Abstract
Maternal adverse childhood experiences (ACEs) are linked to negative health and developmental outcomes in offspring. However, whether maternal ACEs influence infant weight gain in the first months of life, and if this effect differs by infant sex, remains unclear. This study included 352 full-term newborns from low-risk pregnancies and their mothers in low-income settings in Brazil. Anthropometric data (weight, length, head circumference) and other information (feeding type, offspring sex, family income) were collected at delivery (W0), discharge (W1), and up to 8 weeks postpartum (W2). ACEs were assessed using the CDC-Kaiser Questionnaire, and weight gain was calculated as the difference between W2 and W1, divided by the number of days between measurements. The association between maternal ACEs and offspring weight gain was positive only in male offspring (unstandardized coefficient (male) = 1.82, SE = 0.438, p < 0.001); for each 1-point increase in the ACEs score (e.g., from 0 to 1), weight gain increased by 1.8 g/day. These findings indicate that maternal ACEs are associated with increased weight gain in male infants during the first two months of life, potentially increasing the risk of future obesity. Further research is required to investigate the underlying biological mechanisms and their neurodevelopmental implications.
Similar content being viewed by others
Introduction
Adverse childhood experiences (ACEs) encompass potentially traumatic situations, including physical, psychological, and sexual abuse; neglect; parental mental illness; scarcity of resources; and family violence1,2,3,4. Adversities experienced during childhood can impact personal development5,6, further extending beyond directly-exposed individuals, as the impact on an exposed parent can be passed onto the offspring7,8. Studies of White populations have indicated an impact of maternal ACEs on weight gain at 5 and 12 months of age9, obesity risk at 2 and 5 years of age10, and inflammatory markers at 9 years11. Another study evaluating growth trajectories in Brazilian children revealed an increase in the prevalence of overweight and obesity from 10.9 to 11.8% in boys < 5 years and from 9.6 to 10.5% in girls < 5 years; and from 26.8 to 30.0% in boys > 5 years and from 23.9 to 26.6% in girls > 5 years12. This study further identified diet as an important contributing factor, warning of the increase in consumption of ultra-processed foods in recent years, particularly in low- and middle-income countries due to their low cost12,13. Excessive weight gain during early childhood is associated with a higher risk of obesity and chronic diseases in adulthood13,14,15. Obesity is a condition resulting from a disrupted energy balance, which is regulated by neural circuits that begin to develop during pregnancy, and mature in the early postnatal periods, when the brain is highly plastic and sensitive to the environment16. Studies have shown that obesity from the earliest stages can influence neurodevelopment, increasing the risk of neuropsychiatric disorders throughout life17.
Rapid infant weight gain is defined as weight gain above expected values in a short time, and is strongly related to obesity in adulthood13,14,15. Evidence has suggested that rapid weight gain may be associated with early formula feeding, either exclusively or combined with breastfeeding15. One literature review showed that weight gain in the first few days of life differed by sex, with boys gaining approximately 3 g/day more than girls according to the growth z-score used by the World Health Organization18,19.
One study performing prenatal follow-up of pregnant women in the UK, revealed that the risk of obesity can vary between boys and girls, even after adjusting for factors such as low dairy consumption, cotinine levels in the blood of non-smokers, low richness of facilities, and green spaces during pregnancy20. This study further showed that an environment combining these four exposures specifically protects girls against obesity and neurodevelopmental delays, indicating that combining different exposures in multi-exposure profiles, using causal inference, could be useful for identifying at-risk populations20.
Maternal stress can interfere with pre and postnatal development21,22. Two prior studies evaluating the impact of maternal trauma on offspring body size in the first year of life in a cohort of 100 mother-infant dyads from Poland demonstrated an increase of 10% in weight and 2% in head circumference in the children of mothers with higher levels of trauma at 5 and 12 months compared to children of mothers with low trauma levels9,21. Although these previous studies did not report sex differences, other studies have established that male and female fetuses show different responses to challenges faced during pregnancy, such as maternal anxiety and increased cortisol22,23. The scientific literature has identified specific sex-based differences starting from embryonic development, with evidence showing that the placenta in male and female fetuses regulates and expresses genes and proteins differently, thereby directly influencing fetal neurodevelopment24,25,26. Male offspring of anxious pregnant women have higher fetal and birth weights than female offspring as compared to those born to non-anxious mothers, and the same trend was also observed in the first month of life22,23. Evidence has also suggested that pregnant women who have experienced ACEs are more likely to develop anxiety during pregnancy, while their offspring are more vulnerable to the effects of the intergenerational transmission of the trauma27. However, no study has yet investigated the effects of maternal ACEs on the weight gain of the offspring in the first months of life according to infant sex.
