Abstract
Timely and effective rescue of critically ill children no longer solely relies on advanced medical technology; vascular access plays a pivotal role. Best practice recommendations for nursing in vascular access are critical for ICU patients. However, clear guidelines for the maintenance of external infusion connection devices remain lacking. To address this gap, we conducted a prospective observational cohort study to examine the relationship between the number or replacement frequency of external infusion connection devices and catheter-related bloodstream infection (CRBSI). From September 2021 to December 2022, a total of 304 patients with a single non-tunneled central catheter were enrolled in our study. Our findings revealed no significant differences in CRBSI incidence based on the number or replacement frequency of external infusion connection devices during the catheter’s indwelling time (P > 0.05). Notably, coagulase-negative staphylococci, particularly Staphylococcus epidermidis, were the predominant pathogens in CRBSI cases.In real-world clinical settings, adherence to strict aseptic principles during infusion set use and replacement appeared to mitigate the correlation between device replacement frequency or number and CRBSI incidence.
Similar content being viewed by others
Background
Central venous catheters are widely used for vascular access in critically ill children. This vascular access provides significant advantages in patient care, enabling rapid rescue interventions and the efficient administration of large volumes of medications. However, due to their invasive nature, the placement and maintenance of central venous catheters can lead to central venous catheter-related bloodstream infections (CRBSI)1. CRBSI occurs when bacteria or other pathogens enter the bloodstream through a central venous catheter (CVC), resulting in a bloodstream infection (BSI). The occurrence of CRBSI not only extends the duration of hospitalization but also escalates treatment costs. For instance, a single incident of CRBSI has been shown to raise costs by £29,909 per catheter and extend hospital stays by an average of 7 days, according to a study2.
Currently, numerous guidelines and literatures address the prevention of central venous catheter-related bloodstream infections throughout the catheterization, maintenance, and extubation processes3,4. Efforts to prevent infections during catheter insertion aim to minimize the risks posed by invasive procedures are supported by substantial evidence in clinical settings5,6. However, the maintenance phase of catheter indwelling, characterized by a heightened risk of central venous catheter-related bloodstream infections, lacks clear recommendations for clinical practice7. The optimal timing for device replacement during intermittent use remains a topic of debate, as indicated in the updated SHEA 2022 guidelines on preventing catheter-related bloodstream infections suggests that replacements for non-blood, blood products, or nutritional products can extend up to 7 days, while decisions on device replacements for continuous fluid infusion should align with intervals of no more than every 96 h or at least every 7 days according to the 2021 edition of the infusion therapy guideline4,8. A meta-analysis published in 2013 found that the administration set may be left in place for intervals of up to 96 h9.The discord among guidelines from different institutions regarding the frequency of replacing external connections further complicates matters.
In order to prevent CRBSI in critically ill pediatric patients, we conducted an evidence-based CRBSI prevention quality improvement project from 2019 to 202410.Our team derived evidence from prevention guidelines, systematic reviews and evidence summaries and we appraised rigorously. We put the evidence into clinical practice through skill training, media education, and supervision by advanced practice nurses. All the medical staff received updated knowledge and skill training on CVC maintenance. However, some problems had occurred.
In real clinical scenarios, central venous catheter infusion devices often incorporate various external connection devices such as three-ways stopcocks, dual-lumen/single-lumen components, extenders, and diverse infusion connectors10,11,12. Critically ill children frequently required multiple concurrent medications, including vasoactive, sedative, and analgesic drugs. As a result, adjustments to drug regimens continuously occur based on the patient’s condition, with external connection devices being changed, added, or removed very frequently. Consequently, nursing staff encounter confusion when replacing external infusion connections, as there are no clear recommendations available. Dilemmas arise regarding the synchronization of replacement times for connections initiated on different days. For example, should a connection added yesterday be replaced simultaneously with one that has been in used for three days? Additionally, do infusion pathways connected to blood products received earlier in the day required new external connectors? The lack of standardized guidance may lead to wastage of consumables and increased labor costs due to frequent replacements. A randomized controlled trial published by Lancet in 2021 compared the incidence of CRBSI after changing infusion devices every four days versus seven days, concluding that devices can be safely replaced at seven-day intervals13. While the study included infusion bags, strips, and extension tubes, which did not address the issue of replacing external infusion connections connected to blood or blood products. Therefore, a prospective cohort study is proposed to investigate the impact of replacing central venous catheter external connected infusion devices at varying intervals as well as the influence of the number of external devices used on CRBSI. This study aims to provide evidence for tailored clinical practices, ensuring patient safety and cost-effectiveness.
Methods
Study design
This was a prospective observational cohort study to explore the relationship between the type, replacement frequency of external infusion connections devices and CRBSI in critical ill pediatric population.
