Abstract
Parkinson’s disease (PD) is a complex neurodegenerative disorder that affects multiple neurotransmitters, and its exact cause is still unknown. Developing new drugs for PD is a lengthy and expensive process, making it difficult to find new treatments. This study aims to create a detailed dataset to build strong predictive models with various machine learning algorithms. An ensemble modeling approach was employed to screen the DrugBank database, aiming to repurpose approved medications as potential treatments for Parkinson’s disease (PD). The dataset was constructed using pIC50 values of various compounds targeting the inhibition of leucine-rich repeat kinase 2 (LRRK2). The best ensemble model showed exceptional predictive performance, with five-fold cross-validation and external validation metrics exceeding 0.8 (Q2cv = 0.864 and Q2ext = 0.873). The DrugBank screening resulted in three promising drugs—triamterene, phenazopyridine, and CRA_1801—with predicted pIC50 values greater than 7, warranting further investigation as novel PD treatments. Molecular docking and molecular dynamics simulations were performed to provide a comprehensive understanding of the interactions between LRRK2 and the inhibitors in the data set and best molecules of the screening. Free energy of binding calculation along with hydrogen bond occupancy analysis and RMSD of the ligand in the pocket show CRA_1801 as the best candidate to be repurposed as LRRK2 inhibitor.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder that affects approximately 10 million people worldwide. It is characterized by the loss of dopaminergic neurons in the substantia nigra. PD is a multifactorial disease caused by a combination of genetic and environmental factors like head injury and exposure to certain chemicals1. However, its underlying cause, as well as the mechanisms by which it progresses, remain unknown2,3. PD involves several neurotransmitters3 including dopaminergic and non-dopaminergic agents3,4,5, which makes it a complex disorder. In addition, the knowledge of molecular targets to treat PD is narrow and incomplete2. Consequently, developing treatments that offer best results is challenging. In this context, there is an unmet need to discover new compounds with effective pharmacological profiles to control Parkinson’s rapid progression.
Current medicines for PD are limited, fail to stop its evolution, and cause unpleasant side effects. Most focus only on alleviating symptoms and slowing down the disease2, instead of addressing a solution for the root problem6. One of the most used medicines is a combination of levodopa (L-DOPA) and a monoamine oxidase B (MAO-B) inhibitor7. However, L-DOPA gradually loses its efficacy, causes side effects like dyskinesia4, and may worsen symptoms like hallucinations and dysregulation syndrome5. In the same way, MAO-B inhibitors cause hepatotoxicity and the so-called cheese effect8. Moreover, the combination of different compounds can lead to drug-drug interactions, resulting in severe side effects, which in turn reduces patients’ quality of life. Additionally, the development of new medicines is a lengthy and costly process, largely due to the challenges in creating accurate disease models.
In the last two decades, some in silico, in vitro, and in vivo studies have been conducted to target different enzymes involved in PD. To this extent, several molecular targets have been of interest as antiparkinsonian agents. One is the adenosine A2A receptor (AA2A), which belongs to the A family of the G-protein-coupled receptors (GPCRs)2,7,9. Various compounds, such as 1,3,5-triazine-thiadiazole hybrids10,11, 2-benzylidene-1-indanone, and -tetralone derivatives10,11, have been investigated as potent inhibitors of this enzyme. Similarly, monoamine oxidase B (MAO-B) is a significant target due to its crucial role in the deamination of dopamine, which triggers a series of events leading to the development of Parkinson’s disease (PD)12. Moreover, MAO-B inhibitors have demonstrated neuroprotective properties3, making them promising candidates for treatment. Consequently, scientists are actively seeking novel, selective, and reversible inhibitors of this target enzyme.
Recent investigations have focused on acacetin 7-methyl ether13, rutamarin14, coumarins 15 and derivatives from isocarboxazid8, 4-(3-Nitrophenyl)thiazol-2-ylhydrazone16, indole-substituted benzothiazoles and benzoxazoles17, (S)−2-(benzylamino)-propanamide18, and eugenol19, and others.
Researchers have also focused on leucine-rich repeat kinase 2 (LRRK2) as a target for Parkinson’s disease (PD). Mutations in the LRRK2 gene have been linked to familial PD and are a significant genetic risk factor for sporadic PD, as they cause damage to dopaminergic neurons6,20,21. Consequently, it has been hypothesized that inhibiting this enzyme could address a fundamental cause of the disorder and potentially slow its progression by promoting adult neurogenesis 6,21. In this context, Type II kinase inhibitors, specifically derivatives of 5-azaindazole6 and indolinone21, have been investigated for their therapeutic potential.
