Abstract
We aimed to evaluate the relationship between the increase in choroidal microvascular dropout (MvD) toward the disc-fovea axis and the development of central visual field defect (CVFD) in patients with glaucoma. We retrospectively reviewed the longitudinal medical records of patients who underwent at least two optical coherence tomography angiographies (OCTA) with significant macular retinal ganglion cell (RGC) damage at baseline. Patients were divided into three groups: Group 1, no CVFD; Group 2, new CVFD during the follow-up period; and Group 3, CVFD at baseline. The angular extent of MvD and the change were investigated. We had 35 eyes in Group 1, 25 in Group 2, and 43 in Group 3 with OCTA over an average of 3.1 ± 1.3 years. At baseline, MvD was significantly more frequent and larger in Group 3 compared to Groups 1 and 2; there was no difference between Groups 1 and 2, aligning with the absence of baseline CVFD. The mean final MvD extent was significantly smaller in Group 1 (9.9°) than Groups 2 and 3 (43.6 and 44.8°, P < 0.001), also aligning with the presence of final CVFD. Quantitatively, Group 2 with developing CVFD exhibited significantly greater mean increase in MvD (17.8°) than Groups 1 and 3 (0.9 and 6.0°, P < 0.001). In contrast, the changes in OCT-based thicknesses did not differ among the three groups in the period. In conclusion, in patients with glaucoma who had significant RGC damage in the macular area, longitudinal increase in MvD coincided with development of new CVFD, both in terms of the presence and in terms of angular extent, in a three-year follow-up period.
Similar content being viewed by others
Introduction
The recently adopted optical coherence tomography angiography (OCTA) has unveiled parapapillary choroidal microvascular dropout (MvD) as a significant factor in glaucoma1,2,3. Besides the cross-sectionally investigated significance as a valuable biomarker in glaucoma4,5,6,7,8,9,10, recent studied also demonstrated a longitudinal association between changes in MvD and glaucoma progression6,11,12. For example, MvD enlargement was reported to be associated with progressive retinal nerve fiber layer (RNFL) thinning6. The temporal changes in the rates of MvD area and angular circumference were associated with concurrent rates of RNFL loss in glaucoma12. In addition, visual field (VF) progression has been reported to be significantly associated with a greater longitudinal increase in the MvD11.
Therefore, it would be interesting to test whether changing MvD could represent regional functional deterioration, particularly in the central VF area. Abe et al. demonstrated that progressive decline in sensitivity in the central inferior area of the VF had the strongest association with longitudinal decline in quality of life of patients with glaucoma13. Hence, elucidating such relationship would hold clinical significance in comprehensive patient evaluation.
This study adopted a unique comparative design that focuses on patients with significant and comparable macular retinal ganglion cell (RGC) damage at baseline. Through this design, our aim was to examine the longitudinal association between changes in MvD and CVFD, and to test the potential of MvD as a biomarker for regional functional deterioration under given structural damage.
Results
Among the initially enrolled 141 eyes of 127 patients with open angle glaucoma (OAG), we excluded the eyes with (1) minimal peripapillary atrophy (PPA) (6 eyes of 5 patients), (2) unreliable or fluctuating VF (3 eyes of 3 patients), (3) OCTA interval < 1 year (4 eyes of 3 patients), (4) erroneous ganglion cell-inner plexiform layer (GC-IPL) scan (1 eye in 1 patient), and (5) poor quality OCTA (24 eyes of 24 patients). Finally, we included 103 eyes of 92 patients: Group 1 (35 eyes of 33 patients), Group 2 (25 eyes of 25 patients), and Group 3 (43 eyes of 41 patients).
Clinical characteristics of patients
Table 1 shows the baseline and follow-up characteristics of patients. The mean follow-up period within the OCTA observation was 3.1 ± 1.3 years. The average axial length of the patients included in this study was 25.28 ± 1.38 mm, and 76.7% of patients had axial length over 24 mm. No differences were observed in the baseline and final global OCT RNFL and average OCT GC-IPL thicknesses, indicating comparable structural deficits among the three groups. Baseline IOP was significantly higher in Group 2 compared to Groups 1 and 3; however, it was not significantly different at the final follow-up.