Studies of samples in Brazil, a low-middle-income country (LMIC), showed that > 80% of inhabitants experienced one or more ACEs throughout their lives, which is higher than the rates reported in developed countries (under 50%)28,29,30,31. According to the Brazilian Institute of Geography and Statistics32, the Brazilian population predominantly comprises individuals of black or multiracial ethnic backgrounds. In the Brazilian context, socioeconomic and racial profiles are important factors influencing the occurrence of ACEs, with one study showing that black and multiracial children aged < 18 years were more likely to have ≥ 4 ACEs compared with white individuals28.
Recent reports (2020–2023) by Brazilian public security agencies on violence against children and adolescents (0–17 years) revealed an increase in the reporting of the incidence of these practices33,34. Further, reports of child neglect and child maltreatment increased by 14% and 13%, respectively34. Nevertheless, studies investigating correlations between maternal ACEs and gestational outcomes remain scarce, particularly in LMIC, including Brazil, with admixed populations and different vulnerabilities. Thus, our sample has different racial and socioeconomic features than previous studies evaluating ACEs, which predominantly involved high-income and predominantly White populations35. Therefore, this study aimed to evaluate whether maternal ACEs are associated with offspring weight gain in the first 2 months of life, and if such an association depends on the offspring’s sex.
Methods
Sample
The maternal inclusion criteria were as follows: (1) living at high-risk and low-resource settings in the cities of Guarulhos and São Paulo (Brazil); (2) users of the Brazilian public healthcare system (Sistema Único de Saúde, SUS); (3) age 18–38 years old; (4) between 25th–39th gestational weeks; (5) able to read and understand the informed consent form and to provide written informed consent for partaking in the study.
The maternal exclusion criteria were: (1) high-risk pregnancy; (2) BMI (Body Mass Index) > 30; (3) severe psychiatric conditions (e.g. schizophrenia, persistent delusional disorder, bipolar disorder, obsessive–compulsive disorder, dementia, and suicidal ideation); (4) history of head trauma with brain injury, treatment for epilepsy, and/or neurosurgery; (5) decompensated clinical diseases requiring intensive treatment; (6) illicit drug use (except cannabis); (7) presence of toxoplasmosis, other, rubella, cytomegalovirus, and herpes (also known as TORCH) infections.
Offspring were enrolled at birth after their mothers confirmed their interest in continuing the study. The exclusion criteria for offspring were: (1) prematurity (born before the 37th gestational week), (2) low-weight birth (> 2.5 kg), (3) 5-min APGAR score < 7, (4) admission to the neonatal intensive care unit, (5) presence of kernicterus or inborn errors of metabolism.
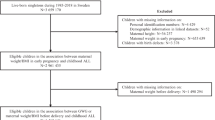
A total of 352 mother–child dyads comprising pregnant women with low-risk pregnancies, both with and without ACEs, and their offspring were enrolled. This research protocol was approved by the Institutional Review Board (IRB) of Duke University (Pro00110664) and Columbia University/New York State Psychiatric Institute (7927). In Brazil, it was sanctioned by the National Research Ethics Committee (CONEP; 78018417.2.0000.5505) and the Research Ethics Committee of Universidade Federal de São Paulo (CEP; 1200/2017). All procedures were conducted in compliance with applicable guidelines and regulations.
In accordance with the principles outlined in the Helsinki Declaration, all maternal participants were provided with comprehensive information regarding the study’s objectives and methodologies, and provided written informed consent for themselves and their newborns to participate. Participants were explicitly informed of their right to withdraw from the study at any stage, with no legal or financial repercussions resulting from their decision to discontinue participation.
Materials and measures
Maternal ACEs, race, maternal educational level, and socioeconomic status
Maternal ACEs were evaluated using the CDC-Kaiser ACE Study Questionnaire2,36, administered at the enrollment. Participants were asked to self-report their own race and their infant’s race, educational and socioeconomic status, assessed using the Brazilian Economic Classification Criteria 201937.
Anthropometric measurements
The following anthropometric measurements of offspring were taken thrice, after birth, immediately after maternity discharge, and during the first 2–8 weeks of life:
Two pediatric neonatologists with more than 10 years of experience in the field performed the following measurements during the first 2 to 8 weeks of the offspring’s life: (1) body length measurement, using an AVANUTRI portable infantometer, (2) body weight measurement, with the infant undressed, using a HOMEIMAGE baby scale model HI-EB522, and (3) head circumference measurement, using a SECA measuring tape, with the glabella and external occipital protuberance as reference points. The intraclass correlations (ICCs) for two-way mixed measures with absolute agreement were calculated. The reliability between the pediatricians’ measurements was high, with an ICC of 0.99 (n = 25; 95% CI 0.99–1.00) for both measurements.