In the quest for new drugs, in silico studies have become increasingly popular among researchers not only because it allows to screen big database in reduced times and at a lower cost but also due to the increased accuracy of current methods. Combining the quantitative structure-activity relationship (QSAR) approach with techniques like pharmacophore analysis, molecular docking, and molecular dynamics has shown promise for various diseases, including type 2 diabetes 22, primary hyperoxaluria type 1 (PHT1)23, tuberculosis24, drug-induced liver injury25, SARS-CoV-226, and cancer27. Machine learning methods have played a pivotal role in drug repurposing since they allow researchers to identify the most promising drug candidates for the novel application and prioritize their experimental validation in preclinical and clinical trials28,29. This approach has also been applied in studies focused on neurodegenerative diseases as the ones reported by Siddiqui, A. et al. Collaborators used machine learning and pharmacophore-based screening techniques, in addition to molecular docking and molecular dynamics simulations to discover new agonist compounds for the GPR40 protein which has a significant role in the development of Alzheimer’s disease29. Similarly, in another study the techniques were applied to find new molecular inhibitors for MAO-B enzyme to treat Parkinson’s and Alzheimer’s disease28. Machine learning models have manage to produce accurate predictions without the use of computer intensive calculations such as quantum mechanics.
However, finding new treatments for Parkinson’s disease (PD) is particularly challenging due to the lack of a comprehensive and diverse dataset needed to build reliable predictive models. This study aims to fill that gap by creating a diverse dataset and developing predictive models using different machine learning algorithms. These models will then be used to screen the DrugBank database to identify existing drugs that could be repurposed as PD treatments. The results will be further validated with molecular docking and molecular dynamics simulations to better understand how these drugs interact with LRRK2. This integrated approach aims to speed up the discovery of effective PD therapies and make the drug development process more efficient and cost-effective.
Methodology
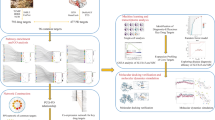
The methodology followed in this study is based on four different approaches of computer-aided drug design (CADD): predictive model development, molecular docking, molecular dynamics, and virtual screening. First, a dataset was constructed based on available information in the literature. Second, the dataset was used as input for the development of possible predictive models. In the same way, the best models were used to perform the virtual screening. Molecular docking and molecular dynamics analyses were executed to get insights into the mode of action of selected compounds. The process is summarized in Fig. 1.
Data set construction
For the construction of the dataset, publications from the last 15 years about Parkinson’s disease treatment and current inhibitors were revised. Then, the biological target and parameters used to measure the inhibition were identified. The collected information was classified according to the target and the parameters used to measure inhibition (Table S1, Supporting Information_1).
Based on the number of inhibitors found for each enzyme and the efficiency of the process, LRRK2 was selected for computational studies. The dataset was polished discarding compounds whose values were not exact or if they came from an article with less than four examples. Finally, a known drug (Sunitinib) was used to normalize the data and used as a reference for the final construction of the dataset.
Modelling process
All molecules were drawn (3D) using GaussView, and optimized using the molecular mechanic level of theory with the Universal Force Field (UFF) parameters23. The ToMoCoMD MIDAS25 software was utilized to compute the 3D topographic descriptors. Initially, an attribute selection process was conducted using specific criteria: a Shannon entropy value of 0.7 for each individual variable and a Pearson correlation coefficient of 0.7 between each pair of descriptors.
Prediction models were built to correlate the descriptors with the pIC50values using different machine learning algorithms available in the Weka software30. Three search methods—Genetic Search (GenSe), Greedy Stepwise (GreedSt), and Best First (BF)—were used with each of the following classifiers of the Wrapper Subset Evaluator: Gaussian Processes (GP), Linear Regression (LR), SMOreg, IBK, and Random Forest (RF). The process was repeated two consecutive times. Models with less than 9 descriptors were selected; partitioned into training and test sets, and their performance was evaluated31. Validation procedures were done by applying the external (Ext) and fivefold cross-validation (CV) methods30. For the case of the best models, a process of hyperparameters optimization was performed to evaluate their effect in the model performance. For the case of the linear regression models, the batchSize [10, 50, 100, 200, 500], and ridge_values = [10−i for i in range (1, 20)] were optimized. For the case of SMOreg and Gaussian Process, the parameters batchSize [10, 50, 100, 200, 500], C (the complexity parameter) in [10−i for i in range(1, 20)], epsilon in [10−ifor i in range(1, 20)], and Kernel [PolyKernel, Puk] were optimized. For the case of the Random Forest, the parameters numTrees in [10, 50, 100], maxDepth in5,10,20, and numFeatures in5,10,20, were optimized. For the case of the IBK, the parameters K_range =1,3,5,7,9,11,13, distanceWeighting_options =1,2 (1 = No, 2 = Weighting), and nearestNeighborsSearch_options [LinearNNSearch, KDTree] were optimized. For each case, a python script was used to run the optimization by Linux terminal.
