Abstract
Zinc deficiency precipitates considerable health problems in developing countries, affecting development, growth, and immunological function. The main issue is that zinc exhibits limited bioavailability in diets, sometimes compounded by the high concentration of phytate molecules in staple foods, which impedes zinc absorption. Nanoparticles offer a promising approach to improve zinc bioavailability and address deficiency through the application of advanced agricultural techniques. The study introduces a novel method for synthesizing Zinc oxide (ZnO) biometallic nanoparticles by employing aqueous extracts of Salvia hispanica L. (Chia seed) as a reducing and capping agent in an environmentally sustainable way. Their active phytoconstituents acted as a stabilising agent and facilitated the conversion of ionic zinc (Zn2+) into elemental zinc. The study synthesized the diverse forms of zinc oxide nanoparticles (NP-α, NP-β, NP-γ, NP-δ, NP-ε, and NP-η) utilising various molar concentrations (0.5mM, 1.0mM, 3.0mM, 5.0mM, 7.0mM, and 9.0mM) of a precursor solution, zinc nitrate [(ZnNO3)2]. The synthesized NPs were evaluated using UV-Vis spectroscopy, FTIR spectroscopy, XRD, SEM, EDX, TEM, SAED, and HR-TEM methods to determine their characteristics. The standard particle size varies from 40 to 80 nm, exhibiting a consistent hexagonal morphology and a polydispersed characteristic with minimal size fluctuation. The molarity substantially influenced the shape of NPs, particularly concerning their size and surface area. An in vitro evaluation was performed to investigate the antibacterial activity against Staphylococcus aureus and the possible degradation of the hazardous dye Congo red. The particles exhibited antibacterial efficacy at a concentration of 40 ppm ZnO, antidiabetic qualities at 10 µl/ml ZnONPs, antioxidant activity at concentrations ranging from 100 to 900 µl/ml showing 89.47 ± 0.022 µg AAE/mg, maximum activity with total antioxidant capacity (TAC), and dye degradation potential at a concentration of 50 mg ZnONPs, revealed 50.78% CR degradation after 90 min of irradiation. Additionally, it had significant inhibitory effects on the enzymes α-amylase (72.93%) and α-glucosidase (60.48%) by ZnONP-η. The efficacy of dye degradation with synthesized nanoparticles seems to enhance with increased particle sizes and reduced specific surface areas. The antioxidant, antidiabetic, and catalytic capabilities improved with an increase in particle size. Nevertheless, it was found that an increase in particle size corresponded with a substantial reduction in antibacterial activity. The study presents an efficient approach for the eco-friendly synthesis of ZnONPs, highlighting their significant potential for many biological applications.
Similar content being viewed by others
Introduction
Nanotechnology has been recognised as a possible catalyst for the forthcoming industrial revolution, offering substantial promise for facilitating many biological developments in the 21st century. In recent decades, there has been a significant rise in the global production and use of nanoscale materials, including various forms metal oxide nanoparticles, carbon nanoparticles, and quantum dots. Metal oxide nanoparticles are particularly significant due to their extensive uses in biomedical and environmental sectors, owing to their distinctive optical, chemical, mechanical, electrical, and magnetic properties1. The defining characteristic of nanomaterials lies in their reduced dimensions, typically ranging from 1 to 100 nm. This nanoscale size results in an increased surface-to-volume ratio, which in turn augments their surface reactivity2. This enhanced reactivity has facilitated their utilization in a wide range of applications, spanning from materials science to biotechnology, solidifying their importance in advancing scientific frontiers.
Among other nanoparticles, zinc oxide nanoparticles (ZnONPs) have been widely employed in the field of medicine, with applications including anti-angiogenesis, anti-inflammatory, anti-platelet, dentistry, medication, gene delivery, and cosmetic3,4. It has gained considerable interest in the domain of metallic nanoparticles owing to their remarkable attributes, including an increased band gap, strong piezoelectric capabilities, and enhanced binding energy5,6. These attributes of ZnONPs have generated considerable interest for diverse technological and industrial applications, such as piezoelectricity, optoelectronics, diagnostics, biological labelling, magnetic sensing, ceramics and rubber production, environmental protection, biology, and pharmaceuticals7,8.