Differences in MvD-related characteristics among the groups
The interobserver agreement was almost perfect for the determination of the presence of MvD (κ = 0.847, P < 0.001) and excellent for the extent of MvD (ICC 0.993, 95% confidence interval 0.989–0.996, P < 0.001). The baseline and final OCTA image scores were 66.8 ± 5.7 and 65.8 ± 6.6, respectively.
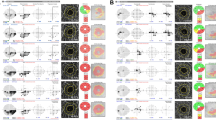
The MvD-related parameters were significantly different among the three groups, both in terms of the presence and in terms of angular extent (Table 2; Fig. 1).
MvD-related and structural OCT parameters in three groups. (A) Different distribution of baseline and final angular extents of MvD corroborating the CVFD status. Note that the change in the distance from the disc-fovea axis was only different between groups 1 and 2, aligning with the disparate final presence of CVFD between these groups. (B) In contrast, OCT structural thickness measures did not demonstrate a significant difference among the groups. Asterisks indicate statistical significance. Error bars indicate a 95% confidence interval.
The baseline MvD was significantly more frequent in Group 3 than in Group 1 or 2, but not different between Groups 1 and 2, aligning with the absence of CVFD at baseline. Final MvD was significantly less frequent in Group 1 than in Group 2 or 3, but not different between Groups 2 and 3, also matching the final CVFD status.
The baseline extent of MvD was significantly larger in Group 3 (38.8 ± 21.3°) compared to Groups 1 and 2 (9.1 ± 15.0° and 25.8 ± 24.7°, respectively, P < 0.001), but not between Groups 1 and 2; the difference matched the baseline CVFD status. The final extent of MvD was significantly greater in Groups 2 and 3 (43.6 ± 25.1° and 44.8 ± 21.6°, respectively), than in Group 1 (9.9 ± 15.4°, P < 0.001), again matching the final presence of CVFD. In addition, the location of the temporal margin was significantly closer to the disc-fovea axis in Groups 2 and 3 compared to Group 1.
Notably, the change in the angular extent of MvD decreased significantly in the order of Group 2, Group 3, and Group 1 (17.8 ± 11.7, 6.0 ± 9.7, and 0.9 ± 3.1°, respectively; P < 0.001). This result corroborated the perimetric conversion observed in Group 2. Interestingly, the change in distance to the disc-fovea axis was only significantly different between Groups 1 and 2 (1.1 ± 6.1 and 10.9 ± 10.2°, P = 0.001), but not between Groups 2 and 3 (10.9 ± 10.2 and 5.8 ± 9.5°, P = 0.181), aligning with the new development in CVFD that was observed specific to Group 2 (Fig. 1).
In contrast, the baseline OCT-based parameters and the changes, such as RNFL and GC-IPL thicknesses and their changes, showed no significant differences among the three groups during the follow-up period (Table 1).
Factors associated with the development of CVFD in groups 1 and 2
Multivariate logistic regression analysis with new development of CVFD as a dependent variable, within Groups 1 and 2, demonstrated that both the changes in the total extent of MvD and the angular location of temporal border of the MvD toward the disc-fovea axis were significant factors (Table 3).
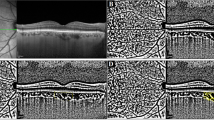
Representative cases in each group are shown in (Fig. 2).
Representative cases. (A) A 45-year-old male diagnosed with NTG. Despite considerable RGC damage, no MvD was observed throughout the follow-up period, with the absence of CVFD. (B) A 42-year-old female diagnosed with NTG. Despite considerable RGC damage in the macular area in the baseline, no CVFD was observed. In 5 years, the MvD expanded toward the disc-fovea axis and a new CVFD developed. (C) A 36-year-old female diagnosed with NTG. We observed distinct MvD and CVFD in the baseline.