For the best models, an applicability domain (AD) analysis was performed. The AD is the theoretical range in which predictions are considered reliable and accurate, being defined by the training set of a model23. AD was determined using AMBIT software using the default consensus approach and 4 methods i.e., Range, Euclidean distance, City-block, and probability density23.
An ensemble model was built by taking the average of the predicted pIC50 values of the best two models and plotting them against the experimental values of the training and test sets.
DrugBank screening
The DrugBank database32 was screened using the best two predictive models. Only compounds within the applicability domain were considered and their pIC50 values were predicted through the ensemble model. The compounds with a pIC50 value higher than 7 were selected for further analysis.
Molecular docking and molecular dynamics (MD) studies
For the molecular docking studies, the X-ray crystal structure of human LRRK2 (PDB: 4YZN) was utilized as the target. PyMOL 33was employed to remove water molecules and other co-crystallized entities, including the natural ligand. All LRRK2 inhibitors from the dataset, along with the top compounds from DrugBank, were docked against the enzyme using AutoDock Vina software34. AutodockTools was used to add polar hydrogens and convert the structures to .PDBQT format23. The coordinates for the calculations were centered on the enzyme’s active site (x= −8.308, y = 16.203, z = 18.565) with a grid box size of 12, 14, and 14 Å for the x, y, and z axes, respectively.
To execute the molecular dynamics (MD) simulations, the compounds from the constructed dataset (Table S2, Supporting Information_2) were ordered based on decreasing pIC50values, and a total of 10 molecules (1_31, 3_7, 1_34, 1_22, 1_26, 2_39, 2_42, 2_47, 1_15, 1_1) were randomly taken from the upper, middle and lower part of the list. The starting structure for this study was obtained from the docking calculations. The topology of the enzyme was constructed using the AMBER99SB-ILDN forcefield employed in Gromacs 2019, while the ligands’ topology was built via ACPYPE server and Generalized Amber Force Field (GAFF)23,35,36. A tree-point water model (TIP3) and a cube-shaped solvation system were used for the system. The system was neutralized and then, equilibrated for 100 ps with a constant number of particles, volume, and temperature (NVT). Subsequently, a constant number of particles, pressure, and temperature (NPT) were used for a second equilibration23,37. Parameters such as potential energy, density, pressure, temperature, and box size were evaluated to spot possible errors during the simulation. In the same way, the Lennard-Jones potential, number of hydrogen bonds, root-mean-square deviation, and other parameters were obtained for the analysis. Additionally, the binding free energy was calculated using the mmpbsa approach by adding the vacuum molecular mechanics potential energies (EMM), the polar solvation energy, and the non-polar solvation energy. Energies were obtained by taking snapshots every 5 ns between 10 and 200 ns using the g_mmpbsa tool38.
Results and discussions
During the literature revision, three enzymes stood as targets of interest in PD treatment (AA2A, MAO-B, and LRRK2). Ki and IC50 were the main parameters used to test biological activity. However, reported values for some of the molecules were inexact; therefore, those compounds were discarded from the dataset. A histogram was constructed based on the information gathered (Figure S1). The category with the greatest number of molecules is the inhibition of MAO-B measured in terms of IC50values. In contrast, just a few studies used Ki to measure the inhibition of this enzyme. Although the number of compounds studied as LRRK2 inhibitors is inferior to MAO-B and AA2A, it is equally reliable and was preferred over them due to their chemical diversity and the use of multi-specific tyrosine kinase inhibitor Sunitinib6,21,39 as control.
More than fifty subsets of descriptors were found with different combinations of machine learning techniques and searching methods. From those, fifteen individual models (M1-M15) with less than 9 descriptors and a QCV2 > 0.7 were selected for further studies (Table S3, Supporting Information_2). Data was partitioned in training (75%) and test (25%) sets, and external and cross-validations performed. The best two models were identified and selected based on their QCV2 and QExt2 values (> 0.79; Table 1). Furthermore, both models present MAE values lower than 0.4.
The AD of models M1 and M10 was obtained where all molecules from the test set entered IN the AD domain. The performance of models M1 and M10 was analyzed by plotting the experimental pIC50 values vs the predicted obtaining Q2CV and Q2EXT (Figure S2). The optimum hyperparameters for M1 are: {R = ridge = 0.1 and batchSize = 100}, and for M10 are: {‘C’: 1.0, ‘EpsilonParameter’: 0.1, ‘BatchSize’: 20, ‘Kernel’: ‘PolyKernel’}.
Model M1, created using a multiple linear regression algorithm, was validated using Tropsha’s test24,40 (Table S4, Supporting Information_2). The assessment of all statistical parameters confirmed the robustness and predictive reliability of the constructed model. It is important to note that Tropsha’s test is specifically performed for models utilizing multiple linear regression algorithms. Consequently, this test was not applied to model M10, which employs the SMOreg algorithm.