There are several physical, chemical and biological methodologies have been known for the preparation of nanoparticles, but researchers are increasingly focusing on biological methods because of its simplicity, environmental friendliness, cost-effectiveness, and absence of hazardous substances or toxic chemical solvents8,9. Other processes involve high thermal conditions, acidic pH and hazardous chemicals which are very toxic and unsafe. Sometimes, hazardous substances adhere to the nanoparticles (NPs), rendering them unsuitable for biological purposes. Moreover, studies suggests that plants exhibit a greater capacity for large-scale nanoparticle synthesis compared to other organisms, including bacteria, fungus, and algae. This potential is attributed to the existence of several bioactive substances in plant bioresources, such as flavonoids, alkaloids, terpenoids, proteins, amino acids, vitamins, enzymes, and glycosides. These chemicals facilitate the bio-reduction and stabilization processes essential for the creation of metallic nanoparticles. Additionally, plant-derived nanoparticles have a broader spectrum of morphological changes regarding their forms and sizes10. Thus, employing plants for the production of ZnO nanoparticles provides a non-toxic, organic alternative to traditional approaches that utilise hazardous chemicals.
Chia (Salvia hispanica L.), a perennial herbaceous plant of the Lamiaceae family, is an abundant source of biologically active compounds. It includes carotenoids, phytosterols, tocopherols, and phenolic compounds like chlorogenic acid, vanillic acid, caffeic acid, ferulic acid, rosmarinic acid, quercetin, myricetin, and kaempferol. Moreover, it possess terpenoids and flavonoids, including apigenin, luteolin, and quercetin11,12. The phytochemicals, especially phenols and flavonoids, found in chia seeds are excellent candidates for effective reducing and stabilising agents in nanoparticle production13. This may enhance the conversion of Zn + into elemental zinc, hence contributing to the reduction and stabilization processes. Therefore, the study selected chia seeds because of their extensive application in health-related operations, such as flour production, mucilage extraction, oil extraction, and intake as whole seeds. The aqueous extracts of chia seeds was employed for the synthesis of ZnONPs at a temperature of 60°C. To validate the synthesis of the nanoparticles, several characterization techniques were employed, including ultraviolet-visible spectroscopy, Fourier-transform infrared (FTIR) spectroscopy, high-resolution transmission electron microscopy (HR-TEM), X-ray diffraction (XRD) analysis, and scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDS). Furthermore, the biological activities of the synthesized ZnONPs, including antioxidant, antibacterial, antidiabetic, and catalytic properties, were assessed in vitro. The study presents a novel method for synthesizing various forms of ZnONPs, with the aim of investigating their potential applications in diverse industries.
Materials and methods
General reagents
The high-purity chemical reagents were procured from Sigma-Aldrich (St. Louis, MA, USA). Zinc nitrate hexahydrate [Zn(NO3)2.6H2O)], Nutrient agar, and Diphenyl Picryl Hydrazyl radical (DPPH˙) were purchased from Hi-media, India. All the solutions were prepared using double distilled water.
Seed collection and extraction
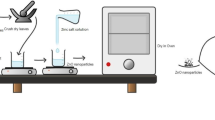
Chia seeds were procured from a local market and subjected to a rigorous cleaning process, involving multiple washes in double-distilled water to eliminate any contaminants. The seeds were then dried in an oven at 50 °C for approximately 30 min. Subsequently, 4 g of the cleaned and dried seeds were immersed in 100 mL of double-distilled water for a period of 1 to 2 h. Following this soaking step, the seeds were crushed and then subjected to a temperature of 80 °C for 15 min. The resulting aqueous suspension of seed extract was filtered through Whatman No. 1 filter paper. The obtained filtrate, designated as the chia seed aqueous extract, was stored at 4 °C for further use in the study12,14.