OCT measurements
Baseline values and changes in sectoral GCIPL thicknesses differed significantly across the three groups, in contrast to global RNFL thickness and average GCIPL thickness (Table 1). However, baseline sectoral GCIPL thicknesses were not significantly different between Group 1 and Group 2 in ad hoc analysis (P = 0.139, 0.981, and 0.440 for temporal, middle, and nasal sectors, respectively). Similarly, the change in sectoral GCIPL thicknesses were not significantly different between Group 1 and Group 2 in ad hoc analysis (P = 1.000, 0.620, and 0.860 for temporal, middle, and nasal sectors, respectively). In the logistic analysis, the difference in temporal sectoral GCIPL thickness was a significant factor for CVFD development in the univariate analysis (Table 3).
Discussion
This study investigated the longitudinal changes in MvD and CVFD in glaucomatous eyes with structural macular RGC damage. Enlargement of MvD was significantly associated with the development of new CVFD under pre-existing structural RGC damage in the macula. Our finding corroborates recent studies that have demonstrated the close proportional relationship between the enlargement of MvD and progressive RNFL thinning as well as VF loss6,11,12. Additionally, our study highlights the significance of MvD as an indicator of regional functional deterioration in the macula, leading to a decrease in central VF sensitivity.
Our results are in line with the cross-sectional relationship between MvD and CVFD. The presence of MvD was a strong predictor for initial parafoveal scotoma in patients with glaucoma10. In addition, the progression rate of CVFD was significantly higher in eyes with MvD than in those without it4.
However, in this study, we recruited patients with similar structural macular RGC damage but with varying CVFD status. The results of our study suggests a potential merit in the MvD parameters in certain circumstances where central VF changes are concerned. In particular, the degree of MvD approaching the disc-fovea axis may provide a visually intuitive parameter for CVFD risk assessment under similar degree of the global RNFL and average GCIPL thicknesses. Moreover, it is known that RNFL thickness measurements and VF results may exhibit poor correlation because of the complex structure–function relationship in glaucoma14. For example, Lee et al. reported the lack of a relationship between the RNFL thinning rate (µm/year) and the rate of VF progression (dB/year).11
From our results, we consider that MvD may provide useful information in addition to the OCT examinations, including global as well as sectoral OCT measurements. In our analysis, the overall difference in sectoral OCT GCIPL thicknesses and the relationship to the development of CVFD were less marked than that of MvD angular extents; however, both modalities may be mutually utilized for a comprehensive evaluation. We consider that our results underscore the potential use of MvD as a visually intuitive parameter of glaucomatous damage approaching the disc-fovea axis, which may be useful as an ancillary imaging modality in addition to the conventional measures.
MvD also merits from the straightforward recognizability. The progression of MvD toward the disc-fovea axis in developing CVFD is topographically understandable based on the structure–function relationship in glaucoma. This correlation is further supported by the topographical correlation observed between MvD and RNFL damage2,15,16. Therefore, MvD may be an easily visualized and intuitively comprehensive biomarker compared with the numerical changes in OCT reports, which are usually less than 10 μm within several years of interval.
However, the pathogenesis of MvD remains unclear, and there is no explanation for how MvD may account for the regional decrease in RGC function.
Investigators have suggested biomechanical explanations for MvD. For instance, increased mechanical stress on the ONH and parapapillary sclera associated with myopia17 and posterior scleral deformation18,19 could cause MvD by predisposing the scleral vessels to tensile stress19. The influence could be exaggerated when IOP is elevated. Investigators have also suggested that MvD may be associated with decreased ocular perfusion. Indocyanine angiography demonstrated that the localized MvD coincided with a perfusion defect in the choroid1. In addition, MvD was associated with a lower diastolic BP3 and an early parafoveal scotoma10, which are associated with systemic vascular risk factors, including hypotension, migraine, and the Raynaud phenomenon10. Similarly, Kim et al. suggested that MvD enlargement could indicate worsened ocular perfusion6. The distinct relationship to CVFD demonstrated in our study may also support its association with decreased ocular perfusion. However, the possible mechanisms leading to CVFD requires further investigation.