To ensure the absence of redundancy or overfitting in the models, the collinearity between descriptors of M1 and M10 was analyzed. A Pearson coefficient of less than 0.7 between descriptors was set as the baseline to confirm the lack of collinearity between features23. The results indicated that all descriptors had a correlation coefficient lower than 0.6 (Fig. 2), thereby confirming the strength of the models. Detailed information on the descriptors, including their full names and occurrences, can be found in the supplementary information (Table S5, Supporting Information_2).
The descriptors were also analyzed by their number of appearances in the models. Between models M1 and M10, there is only one common descriptor (S_NS_p_MID), indicating diversity between the models. Analyzing S_NS_p_MID, it is also present in two of the remaining best 15 models. Similarly, descriptor GV[5]_KA_ps_MID, is present in 40% of the best models, suggesting the potential surface is key in predicting the pIC50 value in this system.
Other properties like softness (s), hardness (h), polarizability (p), electronegativity (e), Van der Waals volume (v), charge (c), and molecular weight (m) were also present in the descriptors of the best models. These parameters have been analyzed in models built for numerous diseases22,23,25. It should be remarked that the property most frequently evaluated by the descriptors of M1 and M10 is polarizability. This is probably due to the presence of highly electronegative atoms like oxygen, nitrogen, and halogens in the inhibitors and their influence on binding.
The diversity of descriptors suggests that an ensemble model could be useful to increase the prediction capacity by analyzing a wider range of structural and physicochemical properties. The results of the ensemble model were obtained by computing the average predicted value of M1 and M10 and showed an enhanced predictability compared to each model; with cross and external validation coefficients of 0.864 and 0.873 respectively (Fig. 3).
DrugBank screening
The screening of the DrugBank database resulted in a list of approved and experimental drugs that could be explored as potential new treatments for PD. The ensemble model was used to predict the pIC50 values of the different drugs. Sixteen compounds entered the AD, where four had a pIC50 greater than 7. Three exhibited properties that make them suitable for a second application as PD treatment (Table 2). Triamterene (pIC50pred = 7.22) and Phenazopyridine (pIC50pred = 7.41) were further studied as potential alternatives due to their pharmacological and toxicological properties. Although CRA_1801 (pIC50pred= 7.67) is an experimental drug and information on its application is not available, it was considered an option for further studies. Antibiotics were not considered in the study due to antimicrobial resistance41,42.
Early this year (2024), Sun and colleagues published a study of the pharmacology of LRRK2 inhibitors using cryo-EM45. Four inhibitors derived from this research i.e., GNE-7915, GZD-824, LRRK2-IN-1, and ponatinib were run in our model. Results show all four compounds present high pIC50 values (8.30, 7.45, 8.04, and 7.43 respectively) serving as an extra validation of the robustness and accuracy of our model to predict possible LRRK2 inhibitors.
Molecular docking
Molecular docking was used to study the possible binding mode between LRRK2 and the ligands in this study. The results from the docking were then used as a starting point in molecular dynamic simulations. The docking methodology was validated by comparing the pose of the co-crystallized inhibitor obtained experimentally with the docking result of the same molecule. As seen in Figure S3, both structures mostly overlap with each other. The morpholine ring is shifted which can be expected and does not affect the accuracy as this part of the molecule is located outside the active site and is flexible.
Once the docking was validated, it was used to dock all the compounds in this study. The results showed that all the compounds fitted inside the active site of LRRK2. Two of the compounds exhibited the same docking scores as LRRK2-IN-1 (1_28 and 1_35; −8.6 kcal/mol), and four of them (1_30, 1_29, 1_33, and 3_12) presented more negative docking scores (from − 8.7 to −9.2 kcal/mol). Plotting the docking scores against the pIC50 values, an R2 of 0.13 was found with no correlation. Regarding the drugs from the DrugBank database, triamterene, phenazopyridine, and CRA_1801 present values of −7.3, −6.4, and − 7.6 kcal/mol, respectively.
ADMET properties
All the molecules selected for molecular dynamics simulation were also used to predict their ADMET properties using the Swiss ADME web server (accessed 21 March 2024)46. Solubility and lipophilicity were evaluated as those are key parameters to assess administration and absorption profiles in the body (Table S6, Supporting Information_2). All molecules showed good solubility using the ESOL 47and Ali 48models. Only half of the compounds showed good solubility using the SILICOS-IT 46 model while the other half were classified as moderately soluble. The lipophilicity measured as the logarithm of the coefficient between n-octanol and water showed seven ligands with values around 2. Interestingly, from the molecules in the dataset, only 2_42 presents a lipophilicity below 1. The molecules in the DB screening present values lower than the ones in the dataset with even negative values for triamterene.