Synthesis of zinc oxide nanoparticles (ZnONPs)
The synthesis of ZnONPs was conducted following the method described by El-Beltagi et al.15 with certain modifications. Briefly, 25 mL of chia seed extract was heated to 60°C on a magnetic stirrer with continuous stirring. Upon reaching 60 °C, zinc nitrate hexahydrate [Zn(NO3)2.6H2O] was introduced at varying molarities: 0.5 mM, 1.0 mM, 3.0 mM, 5.0 mM, 7.0 mM, and 9.0 mM. Each mixture was then left undisturbed on the magnetic stirrer at 60°C for approximately 1 h until a white precipitate formed. The supernatant was discarded, and precipitates were transferred to 1.5 mL Eppendorf tubes. The existence of white-colured particles indicated the formation of ZnO nanoparticles. The precipitate was subjected to multiple washes using a 3:1 mixture of distilled water and ethanol. Subsequently, the samples underwent thermal treatment in a hot air oven at 60°C until a creamy white powder was obtained. The powder was carefully transferred into a hermetically sealed container for further characterization.
Characterization of ZnONPs
Several characterization techniques were used to analyse the physical and chemical properties of synthesized ZnONPs. These techniques involved scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX), transmission electron microscopy (TEM), selected area electron diffraction (SAED), Fourier-transform infrared spectroscopy (FTIR), and X-ray diffraction (XRD) analysis. The reaction between the extract and precursor solution (zinc nitrate) was quantified using a spectrophotometer (Shimadzu UV-1800), which measured the absorbance within the wavelength range of 250–550 nm.
FTIR (FTIR-8400 spectrometer; Shimadzu, Japan) spectroscopy was employed to identify the functional groups that are responsible in the formation of nanoparticles within the spectral range of 400 to 4000 cm−116. The powdered ZnONPs were used in pellet method with potassium bromide (KBr) to record the Fourier transform spectra in transmittance mode at 4.0 cm−1 of resolution16.
XRD was conducted using an eMMA diffractometer (BRUKER, USA), operating at a voltage of 40 kV and current of 20 mA16. The diffraction measurements were performed using CuK-α radiation with a wavelength of 1.54187 nm. The crystalline size (D) of synthesized NPs was calculated using the following Scherrer equation formula:
\({\text{D}}\,=\,{\text{K}{\lambda}}/(\beta{\text{cos}\theta})\)
Where, D is the average crystalline size of ordered crystal grain, K is a dimensionless shape factor of (0.9), X-ray wavelength is 0.15406 nm, full breadth at half maximum intensity was measured in radians, and Bragg angle was measured in degrees.
The morphology and physical dimensions were conducted by using TEM (TEM-4x JEOL, Japan) and SEM (ZEISS-Evo18, Germany), while EDx was used to accomplish the elemental analysis17. SEM was performed at a 20 kV accelerating voltage to analyse the morphological variation including shape and size. To prepare the sample for SEM, a small amount of the sample was dropped on a gold coated copper grid. The excess solution was wiped off using blotting paper and the film on grid was left for 5 min to dry under a mercury lamp17.
Biological applications
Estimation of antioxidant assays
Total antioxidant capacity (TAC) determination
The assay described by Malik et al.18 was used to assess the total antioxidant activity of ZnONPs with some modification. Briefly, 100 µl of the NP sample was introduced into the tubes for the experiment. Then after, 900 µl of TAC reagent including 0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate in 50 ml distilled water were subsequently placed into the tube of tested sample. The reaction mixture was settled at room temperature after being incubated for 2.5 h at 90oC in a water bath. The absorbance of sample was determined using a microplate reader at 630 nm. TAC was represented as µg ascorbic acid equivalent (AAE) per mg of the sample, and the assay was performed three times (triplicates).
Total reducing power (TRP) determination
The method described by Malik et al.18 was used to ensure the total reducing ability of ZnONPs with minor modification. In brief, eppendorff tubes were filled with a 100 µl test sample, 400 µl of 0.2 Molar phosphate buffer (pH 6.6), and 1% w/v potassium ferric cyanide. The tubes with whole mixtures were incubated in a water bath at 55oC for 30 min. Each tube was then filled with 400 µl of 10% w/v trichloroacetic acid, and centrifuged for 10 min at 3000 rpm. The supernatant (140 µl) of each mixture was added to the corresponding wells of a 96-well plate, which already contained 60 µl of ferric cyanide solution (0.1% w/v). Both positive and negative controls followed the previously described method. The expression for total reducing power (TRP) was expressed as µg AAE (ascorbic acid equivalent) per mg of the substance.