Our findings suggest that MvD may occur as a secondary, rather than a primary, change following RGC damage. This interpretation is based on the excessively high correlation observed, which is more indicative of an outcome than a cause, especially given the time required for RGC damage to manifest in chronic diseases like glaucoma. Moreover, studies have reported strong associations between progressive changes in MvD, VF, and RNFL loss6,11,12 and a close topographical correlation between MvD and RNFL defects2,15,20. Furthermore, baseline MvD extent is not associated with the progression of VF or RNFL damage6,11 providing additional evidence against a causative role. The possibility that RNFL defects may lead to MvD could also explain why MvD is observed in various conditions associated with RNFL defects, such as retinal vein occlusion19, compressive optic neuropathy20, and ischemic optic neuropathy15. Nevertheless, this study does not establish a definitive causal relationship between MvD and RGC damage; further prospective studies are warranted to clarify this relationship.
The consequences of defective ocular perfusion should be carefully considered when discussing the clinical significance of MvD. The observed perfusion defect may not necessarily indicate a significant ischemic predisposition of the ONH that leads to glaucomatous damage, and there has been no report of choroidal neovascularization around the MvD. It would be beneficial to study whether a functionally defective parapapillary choroid leads to significant ischemia in the nearby ONH and the connections in the blood supply. It would be beneficial to study whether a functionally defective parapapillary choroid leads to significant ischemia in the nearby ONH and the connections in the blood supply.
Among the OCTA-based parameters, MvD reflects the loss or compromise in choroidal vasculature, while a decrease in peripapillary vessel density indicates compromise in the retinal vasculature, including the capillaries supplying the RNFL. Thus, these two parameters differ anatomically and potentially in their target areas of blood supply and the implications of their alteration. This distinction suggests that MvD may not merely represent RGC loss in glaucoma but could reflect a state of ocular ischemia based on the choroidal blood supply to the ONH. However, the actual vascular network and blood supply relationships between the peripapillary choriocapillaris, larger vessels, and deep ONH vasculature should be investigated to draw conclusions regarding their interrelated significance.
This study has several limitations. First, the study was retrospective, and the sample size was small. Instead, we had highly controlled groups with different VF status despite similar structural damage. Moreover, the enrollment of patients experiencing new-onset CVFD has been limited, reflecting the recent adoption of relatively new OCTA technology. Second, the evaluation of MvD extent on en-face OCTA image could be subjective and can be influenced by artifacts. For example, overlying large retinal vessels could induce projection artifacts, rendering it difficult to detect MvD boundaries. However, we had three examiners, excellent inter-examiner correlation, and used a previously validated method11,21. In addition, we rigorously reviewed the serial en-face images to exclude fluctuating changes. Eyes presentation with any such changes, even in a single image, were excluded. Third, we did not evaluate the patients with 10 − 2 Humphrey VF tests, which could more accurately reflect the CVFD. It would be helpful to perform future studies with 10 − 2 VF tests focusing on the CVFD. Fourth, although the dropout displayed on the choroidal slab grossly matched the perfusion defect observed on indocyanine green angiography1, the slab does not specifically segment the choroid, and the source vasculature for the observed signals is not fully understood. The structure of the ONH and parapapillary tissues is highly complex, especially in the eyes with PPA22,23. Investigations should explore what the observed alterations in the vasculature—be it in the choroid or the sclera—represent, or whether they result from the complex interplay of pathological changes and inherent artifacts associated with the working mechanism of OCTA. Finally, our results did not demonstrate a cause-and-effect relationship between MvD and VF progression. Therefore, future prospective longitudinal studies with larger sample sizes are warranted.
In conclusion, in glaucomatous eyes with significant macular RGC damage, enlargement of MvD coincided with the new development of CVFD, both qualitatively and quantitatively. Future studies are needed to assess the potential of MvD as a biomarker for CVFD and its significance in glaucoma pathogenesis.