A Boiled-Egg graph can be obtained by plotting lipophilicity against the topological polar surface area (Figure S4). Molecules inside the yolk are predicted to have brain penetration while the ones in the white gastrointestinal absorption. Six molecules (1_1, 1_15, 1_22, 1_26, 1_34, 2_39) present brain penetration while four gastrointestinal absorptions (2_42, 3_7, CRA_1801, Phenazopyridine). As expected, due to its Log Po/w value, Triamterene was predicted to have poor gastrointestinal absorption which hinders its oral administration.
Molecular dynamics studies
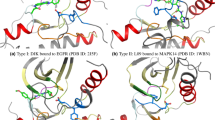
To get insights into the inhibition mechanism of LRRK2, molecular dynamic simulations were performed. First, ten random molecules with different pIC50 values were chosen from the database followed by three molecules derived from the DrugBank screening. Before simulating their behavior, it is worth analyzing the crystal structure of the enzyme and the binding mode of the co-crystallized inhibitor. Looking at the binding mode of the inhibitor, most of the interactions between the ligand and the active site are hydrophobic, except for a double hydrogen bond formed by the ligand with the backbone of valine 1108 (Fig. 4a). The active site is mostly formed by hydrophobic amino acids (I1032, V1040, A1053, V1091, M1105, L1107, and L1161) represented in Fig. 4b49.
Analyzing the simulations for the molecules in the dataset, an RMSD value between 0.2 and 0.4 nm for LRRK2 may indicate that the protein does not change its conformation when binding all the other inhibitors compared with the co-crystallized one (Fig. 5a). Looking at the ligands’ RMSD (Fig. 5b), none of the values is higher than 0.22 nm, suggesting the 3D conformation found during the docking stays stable throughout the simulation.
The trajectory of the simulation over 200 ns was analyzed to evaluate the behavior of various ligands. Most ligands remained within the active site of LRRK2 throughout the simulation, except for molecule 1–26 (Fig. 6a). The initial conformation of molecule 1–26 revealed that its polar groups were not oriented towards V1108, highlighting the critical role of this hydrogen bond in maintaining the ligand’s stability within the open active site of LRRK2 (Fig. 6b).
The Hydrogen Bonds (HBs) were analyzed in the 200ns of simulation (Fig. 7). Interestingly, compounds such as 1–15 that form 2 HBs through most of the time of simulation, and compounds such as 1–34 that lose all of their HBs in most of the last 100 ns of simulation manage to stay in the active site suggesting the importance of hydrophobic interaction for stability. Still, it is also true that losing HBs allows more movement in the active site compared to when the HBs are stable throughout the simulation (1–22 vs. 1.26; Figure S5).
Finally, the free binding energy was calculated for all the molecules except 1–26 because it leaves the active site (Table 3). The result shows a positive interaction for all the compounds with negative binding energies.
The same analysis was done on the best 3 compounds derived from the DrugBank database. RMSD analysis of the enzyme shows greater conformational changes in one of the three ligands i.e. phenazopyridine with values around 0.35 nm (Fig. 8a). For the ligands (Fig. 8b), all of the molecules behave similarly to the ligands in the database.
Following the trajectory throughout the simulation shows that compound phenazopyridine and triamterene leave the active site during the simulation. In the case of phenazopyridine, it leaves the active site after 55 ns of simulation while for triamterene this happens at 85 ns. An HB analysis shows CRA_1801 presents the greatest number of HBs throughout the simulation agreeing with the trajectory result and its ability to stay in the open active site throughout the simulation (Fig. 9).
A free binding energy of −142.50 kcal/mol was obtained for CRA_1801 indicating a positive interaction between the ligands and the enzyme throughout the simulation. Phenazopyridine and triamterene can be used for repurposing due to their local anesthetic and diuretic pharmacological activities, although their lack of interaction during the simulation may indicate they can act as lead compounds to produce new LRRK2 inhibitors from their backbone.
Finally, a HB occupancy analysis was done on CRA_1801 and compound 3–7 (Fig. 10) which has the highest experimental pIC50 value from the training set. This analysis shows the percentage of time that a HB between the ligand and certain amino acid is formed during the time of simulation. In this regard, largest occupancies mean tighter interactions. Results show CrA_1801 makes double the HB compared to compound 3–7. Interestingly, V1108 is in the top occupancies for both compounds showing its importance for inhibition. Due to all the polar groups CRA_1801 presents, it manages to make more interactions, specially with K1084, E1106, and P1158, which may be interesting to study further to assess their importance in inhibition.