Setting and participants
Participants were recruited from two ICUs in our hospital in China, a tertiary university hospital of national children medical center, with 35 and 12 beds in two ICUs, respectively. Patients aged 1 month to 18 years who were admitted to the ICU with only one non-tunneled central venous access device for at least 96 h, with the external infusion connections devices attached, were included. We excluded patients with a bloodstream infection prior to their admission to the ICU, those whose catheter had been inserted in other medical institutions, and those who had a second central venous catheter during treatment, such as hemodialysis ECMO catheters. Patients were screened for eligibility by research nurses and the study was explained to their guardian. Informed written consent was obtained before the research began. The study protocol was approved by the research ethics committee of the Children’s Hospital of Fudan University (IRB number 2020427). The study was conducted in accordance with the Declaration of Helsinki. The study was conducted from September 1,2021 to December 31, 2022 during the COVID-19 period, and all COVID-19 protocols were strictly followed in accordance with government regulations. The research protocol did not change due to the publication of new guidelines or policies.
Procedures
The administration set included fluid bags or syringes. The external infusion connection devices included infusion tubing, transducers, extension tubing, 3-way stopcocks, needless connectors, and single-lumen or multi-lumen extension sets. The infusion set included crystalloids(saline), medication infusions (e.g., sedation or vasoactive agents), non-lipid parenteral nutrition and pressure monitoring infusions (saline). Blood or blood products, and chemotherapy, as well as total parenteral nutrition, were followed by institutional policy or manufacturer advice. For blood or blood products, they were to be replaced at the end of treatment and for TPN or chemotherapy, every 24 h. The fluid bags (e.g. antibiotics) and infusion tubing were replaced every 24 h in accordance with the national policy. The add-on devices were followed by institutional policy or clinical indication.
There were two types of CVC catheter products in our hospital, one of the products without extension sets (product A, ARROWg + ard Blue Central Venous Catheters, CS-24301-E,16-20Ga,1.7 mm, Arrow International, Inc), and the other one (product B, Specath CF-C 20Ga*5 cm, Foshan Special Medical CO.LTD) with multi-lumen extension sets (single lumen, generally) (Fig. 1). The multi-lumen extension sets needed to be added on the end of the catheter in product B. All the add-on devices were labeled, indicating the date of initiation. A blue rectangle label was pasted on the CVC lumen to indicate the CVC insertion time and last time of extension set tubing change (Fig. 2A). A circular sticker of different color marked with different number (e.g. 1,2,3,4,5…) was pasted on the bottom of the 3-way stopcocks to indicate the time of last replacement or added on the extension tube (Fig. 2B). The number on the sticker mean day 1st, day 2nd. A white rectangle label was pasted on the extension tubing and marked with the drug name and initiation date near the patient access site(Fig. 2C).
All the bedside nurses in two ICUs were trained to replace the administration set and external infusion connection devices. The replacement procedure training course included hand hygiene, adherence to Aseptic Non-Touch Technique when connecting, changing, and accessing connection devices, and disinfection of the connection point when connecting to a new administration set or changing administration set. The method of labeling was also included in the training course. Medication infusions were prepared by the Pharmacy Intravenous Admixture Service (PIVAS). Patients were followed until catheter removal, at the end of the treatment, hospital discharge, or transfer to the general ward.
Catheter size and type were chosen based on the patient’s age and weight, and the treatment needed. In-line filters, heparinized surface catheters, and antimicrobial connectors were not used. The catheter insertion procedure was guided by the SHEA guidelines and national practice guideline, including hand hygiene, ultrasound guidance for insertion, maximum sterile barrier precautions, alcoholic chlorhexidine antiseptic for skin preparation, sterile transparent semipermeable polyurethane dressing used for catheter securement, and disinfection of the connection point with 75% alcohol before each disconnection. Blood and tip cultures were sent to the LAB for clinical suspicion of bloodstream infection. Catheter were not routinely changed but removed as clinically indicated. All the maintenance procedures were followed according to the care bundle from the evidence-based CRBSI prevention quality improvement project14.
Data collection
Patient and catheter characteristics were recorded at baseline, including patient age, gender, underlying disease, type of medications (e.g., total parenteral nutrition, liquid formula, blood or blood products, antibiotics), catheter size and type, catheter indwelling time, type of external infusion connections devices after catheter insertion, total number of 3-way stopcocks, extension tubing and multi/single-lumen extension sets during the treatment, and the time interval of replacement of 3-way stopcocks, extension tubing, and administration set. The details of administration set replacement were recorded and audited by the research nurse every day, including the type of administration set (e.g.3-way stopcocks, extension tubing), number and time interval of administration sets added on to or removed from the CVC lumen. Microbiological results were collected from the patient medical records. All the data were summarized in a password-protected document. Bedside nurses could not access the data.