Methods
This was a retrospective, longitudinal study. Data were obtained from patients who visited the Samsung Medical Center (Seoul, Republic of Korea) between January 2018 and October 2023. This study followed all guidelines for experimental investigation in humans, was approved by the Samsung Medical Center Institutional Review Board (#2023-11-147) and adhered to the tenets of the Declaration of Helsinki. The IRB waived informed consent based on the absence of harm to subjects and the retrospective design of the study.
Eligibility criteria
Patients with OAG who had undergone at least two OCTA examinations were enrolled. We included eyes with significant RGC damage in the macular area. Significant macular RGC damage was defined as the presence of a blue-colored region indicating thinning of GC-IPL in OCT thickness map in the macular vulnerability zone (MVZ)24. It has to be extending wide enough to touch the fovea at its temporal margin (Fig. 3). The criterion was set to assess the significance of MvD in VF loss under controlled degree of structural damage.
Patient evaluation for RGC damage, VF defects, and MvD. (A) Glaucomatous RGC damage involving the macula in the baseline. (B) Definition of CVFD as the involvement of the four innermost points on 30 − 2 Humphrey VF test. (C) Grouping of patients according to the longitudinal change in the status of CVFD. (D) Measurement of the angular location and extent of MvD.
OAG was diagnosed according to the following criteria: (1) the presence of glaucomatous optic disc changes, including increased cupping, neuroretinal rim thinning, disc hemorrhage, or RNFL defects; (2) confirmation of an open angle upon gonioscopic examination with no identifiable causes of secondary glaucoma; and (3) glaucomatous VF defects. A glaucomatous VF defect was defined as a second VF defect using more than one reliable test for at least two of the following three criteria: (1) a cluster of three points with a probability of less than 5% on the pattern deviation map, including at least one point with a probability of less than 1% or a cluster of two points with a probability of less than 1%; (2) a glaucoma hemifield test result outside normal limits; or (3) a pattern standard deviation of 95% outside the normal limits.
The exclusion criteria were as follows (1) minimal parapapillary atrophy (PPA) area that did not allow observation of MvD, (2) unreliable VF tests or fluctuation of VF, (3) segmentation errors in macular ganglion cell-inner plexiform layer (GC-IPL) OCT, (4) OCTA interval between the first and last OCTA less than 1 year, and (5) poor-quality OCTA. In particular, poor-quality OCTA images were strictly excluded for a reliable assessment of the changes in MvD. Poor-quality OCT-A images were defined using the following criteria: (1) quality score < 30, (2) poor clarity, (3) local weak signal due to posterior vitreous detachment or floaters, (4) residual motion artifacts visible as an irregular vessel pattern or disc boundary on the en face angiogram, and (5) choroidal layer segmentation errors. Eyes with at least one low-quality OCTA image were excluded. Reliable VF analysis was defined as a false-negative rate of < 15%, a false-positive rate of < 15%, and a fixation loss of < 20%.
Patient evaluation
Comprehensive glaucoma examinations included the measurement of visual acuity and refraction, Goldmann applanation tonometry, slit-lamp biomicroscopy, and gonioscopic examination. Dilated stereoscopic examination of the optic nerve head (ONH), color and red-free fundus photography (Topcon, Paramus, NJ), automated perimetry using a central 30 − 2 Humphrey field analyzer (HFA model 640; Humphrey Instruments, Inc., San Leandro, CA, USA) with the Swedish interactive threshold algorithm standard, ultrasound pachymetry (Tomey SP-3000, Tomey Ltd., Nagoya, Japan), spectral-domain OCT with Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA), and OCTA (DRI OCT Triton Plus; Topcon, Tokyo, Japan) were performed. Axial length was measured using a swept-source OCT biometer (ARGOS, Suntec, Inc., Aichi, Japan).
Patient grouping by CVFD
We defined the presence of CVFD as the involvement of the four central innermost points on the Humphrey 24 − 2 VF test, with at least one point at P < 1% in the pattern deviation plot (Fig. 3), following a previous study25,26. The new CVFD had to be consistent in two consecutive tests.