Information regarding the offspring’s birth and maternity discharge, gestational age, birth weight, length, head circumference, and APGAR score, was obtained from the hospital health records, which had been carefully documented by a medical professional. Data on feeding type (breastfeeding, formula feeding, or mixed feeding) was collected through maternal interviews during the pediatric assessment performed between 2 and 8 weeks of life.
The following formula was used to calculate offspring weight gain: W2-W1/number of days, where W1 is the initial weight of the time interval (maternity discharge day), W2 is the final weight of the time interval (2 to 8 weeks of life), and the number of days corresponds to the time between W2 and W138.
Statistical methods
To test the association between maternal ACEs (focal predictor) and offspring weight gain (outcome), as well as the potential interaction effect between offspring sex and maternal ACEs on offspring weight gain, we applied unconditional and conditional models (i.e. a moderation model). This moderation model was used to evaluate the dependence of the magnitude of the association between maternal ACEs and offspring weight gain on offspring sex. To deal with missing data, as a sensitivity analysis, we assumed missing at random mechanisms and handled missing data under the full information maximum likelihood (FIML) using Mplus39,40, a structural equation modeling software. Therefore, we present estimates considering a complete case analysis (e.g., predictors and outcomes must be complete) and under FIML. All descriptive and frequency analyses were performed using SPSS for Windows (version 21.0; SPSS, Chicago, IL), while the interaction model and plot were generated using PROCESS41.
All models were adjusted for maternal education, socioeconomic status, offspring sex, offspring birth weight, day of maternal discharge, age at W2, and feeding type (exclusive breastfeeding as reference category, formula use, and mixed feeding). For statistical modeling, maternal education and socioeconomic are categorical variables. The adopted statistical significance level was set at 0.05, and effect sizes were reported regarding standardized regression coefficients.
Results
In total, 352 full-term newborns (175 males) and their mothers (mean age 27.24 y/o, SD ± 5.15) enrolled in the Mother Influences on Child Biobehavioral Development Study (Healthy MiNDS) were included in this study. Complete case data, including birth weight, day of maternity discharge, weight at W1, age and weight at W2, weight gain, feeding, maternal ACEs, maternal education, and socioeconomic status, were obtained from 320 participants.
Table 1 presents the final data on infant anthropometrics (weight, length, head circumference), feeding type, offspring sex and race, and birth type. Maternal socio-demographic information (ACEs, race, educational level, and family SES) is presented in Table 2.
Unconditional models
The results of the complete case (N = 320) analysis showed that a higher number of reported maternal ACEs was associated with a greater increase in offspring weight gain (1.19 g/day, unstandardized regression coefficient). Overall, male offspring gained an average of 4.98 g/day more than female offspring. Infants who were mixed-fed had a weight gain 4.95 g/day lower than those who were exclusively breastfed. Estimates derived from the FIML approach (N = 352, presented at the bottom of Table 3), align with those from the complete case analysis, further supporting the model robustness (Table 3).
Conditional models
The test for the interaction effect using the complete case analysis approach showed that maternal ACEs and offspring sex could not independently predict offspring weight gain (F(1,309) = 4.45, p = 0.0357, R-square change for the interaction = 0.0128), as the association between maternal ACEs and offspring weight gain was positive only among male offspring (unstandardized coefficient(male) = 1.82, SE = 0.438, p < 0.001). Consequently, the above-described unconditional model could lead to incorrect inferences regarding the relationships between ACEs and sex on weight gain. Consequently, the nuanced association is best captured and interpreted through a conditional model. In other words, the association between ACEs and gained weight is conditioned to the infant’s sex, where the association between ACEs and weight gain is positive only among males, showing that, depending on the sex, the higher the number of ACEs, the larger is the increasing of weight gain. If the ACEs score increases by 1 point (for example, from 0 to 1), weight gain would increase by 1.8 g/day. Among female offspring, the interaction effect was neither significant nor relevant (unstandardized coefficient(female) = 0.49, SE = 0.457, p = 0.285). These results (Fig. 1) showed that the female offspring group had a less stepped line, while the male offspring group showed a positive slope. Interaction effects under FIML were found to converge with complete case analysis (outputs available under request), (Interaction’s regression coefficient = 1.29, p = 0.038).
Discussion
This study showed that the association between maternal ACEs and offspring weight gain during the first two months of life in an admixed population from a LMIC is conditioned to offspring sex with males experiencing greater daily weight gain in the context of maternal ACEs. Additionally, offspring receiving mixed feeding had lower weight gain than those fed via a single method.