DPPH antioxidant test
The antioxidant capabilities of biosynthesized ZnONPs were evaluated via utilizing the 2, 2-Diphenyl-1-picrylhydrazyl radical (DPPH) along with free radical scavenging assay (FRSA), following Vinotha et al.19. After adding 1 mM of DPPH to 2.5 ml of 50% methanol, the mixture was kept at 20 °C in the dark for 30 min. Further, different concentrations of NPs (100 to 900 µg/ml) were added. Ascorbic acid was used as positive control. The variations in absorbance were computed at 517 nm wavelength using a UV-visible spectrophotometer. The following equation was used to represent the radical scavenging activity as rate movement.
\({\text{Radical scavenging activity }}\% {\text{ }}=~\frac{{\left( {{\text{Control absorbance}}--{\text{sample absorbance}}} \right)}}{{\left( {{\text{Control absorbance}}} \right)}}~{\text{x 1}}00\)
Antioxidant test with ABTS
The ABTS [2, 2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] assay was employed to assess the antioxidant properties of the synthesized particles20. Initially, 2.45 mM potassium persulfate and 7 mM ABTS salt was added to the mixture equally and left in the dark for 16 h at 25°C. The absorbance of the sample was measured at 734 nm. The Trolox reagent was used as positive control. The antioxidant results were expressed as TEAC and the tests were conducted in three replicates.
ZnONPs as antibacterial agent
The antimicrobial efficacy of biogenic ZnONPs was evaluated using conventional techniques21. It was assessed against the gram-positive bacterial strain Staphylococcus aureus (MTCC-29090) using the disc diffusion technique. To making a fresh culture of bacterial strain, the nutrient broth was used as the culture medium and stored at 4 °C. To ensure homogeneity of the medium and bacterial culture, 100 µL of the bacterial strain was inoculated into 100 mL of agar medium and thoroughly mixed. After that, each petri dish was filled with 20 ml of nutritional agar media and left undisturbed to solidify the media in the petri plates without interruption, creating a smooth and homogenous surface. The petri plates with media were punctured using a sterile cork borer to form wells with a diameter of 5 mm. Wick’s paper discs of 9 mm in diameter, were used as ZnONPs loading agents. These discs were subjected to sterilization before being transferred onto petri plates. Thenafter, discs containing ZnONPs at a concentration of 40 ppm were carefully positioned into the wells using sterile forceps and subsequently labelled. The plates of interest were subjected to continuous monitoring for duration of 24 h at a temperature of 37°C in BOD incubator. The zones of inhibition were observed on the petri plates and analyzed. The experiment was conducted with three replicates for each test, and the resulting data were presented as the mean and standard deviation22.
Antidiabetic assay
The anti-diabetic activity of biogenic ZnONPs was examined on basis of inhibitory assays of α-glucosidase and α-amylase enzyme using standard methods.
α-Amylase inhibition assay
The antidiabetic property of ZnONPs in terms of α-amylase inhibition was conducted following Jan et al.23. A 96-well microplate was employed to conduct the test. Each well of the microplate was supplemented with specific volumes of phosphate buffer (15 µl) of 6.8 pH, α-amylase (25 µl), test sample (10 µl), and starch (40 µl). Subsequently, the plate was endured a 30-min incubation period at a temperature of 50°C. Finally, a total of 90 µl of iodine and 20 µl of hydrochloric acid (HCl) solution were carefully transferred into each well. Dimethyl sulfoxide (DMSO) was employed as the negative control, Acarbose served as the positive control, and starch was utilized as a substitute for the buffer solution in the blank. In order to assess the absorbance aptitude of the sample, a microplate photometer was operated at a wavelength of 540 nm. The inhibition percentage was determined using the following formula:
\({\text{Enzyme inhibition }}\% {\text{ }}=\frac{{\left( {{\text{Sample mean }}--{\text{ negative control mean}}} \right)}}{{\left( {{\text{blank mean }}--{\text{ negative control mean}}} \right)}}{\text{x 1}}00\)
α-Glucosidase inhibition assay
The α-glucosidase inhibition test was used to demonstrate their anti-diabetic efficacy by the method of Khyrun et al.24. In the assay, α-glucosidase (Saccharomyces cerevisiae, Sigma-Aldrich) was dissolved by adding 50 mL of phosphate buffer (pH 6.8) supplemented with 100 mg bovine serum albumin (BSA). The reaction mixture was prepared using 490 µl phosphate buffer (pH 6.8), 250 µl of p-nitrophenyl-D-glucopyranoside (5 mM), and 10 µl of the test sample; it was incubated for 5 min at 37 °C. Following a 15-minute re-incubation period at 37 °C, 250 µL of α-glucosidase (0.15 units/mL) was added to each mixture. The reaction was then stopped by adding 2 mL of Na₂CO₃ (200 mM) solution, and the absorbance was measured at 400 nm using a UV-vis spectrophotometer. The assay, performed in triplicate, measured the p-nitrophenol produced from p-nitrophenyl α-D-glucopyranoside. Acarbose was used as a positive control.