We classified the patients into three groups based on the changes in CVFD status. Group 1 consisted of patients with no CVFD throughout the follow-up period, Group 2 included patients with newly developed CVFD during the follow-up, and Group 3 comprised those with CVFD at baseline (Fig. 3). Across the three groups, all eyes had significant structural macular RGC damage in baseline.
Evaluation of MvD
We reviewed the entire serial OCTA images to examine the consistency of changes, and used the first and last OCTA images for the final comparative analysis.
MvD was evaluated as previously reported2,3. Briefly, the optic disc and parapapillary area were imaged with OCTA using 4.5 × 4.5 mm cubes3. The deep-layer microvasculature in the parapapillary area was evaluated using en-face images generated from slabs extending from the retinal pigment epithelium (RPE) to the outer border of the sclera. Microvasculature dropout was defined as a focal sectoral vascular dropout with no visible microvascular networks. The MvD area containing large retinal vessels was included as part of the MvD area if the MvD extended beyond the vessels. In cases where the retinal vessels were located at the border of the MvD, the area covered by the vessels was excluded from the MvD area. Reflectance or shadowing of large vessels on the horizontal and en-face images was excluded from the quantitative analysis. Two independent observers (E.J.L. and D.Y.P.) evaluated MvDs and were blinded to the participants’ clinical information. Disagreements between the two observers were resolved by a third adjudicator (J.C.H.).
The angular extent of the MvD was measured as the angular distance between the temporal and nasal margins of MvD (Fig. 3)2,3. We identified the two points at which the margins of the MvD area met the optic disc margin. The location of each margin was measured against the fovea-disc axis, which was determined by superimposing and manually aligning the OCTA images on the fundus photograph using commercial software (Photoshop CC; Adobe Systems, Mountain View, CA, USA). All measurements were performed using ImageJ software V.1.52 (http://imagej.nih.gov/ij/; National Institutes of Health, Bethesda, Maryland, USA). The measurement was identical for both superior and inferior MvD.
Evaluation of sectoral GCIPL thicknesses
From the OCT examinations performed on the same day with the analyzed OCTA, we obtained sectoral GCIPL thicknesses in the corresponding hemisphere of the MvD or RNFL defect. Temporal, middle, and nasal sectoral GCIPL thicknesses were obtained.
Statistical analysis
The interobserver agreement for the presence of MvD was assessed with Cohen’s kappa (κ), and the circumferential extent of the MvD with intraclass correlation coefficients (ICCs). We used a generalized estimation equation (GEE) to compare the parameters among the groups to consider the potential influence between the two eyes of a patient. In the comparison among the three groups, Group 1 served as the reference. In the multivariate analysis, variables with statistical significance in univariate analysis were included. Among the category of baseline MvD-related parameters, we included baseline extent of MvD, which was the most significant factor, and did not use other parameters to avoid redundancy. From change-related parameters, we included change in temporal GCIPL thickness, change in MvD extent, and the change in MvD margin toward the disc-fovea axis, which were all statistically significant in univariate analysis. Because the change in MvD extent and the change in MvD margin toward the disc-fovea axis were significantly related to each other (Pearson coefficient − 0.709, P < 0.001), we performed separate models for the two variables. The final MvD-related parameters were not included because the outcome of the analysis was the development of CVFD during the follow-up periods. Statistical significance was set at P < 0.05. All statistical analyses were performed using the R Statistical Software (version 4.3.2; R Foundation for Statistical Computing, Vienna, Austria).
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Lee, E. J., Lee, K. M., Lee, S. H. & Kim, T. W. Parapapillary choroidal microvasculature dropout in glaucoma: a comparison between optical coherence tomography angiography and indocyanine green angiography. Ophthalmology 124, 1209–1217. https://doi.org/10.1016/j.ophtha.2017.03.039 (2017).