Outcomes
The primary outcome was CRBSI, which was confirmed by physicians (hospital infectious department, ICU). CRBSI was defined as bacteremia or fungemia in a patient who had an invascular device and>1 positive blood culture result from obtained from the peripheral vein, with the clinical manifestation of infection (e.g. fever, chills, and/or hypotension), and no apparent source for bloodstream infection (with the exception of the catheter), which requires the same organism grow from at least 1 percutaneous blood sample culture and from the catheter tip or 2 blood samples for culture be obtained (1 from a catheter hub and 1 from a peripheral vein)15.Secondary outcomes was catheter tip colonization (>15CFUs)16.
Statistical analysis
IBM SPSS Statistics for MAC, Version 26.0 was used for statistical analysis. The mean and standard deviation or median and interquartile range (IQR) for the continuous variables. Continuous data were analyzed using T-test, when the distribution was abnormal using two-sample Wilcoxon test. Categorical variables were calculated as proportions and percentages, using Chi-square or Fisher exact test. P-values less than 0.05 were considered significant.
Results
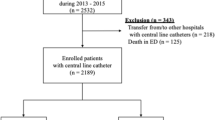
From 1 September 2021 to 31 December 2022, 403 patients were found eligible for the study. After excluding 99 patients who were either transferred from the ICU to general ward or discharged less than 96 h, or had a second central catheter for the treatment, or died in the ICU. We analyzed data for 304 patients. Table 1 summarizes the demographic data., CVC-related information and medications. The type of add-on devices includes multi-lumen/ single lumen extension set, and 3-way stopcocks after insertion. 89.14% of patients added on one multi-lumen extension set and 58.89% of the patients used 0 to 3 3-way stopcocks. No significant difference was detected in terms of those variables (Table 1).
Table 2 shows the average catheter indwelling time was 9 days in the no CRBSI group, whereas the average catheter indwelling time was 9.5 days in the CRBSI group.54.93% of the patient used 0 ~ 3 3-way stopcocks during catheter indwelling time, and 58.89% of the patients added on and removed the 3-way stopcocks for 1–2 times.1 multi-lumen extension set was used in 55.59% of the patients and 94.74% of the patients didn’t change during the catheter indwelling time. 59.21% of patients replaced the administration set for 1 ~ 2 times. Table 3 shows the time interval of the administration set replacement was between 2 ~ 6 days. The average first time interval for administration set replacement was 2 days, and the next 3 administration set replacement average time interval was 4 days. This study found no significant difference between the number of 3-way stopcocks or multi-lumen extension set used or replaced during catheter indwelling time. Different kinds of medication, such as blood or blood products, sedation, analgesics and vasoactive injection from the CVC showed no significant difference during the treatment.
24 (7.89%) of 304 patients had CRBSI (7.06/1000 catheter days), all the CRBSI cases were confirmed by blood culture from peripheral vein or central venous catheter. Coagulase-negative staphylococci, especially staphylococcus epidermidis predominated (Table 4).
Discussion
Infusion therapy is an essential part during the patient treatment, however, CRBSI is related to the intravascular devices. Many guidelines indicate the prevention strategies during the catheter indwelling time. Routine replacement of the administration set can be extended to 7 days but with some restrictions. In our prospective observational cohort study, we found that changing the administration set from 2–6 days had no significant difference, and which is similar to the results of Claire’ s study6.
There is no specific evidence for the replacement frequency of the added-on devices. Most clinical guidelines recommend administration set replacement no more frequently than every 96 h or at least 7 days4,8. A randomized, controlled equivalence study shows that administration set replacement every 7 days is as safe as every 4 days. In our study, administration set replacement from 2 to 6 days showed no significant difference in the incidence of CRBSI. Our findings are consistent with the study and support that administration set replacement could be extended to more than 6 days. More add-on devices may increase misconnections and the risk of contamination. In our study, 45.07% of patients used more than 4 stopcocks and 14.14% of the patients change for more than 3 times during the treatment. The stopcocks and other add-on devices were added or removed from the administration set according to the patients’ conditions.
We did an evidence-based quality improvement project with respect to CVC maintenance since 201910. The care bundle recommended the frequency of administration set changes no more than 72 h17, and the compliance of this criterion was not as good as the other criteria due to the ambiguity in definition of the administration set in different contexts. On the other hand, the condition of critically ill children changed rapidly under clinical scenarios, and the nurses may not have been able to practice according to the recommendations of the care bundle, which reflects the real clinical condition. In our study, the frequency of replacement and the number of add-on devices were not correlated with the incidence of CRBSI when the administration set replaced or used strictly according to the principles of sterility.