\({\text{Enzyme inhibition }}\% {\text{ }}=\frac{{\left( {{\text{Sample mean }}--{\text{ negative control mean}}} \right)}}{{\left( {{\text{blank mean }}--{\text{ negative control mean}}} \right)}}{\text{x 1}}00\)
Catalytic activity
The degradation ability of the toxic dye (Congo red) was evaluated using the procedure described by Hitkari et al.25. In order to evaluate the efficacy of NPs in dye degradation, a dye solution with a concentration of 50 ppm was employed after undergoing several optimisations. During the degradation process, 50 mg of each synthesized ZnO nanoparticles were utilised as catalyst. A stock solution was prepared by mixing 10 mg of the dye with 1 L of distilled water. Furthermore, 90 ml of the stock solution was taken out and 50 mg of NPs were added along with 10 ml of distilled water. Eventually, a mixture containing both dye and NPs was introduced to sonication for 5 min and then subjected under a 100-watt tungsten bulb light as an inducing source for the reaction. A volume of 3 ml of the resultant solution was utilised to quantify the catalytic degradation of dye across various time intervals. The absorbance of the dye was recorded at different time intervals (0 min, 15 min, 30 min, 45 min, 60 min, 75 min, and 90 min) using a UV-visible spectrophotometer at the wavelength of 400 to 800 nm to measure the degradation percentage. The dye solution without ZnONPs was utilised as a control. The “Catalytic Degradation Efficiency” (n) was calculated as:
\(n={C_0} - Ct/{C_0} \times 100\)
C0 denoted the absorbance of the reaction mixture including dye with and without a catalyst (ZnONPs), and Ct represents the absorbance of reaction mixture at t time.
Statistical analysis
The statistical significance was evaluated using one-way analysis of variance (ANOVA) test, followed by p < 0.05 as significance level according to the Tukey’s post hoc test. According to established conventions, the mean and standard deviation were computed in excel.
Results
Characterization of green synthesized ZnONPs
During the synthesis, the solutions (containing the zinc nitrate precursor and seed extract) exhibited colour changes, and a yellowish-white precipitate formed, indicating the reduction of zinc nitrate.
U.V. spectrophotometric analysis
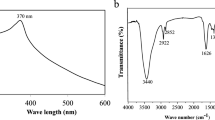
UV-vis spectroscopy was accustomed initially to validate the synthesis of ZnONPs over the wavelength range of 250–550 nm. The absorption spectra of synthesized ZnONPs revealed a distinctive peak at 350–358 nm (Fig. 1).
XRD analysis
X-ray diffraction (XRD) analysis was employed to determine the crystal phases and crystallinity of the synthesized ZnONPs (Fig. 2a). In low-molarity-based synthesis, the produced ZnONPs displayed poor crystalline structure. The preferred diffraction peaks of NPs were found at 2 theta (2Ө) = 47.42, 56.40, 62.76, 66.38, 67.82, and 69.42o, which, respectively, corresponded to the lattice planes (102), (110), (103), (200), (112), and (201). All of the diffraction peaks (in Fig. 2a) were accurately matched to the phase of hexagons (wurtzite pattern) of ZnONPs and are quite near to the approved values (Joint Committee on Powder Diffraction Standards (JCPDS) number 36–1451). These results supported strong crystallinity and semi-spherical to hexagonal indices of particles. The size of the crystals and the intensity of the diffraction peaks both increased as the molarity ascended from 5.0 mM to 9.0 mM. At the (101) plane, the average crystalline sizes of NP-α, β, γ, δ, ε and η were determined using Scherrer’s Equation, which were 4.83 nm, 8.23 nm, 11.31 nm, 16.35 nm, 22.38 nm, and 24.45 nm respectively. The sample of NP-α (low molarity-based NPs) had the least average crystalline size, measuring 4.83 nm. As the molarity increased, the average crystalline size progressively increased.