The current WHO guidelines indicate that children’s daily weight gain in the first 3 months after birth should range from 25-30 g/day19. However, infants in our cohort had a higher average daily weight gain of 35 g/day, which may be a reflection of the increase in weight gain influenced by maternal ACEs, a factor that could contribute to the development of childhood overweight and obesity.
One prior study involving 100 mother-infant dyads reported that the offspring of mothers who experienced childhood trauma were bigger at 5 and 12 months of age compared to those of mothers without trauma 9. This difference may be attributed to epigenetic changes and the intergenerational transmission of trauma, potentially aligning with theories concerning the pace-of-life syndrome, which posits that hostile and inhospitable environments characterized by threats to life may prompt a faster developmental trajectory23,42,43. It is also important to consider the life history theory, which posits that metabolic resource expenditure can lead to functional trade-offs due to the physiological architecture of the human body, which seeks to maintain homeostasis44,45. In this context, this theory predicts that the early-life signals of mortality or environmental unpredictability may influence faster developmental trajectories, serving as a basis for longitudinal studies investigating the effects of childhood adversities on postnatal outcomes44.
A cohort study conducted on a rural population in Pakistan examined the impact of maternal ACEs (with an average of 2.3 ACEs in the sample) on offspring development at 36 months of age46. This study assessed child growth using WHO z-scores, in addition to fine motor development and receptive language skills, with results indicating that maternal ACEs were associated with better fine motor development and receptive language skills, as well as poorer socio-emotional and behavioral outcomes46. Additionally, the study found that as the number of ACEs increased, the effect on child growth became more pronounced, although no statistically significant relationship was observed in the study sample46. The present study supports this evidence, showing that the impact of maternal ACEs (with an average of 3.5 ACEs in the sample) could intensify with their magnitude, as reflected in the observed increase in infant weight, particularly among males.
Additionally, one study involving African American mother–offspring dyads evaluated the effects of maternal ACEs on the development of psychopathology in offspring, finding that male offsprings were more prone to displaying externalizing symptoms of psychopathologies related to maternal ACEs25. Maternal ACEs can also significantly impact offspring development through changes in the functioning of the hypothalamic–pituitary–adrenal axis and pro-inflammatory cytokines levels11,23,25. Indeed, one recent review indicated that the male offspring of mothers with a history of childhood trauma may be more sensitive to dysregulation of the hypothalamic–pituitary–adrenal axis during fetal development23. As this axis is responsible for integrating diverse functions between the nervous and endocrine systems, this could compromise development and could explain how maternal trauma and stress could interfere with offspring weight gain, possibly contributing to neurophysiological dysfunctions in adult life25. These results are consistent with our findings, showing that male offspring are more sensitive to maternal ACEs, resulting in higher weight gain in early life.
Differences in the regulation and expression of genes, steroids, proteins, and placental structure between males and females are well recognized24,47. Male offspring develop strategies to maintain constant growth in the face of an adverse intrauterine environment. They are more susceptible to environmental effects, such as increased levels of maternal cortisol. Conversely, female offspring can adapt to the inhospitable intrauterine environment, via a mechanism involving growth rate/size reduction without developing Intrauterine Growth Restriction (IUGR), resulting in a higher survival rate24,47,48. This discrepancy contributes to the higher prematurity and fetal mortality rates in male fetuses, which are also impacted by events that may affect mothers throughout life and pregnancy48,49. This potentially explains why maternal ACEs have a greater effect on weight gain in male offspring.
Our data indicate that maternal ACEs are associated with weight gain in male offspring, potentially due to the greater exposure of male fetuses to the effects of maternal ACEs. These results are in line with the life history theory, which posits the existence of a compensatory response to the challenges faced during pregnancy44. This compensatory mechanism could manifest itself in an increase in weight gain during the neonatal period in term newborns. Rapid infant weight gain can have a positive effect in premature newborns; however, in full-term newborns it has been linked with a higher risk of health problems, including obesity50.
Another study evaluating the effects of maternal ACEs, pre-pregnancy, and gestational body mass index (BMI) on the risk of obesity in preschool-aged offspring found that the offspring of mothers who experienced childhood physical abuse were at a higher risk of obesity at 2–5 years of age, regardless of maternal BMI10. A positive correlation has also been found between maternal ACEs, interleukin-6 (a pro-inflammatory cytokine), and obesity in offspring aged 9 years11. Moreover, rapid early weight gain in offspring has been strongly associated with cardiovascular and metabolic risks and childhood obesity. Additionally, maternal ACEs may impact offspring weight gain and the aforementioned outcomes through changes in metabolic pathways, inflammatory factors, glucose metabolism, and adipose tissue formation10,11,51,52. These findings indicate that the effects of maternal ACEs could be important from the early stages of offspring development. In summary, our existing results emphasize the importance of closely caring for and monitoring the offspring of mothers who experienced ACEs, including in the first months of their lives.