Lee, E. J., Lee, S. H., Kim, J. A. & Kim, T. W. Parapapillary deep-layer microvasculature dropout in glaucoma: topographic association with glaucomatous damage. Invest. Ophthalmol. Vis. Sci. 58, 3004–3010. https://doi.org/10.1167/iovs.17-21918 (2017).
Suh, M. H. et al. Deep retinal layer microvasculature dropout detected by the optical coherence tomography angiography in glaucoma. Ophthalmology 123, 2509–2518. https://doi.org/10.1016/j.ophtha.2016.09.002 (2016).
Jo, Y. H., Kwon, J., Jeong, D., Shon, K. & Kook, M. S. Rapid central visual field progression rate in eyes with open-angle glaucoma and choroidal microvasculature dropout. Sci. Rep. 9, 8525. https://doi.org/10.1038/s41598-019-44942-5 (2019).
Kim, C. Y., Lee, E. J., Kim, J. A., Kim, H. & Kim, T. W. Progressive retinal nerve fibre layer thinning and choroidal microvasculature dropout at the location of disc haemorrhage in glaucoma. Br. J. Ophthalmol. 105, 674–680. https://doi.org/10.1136/bjophthalmol-2020-316169 (2021).
Kim, J. A., Lee, E. J. & Kim, T. W. Evaluation of parapapillary choroidal microvasculature dropout and progressive retinal nerve fiber layer thinning in patients with glaucoma. JAMA Ophthalmol. 137, 810–816. https://doi.org/10.1001/jamaophthalmol.2019.1212 (2019).
Park, H. L., Kim, J. W. & Park, C. K. Choroidal microvasculature dropout is associated with progressive retinal nerve fiber layer thinning in glaucoma with disc hemorrhage. Ophthalmology 125, 1003–1013. https://doi.org/10.1016/j.ophtha.2018.01.016 (2018).
Park, H. Y., Shin, D. Y., Jeon, S. J. & Park, C. K. Association between parapapillary choroidal vessel density measured with optical coherence tomography angiography and future visual field progression in patients with glaucoma. JAMA Ophthalmol. 137, 681–688. https://doi.org/10.1001/jamaophthalmol.2019.0422 (2019).
Kim, M., Choung, H. K., Lee, K. M., Oh, S. & Kim, S. H. Longitudinal changes of optic nerve head and peripapillary structure during childhood myopia progression on OCT: Boramae myopia cohort study report 1. Ophthalmology 125, 1215–1223. https://doi.org/10.1016/j.ophtha.2018.01.026 (2018).
Park, S. C. et al. Initial parafoveal versus peripheral scotomas in glaucoma: risk factors and visual field characteristics. Ophthalmology 118, 1782–1789. https://doi.org/10.1016/j.ophtha.2011.02.013 (2011).
Lee, J. Y., Shin, J. W., Song, M. K., Hong, J. W. & Kook, M. S. An increased choroidal microvasculature dropout size is associated with progressive visual field loss in open-angle glaucoma. Am. J. Ophthalmol. 223, 205–219. https://doi.org/10.1016/j.ajo.2020.10.018 (2021).
Micheletti, E. et al. Rates of choroidal microvasculature dropout and retinal nerve fiber layer changes in glaucoma. Am. J. Ophthalmol. 241, 130–138. https://doi.org/10.1016/j.ajo.2022.04.024 (2022).
Abe, R. Y. et al. The impact of location of progressive visual field loss on longitudinal changes in quality of life of patients with glaucoma. Ophthalmology 123, 552–557. https://doi.org/10.1016/j.ophtha.2015.10.046 (2016).
Harwerth, R. S., Wheat, J. L., Fredette, M. J. & Anderson, D. R. Linking structure and function in glaucoma. Prog. Retin. Eye Res. 29, 249–271. https://doi.org/10.1016/j.preteyeres.2010.02.001 (2010).
Lee, E. J., Kee, H. J., Park, K. A., Han, J. C. & Kee, C. Comparative topographical analysis of choroidal microvascular dropout between glaucoma and nonarteritic anterior ischemic optic neuropathy. Invest. Ophthalmol. Vis. Sci. 62, 27. https://doi.org/10.1167/iovs.62.13.27 (2021).