FTIR spectroscopic analysis
FTIR was used in order to validate the synthesis of nanoparticles at the nanoscale level. Furthermore, it was used to analyze the rotational and vibrational modes of the molecules to identify the functional groups and potential phytoconstituents involved in the reduction and stabilization of the ZnONPs. The analysis was aimed to ascertain the potential interactions between phytochemicals, such as proteins and secondary metabolites, present in the extract. The FTIR spectrum of the aqueous seed extract displayed absorption peaks at specific wavenumbers, namely 1742.19 cm−1, that indicating the presence of a ketone group, 1646.34 cm−1, demonstrating the presence of a C = C group, 1157.16 cm−1, pointing to C-O stretching, and 2852.14 cm−1 and 2922.49 cm−1, suggesting the presence of an amine (C-H) group (Table-1). In addition to smaller peaks at 1362.39 cm⁻¹ indicative of phenol and nitro compounds (commonly found in proteins and enzymes), further peaks were observed at 2314.90 cm⁻¹ and 1636.45 cm⁻¹. The peak at 2314.90 cm⁻¹ corresponds to C–H stretching vibrations, while the peak at 1636.45 cm⁻¹ can be attributed to primary and secondary amines, polysaccharide C–O stretching, and phenolic groups. These results suggest that various biological components are involved in the formation and stabilization of the ZnO nanoparticles26. Further evidence for the involvement of biomolecules in the synthesis of ZnONPs was provided by the observation of minor peak shifts across all samples. Table 1 provides a comparison of the observed functional groups with their respective standard forms. Overall, C–H and O–H were the predominant functional groups, exhibiting slight peak shifts with increasing molarity of the NPs. Figure 2b displays peaks at 510 cm⁻¹ and 526 cm⁻¹, corresponding to the metal oxide stretching vibrations of Zn–O bonds, as well as a peak at 886 cm⁻¹ representing the formation of tetrahedral coordination of Zn. The stretching vibrations of the ZnO nanoparticles were indicated by peaks ranging from 627.25 to 756.47 cm⁻¹. Broad peaks were observed at 3450.38 cm⁻¹ and 1157.16 cm⁻¹, indicative of O–H and C–O stretching vibrations, respectively. The increase in crystalline size of the NPs has been attributed to the increasing molarity, which may induce reorientation of the particles and reduce defects in the NP grain boundaries. These findings are consistent with the study conducted by Liang et al.27. XRD analysis of the ZnO nanoparticles showed that the average particle size increased with increasing molarity. Therefore, nanoparticles synthesized with higher molarities (5.0 mM, 7.0 mM, and 9.0 mM), denoted as NP-δ, NP-ε, and NP-η, respectively, were selected for further characterization using SEM, TEM, SAED, and HR-TEM.
SEM analysis
Morphological structure plays a crucial role in determining the electro-optic and biological properties of nanoparticles. SEM analysis revealed that the biosynthesized ZnONPs were semi-spherical to hexagonal in shape, exhibiting polydispersity and some aggregation (Fig. 3a-c). ZnONP-η, synthesized with 9.0 mM molarity (Figs. 4 and 5), exhibited an amorphous and porous nature (Fig. 6b). The presence of both agglomerates and dispersed nanoparticles (Fig. 3) is consistent with the findings of Khan et al.28.