The existing literature on the impact of ACEs on adults has revealed sexual and racial disparities in weight gain, with direct implications on obesity53. Notably, the black population is more vulnerable than the white population to these effects. Furthermore, men show an increase in waist circumference and central adiposity compared to women53. This evidence aligns with our findings, which showed that maternal ACEs influence offspring weight gain differently by sex, with males being more susceptible. Additionally, one study conducted in the United States examined the impact of maternal ACEs on birth outcomes54. This study found that women who identified as Black or African-American exhibited a negative association between the age of menarche and infant cephalization, particularly among those with a high number of ACEs. In contrast, women with a low ACE score did not show any such relationship54. This finding further underscores the relevance of our study, which was conducted in Brazil, which encompasses a mixed-race population consisting of approximately 60% women who self-identified as Black or multiracial.
The current consensus is that exclusive breastfeeding in the first 6 months of life is the optimal form of feeding12,14,52. Further, postpartum depression may be indicative of early cessation of exclusive breastfeeding, while promoting this practice in the long term could help mitigate depressive symptoms55,56. In the present study, 72% of offspring were breastfed exclusively. Further, a previous study examining the impact of maternal ACEs on offspring weight gain at 5 months of age in a sample of 100 mother-baby dyads reported that all offspring were exclusively breastfed in the first few months of life9. Our analysis indicated that offspring that received a mixed diet (breast milk with infant formula) showed lower weight gain compared with those who received only breast milk, possibly due to the physicians’ recommendations for mothers to supplement the diets of specific offspring who show weight gain below expectations with infant formula. As the weight gain assessment in our study was performed between the first 2 and 8 weeks of life, this may explain the contrast with other studies, which showed that the use of formula feeding + breastfeeding impacts rapid weight gain from the age of 3 months15.
This study had several limitations. First, we relied on retrospective reporting of ACEs about adversities that occurred during mothers’ childhood. Further, this study only included full-term newborns weighing > 2,500 g, excluding premature and low birth weight. This was performed to isolate the direct effects of maternal ACEs on offspring development; however, it may have prevented the assessment of potential associations between low birth weight and maternal ACEs. Furthermore, employing a variable encompassing various traumas precluded us from determining the specific type of trauma most strongly associated with neurobiological alterations in weight gain. Weight gain appears to correlate cumulatively with the number of maternal ACEs. Additionally, our model did not account for pre- and post-gestational maternal nutrition, gestational weight gain (GWG), maternal or infant cortisol levels, or inflammatory markers such as cytokines, all of which may be directly involved in weight gain.
Nevertheless, this study has several notable strengths. Firstly, our cohort aimed to better understand the effects of maternal ACEs on offspring neurodevelopment by including only low-risk pregnant women with a BMI under 30, thus excluding maternal obesity as a potential confounder. We further controlled for birth weight and infant age, as well as the type of feeding provided during the assessment period. Our data support the hypothesis that maternal ACEs independently influence postnatal weight gain in offspring during the first months of life, with significant differences observed based on sex. Furthermore, the target population (primarily Black and mixed-race individuals) differed substantially from the predominantly White populations enrolled in previous studies, adding diversity and heterogeneity to the current research. As such, this study enhances the literature on maternal ACEs and their potential impact on offspring.
Conclusion
Overall, the findings of the present study suggest that maternal ACEs are associated with offspring weight gain, with variations based on the infant’s sex, in an admixed Brazilian cohort. Specifically, a higher number of maternal ACEs correlated with increased weight gain during the first two months of life, particularly in male infants. This early weight gain may signal potential future metabolic health issues, including the risk of overweight and childhood obesity, thus underscoring the need for increased attention from the pediatric clinical community. In conclusion, our findings support the intergenerational transmission of maternal trauma in a mixed-race Brazilian cohort, and suggest that future research should explore the underlying epigenetic and metabolic mechanisms driving the observed increase in weight gain.
Data availability
The raw data supporting the findings of this study are available from the corresponding author (AJ) upon request. Raw data are not publicly available due to restrictions in the written consent signed by the participants of our study.
Abbreviations
- ACEs:
-
Adverse childhood experiences
References
Cronholm, P. F. et al. Adverse childhood experiences. Am. J. Prev. Med. 49, 354–361. https://doi.org/10.1016/j.amepre.2015.02.001 (2015).
Felitti, V. J. et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. Am. J. Prev. Med. 14, 245–258. https://doi.org/10.1016/S0749-3797(98)00017-8 (1998).
Holman, D. M. et al. The association between adverse childhood experiences and risk of cancer in adulthood: A systematic review of the literature. Pediatrics 138, S81–S91. https://doi.org/10.1542/peds.2015-4268L (2016).