Experimental drug CRA_1801 (DB03016) is suggested to be the best candidate to be tested as an LRRK2 inhibitor in experimental in vitro studies. Interestingly, comparing the structure of CRA_1801, phenazopyridine, and triamterene against the co-crystallized inhibitor (Fig. 11); the four structures present nitrogenates heterocycles that are likely to create HBs with V1108 being able to inhibit LRRK2.
Conclusions
The dataset proposed in this study facilitated the development of an ensemble prediction model capable of identifying potential LRRK2 inhibitors. Model M1, comprising seven descriptors, demonstrated reliable prediction of pIC50 values within its applicability domain. The model exhibited strong predictive performance, with internal fivefold cross-validation, leave-one-out (LOO) method, and model equation yielding determination coefficients (R2 and Q2) greater than 0.79. Additionally, it achieved an impressive prediction accuracy for the test set, with a QExt2 of 0.795. The model’s robustness and consistency were further validated by passing all parameters in Tropsha’s test. Similarly, Model M10, which includes nine descriptors, showed excellent predictability for the test set with a QExt2 of 0.803. The lack of significant correlation between the descriptors of M1 and M10 (correlation coefficient < 0.6) indicated no redundancy or overfitting. Notably, only one of the seven descriptors in M1 overlapped with those in M10, suggesting that the ensemble model enhances predictive capacity (QExt2 = 0.873).
The DrugBank database screening identified three existing drugs—triamterene, phenazopyridine, and CRA_1801—as potential new treatments for PD, based on their predicted pIC50 values. Although their docking scores and molecular dynamics studies were not favorable for triamterene and phenazopyridine, these compounds warrant experimental testing as LRRK2 inhibitors to assess their feasibility as PD therapies. Furthermore, they may be used as lead compounds requiring several rounds of optimization. Additionally, CRA_1801 emerged as a promising candidate due to its high pIC50 value, negative free binding energy, and the long-lasting HB formed during the simulation.
Molecular docking and molecular dynamics simulations provided valuable insights into the ligand-enzyme interactions in this type of system where V1108 seems to be important for inhibition. Despite limited information on the binding mechanisms, these computational techniques offer a deeper understanding of the inhibition process. Continued research in this area holds promise for the development of more effective treatments for Parkinson’s disease.
Data availability
All data used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Parkinson’s Foundation, U. S. A. (2023). https://www.parkinson.org/understanding-parkinsons/causes. Accessed 9 Sep 2024.
Koszła, O. et al. Current approaches and tools used in drug development against parkinson’s disease. Biomolecules 11, 1–16. https://doi.org/10.3390/biom11060897 (2021).
Schapira, A. H. V., Bezard, E., Brotchie, J. & Calon, F. Novel pharmacological targets for the treatment of Parkinson ’ s disease. 5:845–854. (2006). https://doi.org/10.1038/nrd2087
Loza, M. I. & Carlsson, J. Docking screens for dual inhibitors of Disparate Drug targets for Parkinson ’ s Disease. (2018). https://doi.org/10.1021/acs.jmedchem.8b00204
Meissner, W. G. et al. Priorities in Parkinson ’ s disease research. 10:. (2011). https://doi.org/10.1038/nrd3430
Osborne, J. et al. Discovery of potent and selective 5-azaindazole inhibitors of leucine-rich repeat kinase 2 (LRRK2) – part 1. Bioorg. Med. Chem. Lett. 29, 668–673. https://doi.org/10.1016/j.bmcl.2018.11.058 (2018).
Cruz-Vicente, P., Passarinha, L. A., Silvestre, S. & Gallardo, E. Recent developments in new therapeutic agents against alzheimer and parkinson diseases: In-silico approaches. Molecules 26 https://doi.org/10.3390/molecules26082193 (2021).
Agrawal, N. & Mishra, P. Synthesis, monoamine oxidase inhibitory activity and computational study of novel isoxazole derivatives as potential antiparkinson agents. Comput. Biol. Chem. 79, 63–72. https://doi.org/10.1016/j.compbiolchem.2019.01.012 (2019).
Masih, A. et al. Design and development of 1,3,5-triazine-thiadiazole hybrids as potent adenosine A2A receptor (A2AR) antagonist for benefit in Parkinson’s disease. Neurosci. Lett. 735, 135222. https://doi.org/10.1016/j.neulet.2020.135222 (2020).
Van Rensburg, H. D. J., Legoabe, L. J., Terre’Blanche, G. & Van Der Walt, M. M. 2-Benzylidene-1-Indanone analogues as dual Adenosine A 1 /A 2a receptor antagonists for the potential treatment of neurological conditions. Drug Res. 69, 382–391. https://doi.org/10.1055/a-0808-3993 (2019).
Van Janse, H. D., Legoabe, L. J. & Terre’Blanche, G. Synthesis and structure Activity relationships of Chalcone based Benzocycloalkanone Derivatives as Adenosine A 1 and/or a 2A receptor antagonists. Drug Res. 70, 243–256. https://doi.org/10.1055/a-1146-2996 (2020).