TEM and HR-TEM analysis
Transmission electron microscopy (TEM) (ZEISS-Evo18) was employed to characterize the morphology of the ZnONPs, including size distribution, grain size, and individual particle dimensions. TEM images of the synthesized ZnONP-δ (Fig. 4a), ZnONP-ε (Fig. 5a), and NP-η (Fig. 6a) demonstrated that the average particle size varied between around 40 to 60 nm, 50 to 60 nm, and 60 to 70 nm, respectively. Furthermore, the analysis of the average size and area distribution curves of the synthesized ZnONP-δ (Fig. 4d and e), ZnONP-ε (Fig. 5d and e), and ZnONP-η (Fig. 6e and f) was measured. These curves depict the diversity of particles in terms of their size and area distribution which were generated through the utilization of ImageJ software, which analyses on TEM images. TEM images, acquired at a magnification of 200 nm, revealed a predominant distribution of particle sizes ranging from 40 to 70 nm7. An analysis of the crystalline structure and interplanar distances (d) of ZnONP-δ, NP-ε, and NP-η was conducted using high-resolution transmission electron microscopy (HR-TEM) images. The measurements revealed interplanar distances of 0.180 nm, 0.250 nm, and 0.261 nm for the structures of the particles. The interplanar distances (d) of ZnONP-δ (Fig. 4c), NP-ε (Fig. 5c), and NP-η (Fig. 6d) were determined to be 0.180 nm, 0.250 nm, and 0.261 nm, respectively. In addition, it was found that the SAED pattern for ZnONP-η (Fig. 6c) displayed a higher quantity of concentric rings compared to the diffraction peaks observed in ZnONP-δ and ZnONP-ε (Figs. 4b and 5b). SAED analysis confirmed the presence of the (100), (002), and (101) planes, consistent with the XRD results of this study29.
Energy dispersive X-ray analysis (EDX)
The elemental composition and stereochemistry of NPs were analysed using energy-dispersive X-ray spectroscopy (EDX). The presence of distinct zinc and oxygen signals confirmed the successful synthesis of ZnONPs in a pure chemical state. EDX analysis further revealed a distinct peak corresponding to zinc, confirming the effective synthesis and purity of the plant-synthesized ZnONPs. The current mass of oxygen and zinc in NP-η (Zn = 59.41%) (Fig. 7a and b) was greater than that in NP-δ (Zn = 39.31%, O = 19.07%) (Fig. 8a and b) and NP-ε (Zn = 40.93%, O = 26.63%) (Fig. S1a & S1b). A previous study on ZnO NPs synthesized from Syzygium cumini reported an elemental composition of 65% zinc and 25% oxygen30. EDX analysis in the current study showed similar results, with zinc constituting more than 74% of the total elemental composition and oxygen accounting for over 26%.
The elemental mapping of the synthesized ZnONP-δ, NP-ε, and NP-η is depicted in Figs. 7c and 8c, and Fig. S1c, respectively. These figures demonstrate the uniform appearance of the grain and the corresponding distribution of Zn, O, and C elements. Additional peaks may be due to the presence of capping agents on the ZnONPs, the elemental composition of the glass used for sample storage, the presence of other elements in the salts, or the inherent characteristics of the photo-structures. The findings are remarkably comparable to earlier reports of Ali et al.31 and Rahman et al.9.
Biological applications
Anti-oxidant assays
Reactive oxygen species (ROS), molecules with unpaired electrons, are produced within body cells and tissues. These free radicals can arise from both internal factors, such as autoimmune disorders, and external sources like pollution, radiation, toxic chemicals, and nicotine inhalation. Oxidative stress, resulting from an imbalance between ROS production and the body’s antioxidant defences, has been implicated in numerous diseases, including Parkinson’s disease, arthritis, cancer, and heart disease. Antioxidants play a crucial role in mitigating oxidative stress by neutralizing free radicals, thereby protecting cells and tissues from damage32,33. This study investigated the antioxidant properties of synthesized zinc oxide nanoparticles (ZnONPs) using four different assays: total antioxidant capacity (TAC), total reducing power (TRP), ABTS radical scavenging assay, and DPPH free radical scavenging assay (Table 2).