Zhang, L., Mersky, J. P., Gruber, A. M. H. & Kim, J.-Y. Intergenerational transmission of parental adverse childhood experiences and children’s outcomes: A scoping review. Trauma Violence Abus. 24, 3251–3264. https://doi.org/10.1177/15248380221126186 (2023).
Basu, A., McLaughlin, K. A., Misra, S. & Koenen, K. C. Childhood maltreatment and health impact: The examples of cardiovascular disease and type 2 diabetes mellitus in adults. Clin. Psychol. Sci. Pract. 24, 125–139. https://doi.org/10.1111/cpsp.12191 (2017).
Hughes, K. et al. The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Health 2, e356–e366. https://doi.org/10.1016/S2468-2667(17)30118-4 (2017).
Bellis, M. A. et al. Measuring mortality and the burden of adult disease associated with adverse childhood experiences in England: A national survey. J. Public Health 37, 445–454. https://doi.org/10.1093/pubmed/fdu065 (2015).
Fang, L., Chuang, D.-M. & Lee, Y. Adverse childhood experiences, gender, and HIV risk behaviors: Results from a population-based sample. Prev. Med. Rep. 4, 113–120. https://doi.org/10.1016/j.pmedr.2016.05.019 (2016).
Apanasewicz, A. et al. Maternal childhood trauma is associated with offspring body size during the first year of life. Sci Rep 12, 19619. https://doi.org/10.1038/s41598-022-23740-6 (2022).
Leonard, S. A., Petito, L. C., Rehkopf, D. H., Ritchie, L. D. & Abrams, B. Maternal history of child abuse and obesity risk in offspring: Mediation by weight in pregnancy. Child. Obes. 13, 259–266. https://doi.org/10.1089/chi.2017.0019 (2017).
Lacey, R. E. et al. Adverse childhood experiences and early life inflammation in the Avon longitudinal study of parents and children. Psychoneuroendocrinology 122, 104914. https://doi.org/10.1016/j.psyneuen.2020.104914 (2020).
Santiago-Vieira, C. et al. Recent changes in growth trajectories: a population-based cohort study of over 5 million Brazilian children born between 2001 and 2014. Lancet Reg. Health Americas 32, 100721. https://doi.org/10.1016/j.lana.2024.100721 (2024).
Kenney, E., Frongillo, E. A., McIver, K. L., Dowda, M. & Pate, R. R. Child and mother characteristics associated with 6-month weight gain for infants and toddlers during 6 to 36 months. Pediatr. Obes. 19, e13148. https://doi.org/10.1111/ijpo.13148 (2024).
Kerkhof, G. F., Willemsen, R. H., Leunissen, R. W. J., Breukhoven, P. E. & Hokken-Koelega, A. C. S. Health profile of young adults born preterm: Negative effects of rapid weight gain in early life. J. Clin. Endocrinol. Metab. 97, 4498–4506. https://doi.org/10.1210/jc.2012-1716 (2012).
Ortega-Ramírez, A. D., Murillo-Zamora, E., Trujillo-Hernández, B., Delgado-Enciso, I. & Sánchez-Ramírez, C. A. Birth weight, slowness in eating and feeding practices as independent determinants of rapid weight gain. Acta Paediatr. https://doi.org/10.1111/apa.17330 (2024).
Colleluori, G., Galli, C., Severi, I., Perugini, J. & Giordano, A. Early life stress, brain development, and obesity risk: Is oxytocin the missing link?. Cells 11, 623. https://doi.org/10.3390/cells11040623 (2022).
Flores-Dorantes, M. T., Díaz-López, Y. E. & Gutiérrez-Aguilar, R. Environment and gene association with obesity and their impact on neurodegenerative and neurodevelopmental diseases. Front. Neurosci. 14, 863. https://doi.org/10.3389/fnins.2020.00863 (2020).
Jaldin, M. D. G. M., Pinheiro, F. S., Santos, A. M. D. & Muniz, N. C. Crescimento infantil comparado com as referências NCHS e o padrão WHO/2006. Rev. Nutr. 26, 17–26. https://doi.org/10.1590/S1415-52732013000100002 (2013).
Weffort, V. R. S. Manual de Avaliação Nutricional (Sociedade Brasileira de Pediatria, 2021).
Cáceres, A. et al. Prenatal environmental exposures associated with sex differences in childhood obesity and neurodevelopment. BMC Med. 21, 142. https://doi.org/10.1186/s12916-023-02815-9 (2023).
Apanasewicz, A. et al. Traumatized women’s infants are bigger than children of mothers without traumas. anthranz 77, 359–374. https://doi.org/10.1127/anthranz/2020/1285 (2020).