Naidoo, D. et al. New role for crinamine as a potent, safe and selective inhibitor of human monoamine oxidase B: in vitro and in silico pharmacology and modeling. J. Ethnopharmacol. 248, 112305. https://doi.org/10.1016/j.jep.2019.112305 (2020).
Chaurasiya, N. D. et al. Selective inhibition of human monoamine oxidase B by Acacetin 7-Methyl ether isolated from Turnera diffusa (Damiana). Molecules 24, 1–15. https://doi.org/10.3390/molecules24040810 (2019).
Kozioł, E. et al. Rutamarin: efficient liquid–liquid chromatographic isolation from ruta graveolens L. and evaluation of its in vitro and in silico MAO-B inhibitory activity. Molecules 25, 1–12. https://doi.org/10.3390/molecules25112678 (2020).
Rizakov, A. Z., Kolev, M. K. & Velkov, Z. A. QSAR analysis of coumarins, flavones and their bicyclo ethers as monoamine oxidases inhibitors. Bul. Chem. Commun. 52, 93–100. https://doi.org/10.34049/bcc.52.A.330 (2020).
Secci, D. et al. 4-(3-Nitrophenyl)thiazol-2-ylhydrazone derivatives as antioxidants and selective hMAO-B inhibitors: synthesis, biological activity and computational analysis. J. Enzyme Inhib. Med. Chem. 34, 597–612. https://doi.org/10.1080/14756366.2019.1571272 (2019).
Nam, M. H. et al. Indole-substituted benzothiazoles and Benzoxazoles as selective and reversible MAO-B inhibitors for treatment of Parkinson’s Disease. ACS Chem. Neurosci. 8, 1519–1529. https://doi.org/10.1021/acschemneuro.7b00050 (2017).
Jin, C. F. et al. Computational fragment-based design facilitates Discovery of Potent and Selective Monoamine Oxidase-B (MAO-B) inhibitor. J. Med. Chem. 63, 15021–15036. https://doi.org/10.1021/acs.jmedchem.0c01663 (2020).
Dhiman, P., Malik, N. & Khatkar, A. Lead optimization for promising monoamine oxidase inhibitor from eugenol for the treatment of neurological disorder: synthesis and in silico based study. BMC Chem. 13, 1–20. https://doi.org/10.1186/s13065-019-0552-4 (2019).
Khamouli, S. et al. Multi-combined 3D-QSAR, docking molecular and ADMET prediction of 5-azaindazole derivatives as LRRK2 tyrosine kinase inhibitors. J. Biomol. Struct. Dyn. 40, 1285–1298. https://doi.org/10.1080/07391102.2020.1824815 (2020).
Salado, I. G. et al. Leucine rich repeat kinase 2 (LRRK2) inhibitors based on indolinone scaffold: potential pro-neurogenic agents. Eur. J. Med. Chem. 138, 328–342. https://doi.org/10.1016/j.ejmech.2017.06.060 (2017).
Cabrera, N. et al. In Silico Searching for alternative lead compounds to treat type 2 diabetes through a QSAR and Molecular Dynamics Study. Pharmaceutics 14 https://doi.org/10.3390/pharmaceutics14020232 (2022).
Cabrera, N. et al. Searching glycolate oxidase inhibitors based on QSAR, molecular docking, and molecular dynamic simulation approaches. Sci. Rep. 12, 1–19. https://doi.org/10.1038/s41598-022-24196-4 (2022).
Valencia, J. et al. QSAR Studies, Molecular Docking, Molecular Dynamics, Synthesis, and Biological evaluation of Novel Quinolinone-based thiosemicarbazones against Mycobacterium tuberculosis. Antibiotics 12 https://doi.org/10.3390/antibiotics12010061 (2023).
Mora, J. R. & Marrero-ponce, Y. Ensemble models based on QuBiLS-MAS features and shallow learning for the Prediction of Drug-Induced Liver toxicity: improving Deep Learning and Traditional Approaches ́. (2020). https://doi.org/10.1021/acs.chemrestox.0c00030
Cuesta, S. A., Mora, J. R. & Márquez, E. A. In silico screening of the drugbank database to search for possible drugs against sars-cov-2. Molecules 26 https://doi.org/10.3390/molecules26041100 (2021).
Sharma, T. et al. Combining structure-based pharmacophore modeling and machine learning for the identification of novel BTK inhibitors. Int. J. Biol. Macromol. 222, 239–250. https://doi.org/10.1016/j.ijbiomac.2022.09.151 (2022).
Siddiqui, A. J. et al. Targeting Monoamine Oxidase B for the treatment of Alzheimer’s and Parkinson’s diseases using novel inhibitors identified using an Integrated Approach of Machine Learning and computer-aided Drug Design. Mathematics 11 https://doi.org/10.3390/math11061464 (2023).
