Affiliations
Contributions
Study concept and design: C.I.K., S.P.S., K.J.L.; principal investigation: C.I.K.; acquisition of data: S.P.S., K.J.L., J.C.K., G.B.K.; analysis and interpretation of data: S.P.S., K.J.L., J.C.K., G.B.K., M.J.S.; drafting of the manuscript: C.I.K.; critical revision of the manuscript for important intellectual content: M.T.; and review of the manuscript: M.Y.K., S.Y.H., S.I.J. All authors read, corrected, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was conducted in accordance with the principles of the Declaration of Helsinki. The acquisition of human data for this study was approved by the CHA Bundang Medical Center Institutional Review Board (no. CHAMC 2019–08–025) and Hallym Dongtan Sacred Hospital Institutional Review Board (no. 2023-05-003). The aims and the methods of the study were verbally explained to the participants. Participation in the study was voluntary, and participants could withdraw from the study at will. They were assured of the confidentiality of their data. Written informed consent was obtained from all the participants.
Competing interests
Mr. Jong Chan Kim and Guk Bae Kim are research workers at Anymedi Inc., which is developing 3D-printing products related to the research being reported. Drs. Suk Pyo Shin, Kyong Joo Lee, Min Je Sung, Moo Yeop Kim, Sung Yong Han, Sung Ill Jang, Mamoru Takenaka and Chang-Il Kwon have no conflicts of interest or financial ties to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary Material 2
Supplementary Material 3
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shin, S.P., Lee, K.J., Sung, M.J. et al. Endoscopic retrograde cholangiopancreatography training using a silicone simulator fabricated using a 3D printing technique (with videos). Sci Rep 15, 2619 (2025). https://doi.org/10.1038/s41598-025-86755-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-86755-9
Keywords
This article is cited by
-
Endoscopic retrograde cholangiopancreatography consultation after digestive tract reconstruction and risk factors for complications
European Journal of Medical Research (2025)