Son, K. Y., Han, J. C. & Kee, C. Parapapillary deep-layer microvasculature dropout is only found near the retinal nerve fibre layer defect location in open-angle glaucoma. Acta Ophthalmol. 100, e174–e180. https://doi.org/10.1111/aos.14856 (2022).
Cahane, M. & Bartov, E. Axial length and scleral thickness effect on susceptibility to glaucomatous damage: a theoretical model implementing Laplace’s law. Ophthalmol. Res. 24, 280–284. https://doi.org/10.1159/000267179 (1992).
Shin, D. Y. et al. Association between peripapillary scleral deformation and choroidal microvascular circulation in glaucoma. Sci. Rep. 9, 18503. https://doi.org/10.1038/s41598-019-54882-9 (2019).
Baek, J. et al. Parapapillary choroidal microvasculature dropout in branched retinal vein occlusion and glaucoma. Invest. Ophthalmol. Vis. Sci. 63, 27. https://doi.org/10.1167/iovs.63.3.27 (2022).
Lee, E. J. et al. Glaucoma-like parapapillary choroidal microvasculature dropout in patients with compressive optic neuropathy. Ophthalmology 127, 1652–1662. https://doi.org/10.1016/j.ophtha.2020.06.001 (2020).
Lee, E. J., Kim, T. W., Kim, J. A. & Kim, J. A. Central visual field damage and parapapillary choroidal microvasculature dropout in primary open-angle glaucoma. Ophthalmology 125, 588–596. https://doi.org/10.1016/j.ophtha.2017.10.036 (2018).
Wang, Y. X., Panda-Jonas, S. & Jonas, J. B. Optic nerve head anatomy in myopia and glaucoma, including parapapillary zones alpha, beta, gamma and delta: histology and clinical features. Prog. Retin. Eye Res. 83, 100933. https://doi.org/10.1016/j.preteyeres.2020.100933 (2021).
Curcio, C. A., Saunders, P. L., Younger, P. W. & Malek, G. Peripapillary chorioretinal atrophy: Bruch’s membrane changes and photoreceptor loss. Ophthalmology 107, 334–343. https://doi.org/10.1016/s0161-6420(99)00037-8 (2000).
Hood, D. C., Raza, A. S., de Moraes, C. G., Liebmann, J. M. & Ritch, R. Glaucomatous damage of the macula. Prog. Retin. Eye Res. 32, 1–21. https://doi.org/10.1016/j.preteyeres.2012.08.003 (2013).
Chakravarti, T., Moghimi, S., De Moraes, C. G. & Weinreb, R. N. Central-most visual field defects in early glaucoma. J. Glaucoma 30, e68–e75. https://doi.org/10.1097/IJG.0000000000001747 (2021).
Huh, M. G. et al. Papillomacular bundle defect (PMBD) in glaucoma patients with high myopia: frequency and risk factors. Sci. Rep. 13, 21958. https://doi.org/10.1038/s41598-023-48687-0 (2023).
Acknowledgements
The authors thank Jinseob Kim, MD, MPH, for his professional advice on statistical analysis.
Funding
This work was partly supported by MSIT (Ministry of Science and ICT), Korea, under the ICT Creative Consilience program (IITP-2022-2020-0-01821) supervised by the IITP (Institute for Information & communications Technology Planning & Evaluation). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
E.J.L. and J.C.H. designed the study. E.J.L. and D.Y.P. acquired and analyzed the data. E.J.L. wrote the manuscript. J.C.H. revised the manuscript. D.Y.P., E.J.L., J.C.H., C.K., and H.M.P. provided materials. E.J.L. performed statistical analysis. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, E.J., Park, D.Y., Park, H.M. et al. Enlargement of microvascular dropout toward the disc-fovea axis indicates new central visual field defect in glaucoma. Sci Rep 15, 11052 (2025). https://doi.org/10.1038/s41598-025-87245-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87245-8