Siddiqui, A. J. et al. A computational and machine learning approach to identify GPR40-targeting agonists for neurodegenerative disease treatment. PLoS One. 19, e0306579. https://doi.org/10.1371/journal.pone.0306579 (2024).
Hall, M. et al. The WEKA data mining software: an update. SIGKDD Explor. 11, 10 (2009).
Tropsha, A. Best practices for QSAR model development, validation, and exploitation. Mol. Inf. 29, 476–488. https://doi.org/10.1002/minf.201000061 (2010).
Wishart, D. S. et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 46, D1074–D1082. https://doi.org/10.1093/nar/gkx1037 (2018).
Schrodinger, L. The PyMOL molecular graphics system, version 1.3r1 (2010).
Version, A. et al. AutoDock Version 4.2. 1–69 (2014).
Sousa da Silva, A. W. & Vranken, W. F. ACPYPE-AnteChamber PYthon Parser interfacE (In: BMC Res Notes, 2012).
Lindorff-Larsen, K. & Al, E. Improved side-chain torsion potentials for the Amber ff99SB protein force field: improved protein sidechain potentials. Proteins 78, 1950–1958. https://doi.org/10.1002/prot.22711 (2010).
Berendsen, H. J. C. et al. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690. https://doi.org/10.1063/1.448118 (1984).
Van Der Spoel, D. et al. GROMACS: fast, flexible, and free. J. Comput. Chem. 26, 1701–1718. https://doi.org/10.1002/jcc.20291 (2005).
Troxler, T. et al. Discovery of novel indolinone-based, potent, selective and brain penetrant inhibitors of LRRK2. Bioorg. Med. Chem. Lett. 23, 4085–4090. https://doi.org/10.1016/j.bmcl.2013.05.054 (2013).
Golbraikh, A. & Tropsha, A. Beware of q2! J. Mol. Graph Model. 20, 269–276. https://doi.org/10.1016/S1093-3263(01)00123-1 (2002).
Kormuth, K. A. & Lakdawala, S. S. Emerging antiviral resistance. Nat. Microbiol. 5, 4–5. https://doi.org/10.1038/s41564-019-0639-7 (2020).
Murray, C. J. et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655. https://doi.org/10.1016/S0140-6736(21)02724-0 (2022).
National Center for Biotechnology Information. PubChem Compound Summary for CID 5546, Triamterene. (2023). https://pubchem.ncbi.nlm.nih.gov/compound/Triamterene. Accessed 24 Apr 2023.
National Center for Biotechnology Information. PubChem Compound Summary for CID 4756, Phenazopyridine. (2023). https://pubchem.ncbi.nlm.nih.gov/compound/Phenazopyridine. Accessed 24 Apr 2023.
Zhu, H., Hixson, P., Ma, W. & Sun, J. Pharmacology of LRRK2 with type I and II kinase inhibitors revealed by cryo-EM. Cell. Discov. 10, 10. https://doi.org/10.1038/s41421-023-00639-8 (2024).
Daina, A., Michielin, O. & Zoete, V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 7 https://doi.org/10.1038/srep42717 (2017).
Delaney, J. S. ESOL: estimating aqueous solubility directly from molecular structure. J. Chem. Inf. Comput. Sci. 44, 1000–1005. https://doi.org/10.1021/ci034243x (2004).
Ali, J. et al. Revisiting the general solubility equation: in silico prediction of aqueous solubility incorporating the effect of topographical polar surface area. J. Chem. Inf. Model. 52, 420–428. https://doi.org/10.1021/ci200387c (2012).
Eisenberg, D., Schwarz, E., Komaromy, M. & Wall, R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J. Mol. Biol. 179, 125–142. https://doi.org/10.1016/0022-2836(84)90309-7 (1984).
Acknowledgements
The authors are grateful to the USFQ-POLI grants 2025 for financial support. The authors have used the high-performance computing (HPC) system available in the USFQ and Uninorte, for the development of this project.
Author information
Authors and Affiliations
Contributions
M.C.G, S.A.C.: Conceptualization, Methodology, Software J.R.M, Y.M, J.L.P.: Data curation, Writing- Original draft preparation. F.A., E.A.M, J.R.M.: Visualization, Investigation. J.R.M.: Supervision. E.A.M.: Software, Validation.: S.A.C., F.A., J.R.M.: Writing- Reviewing and Editing,
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
García, M.C., Cuesta, S.A., Mora, J.R. et al. Using computer modeling to find new LRRK2 inhibitors for parkinson’s disease. Sci Rep 15, 4085 (2025). https://doi.org/10.1038/s41598-025-86926-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-86926-8