Kaitz, M., Mankuta, D., Rokem, A. M. & Faraone, S. V. Relation between maternal antenatal anxiety and infants’ weight depends on infants’ sex: A longitudinal study from late gestation to 1-month post birth. J. Psychosom. Res. 79, 620–627. https://doi.org/10.1016/j.jpsychores.2015.07.006 (2015).
Sutherland, S. & Brunwasser, S. M. Sex differences in vulnerability to prenatal stress: A review of the recent literature. Curr. Psychiatry Rep. 20, 102. https://doi.org/10.1007/s11920-018-0961-4 (2018).
Stark, M. J., Wright, I. M. R. & Clifton, V. L. Sex-specific alterations in placental 11β-hydroxysteroid dehydrogenase 2 activity and early postnatal clinical course following antenatal betamethasone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R510–R514. https://doi.org/10.1152/ajpregu.00175.2009 (2009).
McKenna, B. G. et al. Infant epigenetic aging moderates the link between Black maternal childhood trauma and offspring symptoms of psychopathology. Dev. Psychopathol. 36, 1–13. https://doi.org/10.1017/S0954579423001232 (2023).
Tsompanidis, A. et al. Sex differences in placenta-derived markers and later autistic traits in children. Transl. Psychiatry 13, 256. https://doi.org/10.1038/s41398-023-02552-w (2023).
Ward, L. G., Bublitz, M., Sokol, N., Brown, S. & Stroud, L. R. Experiences of maltreatment in childhood are associated with increasing anxiety and lower body acceptance over pregnancy. J. Psychosom. Res. 172, 111414. https://doi.org/10.1016/j.jpsychores.2023.111414 (2023).
Soares, A. L. G. et al. Adverse childhood experiences: Prevalence and related factors in adolescents of a Brazilian birth cohort. Child Abuse Neglect 51, 21–30. https://doi.org/10.1016/j.chiabu.2015.11.017 (2016).
Barbosa, L. P. et al. Childhood trauma and suicide risk in a sample of young individuals aged 14–35 years in southern Brazil. Child Abuse Neglect 38, 1191–1196. https://doi.org/10.1016/j.chiabu.2014.02.008 (2014).
Bellis, M. A., Hughes, K., Leckenby, N., Perkins, C. & Lowey, H. National household survey of adverse childhood experiences and their relationship with resilience to health-harming behaviors in England. BMC Med. 12, 72. https://doi.org/10.1186/1741-7015-12-72 (2014).
Bethell, C. D., Newacheck, P., Hawes, E. & Halfon, N. Adverse childhood experiences: Assessing the impact on health and school engagement and the mitigating role of resilience. Health Affairs 33, 2106–2115. https://doi.org/10.1377/hlthaff.2014.0914 (2014).
Desigualdades Sociais Por Cor Ou Raça No Brasil. vol. 41 (Instituto Brasileiro de Geografia e Estatística IBGE, Rio de Janeiro, 2019). https://biblioteca.ibge.gov.br/index.php/biblioteca-catalogo?view=detalhes&id=2101681
ANUÁRIO BRASILEIRO DE SEGURANÇA PÚBLICA 2022. (Fórum Brasileiro de Segurança Pública, São Paulo, 2022). https://publicacoes.forumseguranca.org.br/handle/fbsp/58
ANUÁRIO BRASILEIRO DE SEGURANÇA PÚBLICA 2023. (Fórum Brasileiro de Segurança Pública, São Paulo, 2023). https://publicacoes.forumseguranca.org.br/handle/fbsp/57
Benjet, C. Childhood adversities of populations living in low-income countries: Prevalence, characteristics, and mental health consequences. Curr. Opin. Psychiatry 23, 356–362. https://doi.org/10.1097/YCO.0b013e32833ad79b (2010).
Pereira, F. G. & Viana, M. C. Adaptação transcultural do adverse childhood experiences international questionnaire. Rev. saúde pública 55, 79. https://doi.org/10.11606/s1518-8787.2021055003140 (2021).
ABEP - Associação Brasileira de Empresas de Pesquisa. Critério Brasil: Critério de Classificação Econômica Brasil. [Internet] (2019). https://www.abep.org/criterio-brasil
Fenton, T. R., Senterre, T. & Griffin, I. J. Time interval for preterm infant weight gain velocity calculation precision. Arch. Dis. Child. Fetal Neonatal Ed. 104, F218–F219. https://doi.org/10.1136/archdischild-2018-314843 (2019).
Enders, C. & Bandalos, D. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct. Equ. Model. Multidiscip. J. 8, 430–457. https://doi.org/10.1207/S15328007SEM0803_5 (2001).
Muthén, L. & Muthén, B. Mplus User’s Guide. (2020).