The strength of this study lies in its occurrence in a real clinical scenario, without any guidance from clinical practice allowing the practice of nurses to adapt to the condition of the patients. Generalizability included different kinds of diseases and the diversity of usage of the administration set. The limitations included the study was implemented in two pediatric intensive care centers. CRBSI might have been under-reported if the clinicians did not order blood cultures and catheter tip cultures. Our results apply to types of infusions, such as blood, blood products, total parenteral nutrition and liquid formula. Furthermore, our results apply specifically to non-tunneled central venous catheters, not to PICC, PORT etc.
Conclusion
Millions of vascular catheters are used around world each year and clear CVC maintenance recommendations for medical workers are valuable. Replacement frequence and number of external infusion connection devices used during the treatment was not the main cause to the incidence of CRBSI if under strict aseptic principle. Reducing the frequence of this nursing procedure is benefit for patient and the saving time is available for other prevention measures.
Data availability
The availability of the data can be accessed upon request from the corresponding author.
References
Chou, E. H. et al. Incidence, trends, and outcomes of infection sites among hospitalizations of sepsis: A nationwide study. PLoS One. 15, e0227752 (2020).
Leistner, R. et al. Costs and prolonged length of stay of central venous catheter-associated bloodstream infections (CVC BSI): A matched prospective cohort study. Infection 42, 31–36. https://doi.org/10.1007/s15010-013-0494-z (2014).
Loveday, H. P. et al. epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J. Hosp. Infect. 86 (suppl 1), S1–70 (2014).
Gorski, L. A., Hadaway, L., & Hagle, M. E., et al. Infusion nursing standards of Practice, 8th edition. J. Infus. Nurs. 44 (15), 1–230 (2021).
American Society of Anesthesiologists. Practice guidelines for central venous Access. Anesthesiology 116 (3), 539–573 (2012).
Lobo, D. N. & Lobo, A. R. Central venous catheter insertion: A practical guide. Contin Educ. Anaesth. Crit. Care Pain. 8 (6), 195–199 (2008).
Mihalopoulos, N. L. et al. Central venous catheter care: A review of the evidence and best practice recommendations. J. Infus Nurs. 37 (3), 174–183 (2014).
Buetti, N. et al. Strategies to prevent central line-associated bloodstream infections in acute-care hospitals: 2022 update. Infect. Control &Hospital Epidemiol. 43, 553–569 (2022).
Ullman, A. J. et al. Optimal timing for intravascular administration set replacement. Cochrane Database of Systematic Reviews. Issue 9. Art. No.: CD003588 (2013).
Safdar, N., Fine, J. P. & Maki, D. G. The use of needleless connectors and their impact on central line-associated bloodstream infections: a systematic review. Am. J. Infect. Control. 33 (5), 256–263. https://doi.org/10.1016/j.ajic.2005.03.008 (2005).
Pittet, D. et al. Evaluation of the performance and microbial contamination of different types of needleless connectors. Am. J. Infect. Control. 35 (10), 672–682 (2007).
Mermel, L. A. et al. Evaluation of the bacterial colonization of three-way stopcocks used for the administration of intravenous therapy. Infect. Control Hosp. Epidemiol. 17 (12), 796–801 (1996).
Rickard, C. M. et al. Effect of infusion set replacement intervals on catheter-related bloodstream infections (RSVP): a randomized, controlled, equivalence (central venous access device)- non-inferiority (peripheral arterial catheter) trial. Lancet 397, 1447–1458 (2021).
Wang, W. C., Fu, Q. & Shen, W. J., et al. Using i-PARIHS theoretical framework to develop evidence implementation strategies for central venous catheter maintenance: A multi-site quality improvement project. JBI Evid. Implement. 22 (2), 195–204 (2024).
Mermel, L. A., Allon, M., & Bouza, E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 49(1), 1–45 (2009).
Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement. CLSI, 2013. CLSI Document M100-S23.
Stephanie & Obeid BMedSc (Hons), PhD. Evidence Summary. Central Venous Catheterization (Primary and Community Care): Infection ControlJBI1543 (The Joanna Briggs Institute EBP Database, 2017).
Funding
Scientific Research Foundation of Shanghai Local High-Level University Construction Project–Evidence-based Nursing Innovation Research Institute: the whole chain innovation research project of evidence-based nursing in 2020 (Grant Number: FNDGJ202008).
Shanghai Hospital Development Center: The promotion and optimization management of diagnosis and treatment technology in municipal hospitals (Grant number: SHDC22023240).
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.G WC W, and GP L; Investigation: YQ.W, WJ.S and XY. LWriting-original draft: WC.W ; Writing-review and editing: Y.G, WC.W, and GL P. All authors have read and agreed the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, W., Wang, Y., Xu, Y. et al. Effect of external infusion connection devices replacement frequency on catheter related bloodstream infection. Sci Rep 15, 3315 (2025). https://doi.org/10.1038/s41598-025-87310-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87310-2