Hayes, A. F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach (The Guilford Press, 2022).
Dammhahn, M., Dingemanse, N. J., Niemelä, P. T. & Réale, D. Pace-of-life syndromes: A framework for the adaptive integration of behaviour, physiology and life history. Behav. Ecol. Sociobiol. 72, 62. https://doi.org/10.1007/s00265-018-2473-y (2018).
Wikelski, M., Spinney, L., Schelsky, W., Scheuerlein, A. & Gwinner, E. Slow pace of life in tropical sedentary birds: A common-garden experiment on four stonechat populations from different latitudes. Proc. R. Soc. Lond. B 270, 2383–2388. https://doi.org/10.1098/rspb.2003.2500 (2003).
Kuzawa, C. W. et al. Evolutionary life history theory as an organising framework for cohort studies: Insights from the Cebu Longitudinal Health and Nutrition Survey. Ann. Human Biol. 47, 94–105. https://doi.org/10.1080/03014460.2020.1742787 (2020).
Urlacher, S. S. et al. Constraint and trade-offs regulate energy expenditure during childhood. Sci. Adv. 5, eaax1065. https://doi.org/10.1126/sciadv.aax1065 (2019).
Chung, E. O. et al. Maternal adverse childhood experiences on child growth and development in rural Pakistan: An observational cohort study. PLOS Glob. Public Health 3, e0001669. https://doi.org/10.1371/journal.pgph.0001669 (2023).
Clifton, V. L. Review: Sex and the human placenta: Mediating differential strategies of fetal growth and survival. Placenta 31, S33–S39. https://doi.org/10.1016/j.placenta.2009.11.010 (2010).
DiPietro, J. A. & Voegtline, K. M. The gestational foundation of sex differences in development and vulnerability. Neuroscience 342, 4–20. https://doi.org/10.1016/j.neuroscience.2015.07.068 (2017).
Mueller, B. R. & Bale, T. L. Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 28, 9055–9065. https://doi.org/10.1523/JNEUROSCI.1424-08.2008 (2008).
Singhal, A. Long-term adverse effects of early growth acceleration or catch-up growth. Ann. Nutr. Metab. 70, 236–240. https://doi.org/10.1159/000464302 (2017).
Brown, M., Worrell, C. & Pariante, C. M. Inflammation and early life stress: An updated review of childhood trauma and inflammatory markers in adulthood. Pharmacol. Biochem. Behav. 211, 173291. https://doi.org/10.1016/j.pbb.2021.173291 (2021).
Lyons-Reid, J., Albert, B. B., Kenealy, T. & Cutfield, W. S. Birth size and rapid infant weight gain—where does the obesity risk lie?. J. Pediatr. 230, 238–243. https://doi.org/10.1016/j.jpeds.2020.10.078 (2021).
Leachman, J. R. et al. Sex and race define the effects of adverse childhood experiences on self-reported BMI and metabolic health biomarkers. Biol. Sex Differ. 13, 29. https://doi.org/10.1186/s13293-022-00439-x (2022).
Holdsworth, E. A. & Appleton, A. A. Adverse childhood experiences and reproductive strategies in a contemporary U.S. population. Am. J. Phys. Anthropol. 171, 37–49. https://doi.org/10.1002/ajpa.23967 (2020).
Figueiredo, B., Canário, C. & Field, T. Breastfeeding is negatively affected by prenatal depression and reduces postpartum depression. Psychol. Med. 44, 927–936. https://doi.org/10.1017/S0033291713001530 (2014).
Hart, S. L., Jackson, S. C. & Boylan, L. M. Compromised weight gain, milk intake, and feeding behavior in breastfed newborns of depressive mothers. J. Pediatr. Psychol. 36, 942–950. https://doi.org/10.1093/jpepsy/jsr031 (2011).
Funding
This study was mainly funded by both the National Institute of Mental Health (NIMH; R01 MH121070-01A1), and the São Paulo Research Foundation (FAPESP), Brasil. Process Number 2019/21612-0, and in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
Author information
Authors and Affiliations
Contributions
VS, CD, JP and AJ contributed to study conception and design, material preparation, and data collection/analysis, and prepared the first draft of the manuscript. AR, HCM, CA, and IS contributed to material preparation, and data collection/analysis, and prepared the first draft of the manuscript. BS, LR, AL, and AM contributed to material preparation and data collection/analysis. All authors commented on previous versions of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santana, V.O., Ramos, A.C., Cogo-Moreira, H. et al. Sex-specific association between maternal childhood adversities and offspring’s weight gain in a Brazilian cohort. Sci Rep 15, 2960 (2025). https://doi.org/10.1038/s41598-025-87078-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87078-5