The TAC assay, based on the phosphomolybdenum method, measures the reduction of Mo (VI) to Mo (V) by antioxidants. ZnONPs exhibited a TAC value of 89.47 ± 0.022 µg ascorbic acid equivalents (AAE)/mg at a concentration of 900 µg/ml. The TRP assay assesses the ability of ZnO NPs to reduce Fe³⁺ ions to Fe²⁺ ions, indicating their redox potential. The maximum TRP value observed was 75.44 ± 0.021 µg AAE/mg at 900 µg/ml. Furthermore, the DPPH and ABTS assays confirmed the antioxidant capacity of ZnO NPs. These spectrophotometric methods evaluate the ability of antioxidants to quench stable colored radicals. The highest radical scavenging activity was observed with DPPH (78.19 ± 0.02%) and ABTS (84.34 ± 0.023%). All assays were performed in triplicate. The results demonstrate that ZnO NPs possess significant antioxidant properties, with a dose-dependent response observed in all assays. These findings highlight the potential of ZnO NPs as effective free radical scavengers, suggesting their possible therapeutic applications in mitigating oxidative stress-related diseases.
Antibacterial activity
The antibacterial activity of six ZnONP types (η, ε, δ, γ, β, and α) against Staphylococcus aureus was assessed using the zone-of-inhibition method. The measured inhibition zones for the different ZnONPs were 3.75 ± 0.95, 5 ± 2.16, 5.75 ± 1.70, 6.25 ± 1.5, 7.75 ± 1.89, and 9.63 ± 1.08 mm, respectively (Figures S2 and S3). ZnONP-α exhibited the most pronounced antibacterial efficacy, with an inhibition zone of 9.63 mm, compared to other NP types. The antibacterial plates and graphs in Figure S3 clearly demonstrate that ZnONP-α, synthesized with lower molarity zinc nitrate, produced larger zones of inhibition against S. aureus. These findings suggest that nanoparticle dimensions and density play a substantial role in influencing antibacterial efficacy. Zinc oxide nanoparticles have demonstrated strong antibacterial activity against the Gram-positive bacterium, S. aureus. As particle size decreases, a greater number of particles can penetrate the bacterial cell membrane, leading to cell rupture. The surface area of NPs is another notable characteristic that significantly affects their antibacterial activity34,35.
Regarding ZnONPs’ antibacterial mechanism, it has been established that the production of ROS from ZnONPs can cause oxidative stress in bacterial cells, which inhibits DNA replication and protein synthesis. In this case, the ZnO conductivity rises near the band gap of UV spectrum, which is defined by high emission energy. The electronic excitation can destabilize the charges present in the cytoplasmic membrane resulting in their rupture. The charge of cytoplasmic membrane may become unstable due to the electrical excitation, it results the rupture of cell. The release of Zn²⁺ ions from the dissolution of ZnO in aqueous solutions can induce damage to the cytoplasmic membrane35. This damage is attributed to the Zn²⁺ ion’s affinity for sulfur groups, leading to the oxidation of thiol groups and subsequent inhibition of glycolytic enzymes. Furthermore, the surface area of nanoparticles plays a crucial role in their antibacterial activity34,35. Notably, the presented data demonstrate that these plant-based ZnO NPs exhibit higher antibacterial activity compared to previous research utilizing Camellia sinensis-mediated ZnO NPs against various Gram-positive and Gram-negative bacterial strains36.
Antidiabetic activity
The particles exhibited inhibitory activity against α-glucosidase and α-amylase (Table 3). A maximum α-amylase inhibition of 72.93% was observed at a ZnONP-η, concentration of 280 µg/ml. At the same concentration, α-glucosidase inhibition was 60.48%. These findings provide empirical evidence supporting the potent antidiabetic properties of bio-based ZnO nanoparticles23 and suggest a potentially advantageous therapeutic strategy for diabetes, offering a viable alternative to conventional pharmaceutical interventions.
Catalytic activity
The photocatalytic activity of the synthesized ZnONPs was evaluated by monitoring the degradation of Congo Red (CR) dye at various time intervals, with absorbance measurements taken between λmax 450 and 500 nm. As illustrated in Figure S4, the concentration of CR dye decreased with increasing irradiation time, indicating photocatalytic degradation. Control experiments confirmed that dye degradation occurred solely in the presence of the photocatalyst and upon exposure to irradiation. UV-Vis spectrophotometric analysis was employed to investigate the influence of irradiation time on the photocatalytic degradation of Congo Red (CR) dye. The experimental setup involved the addition of 50.0 mg of ZnONPs to the CR dye solution, which was subsequently exposed to irradiation from a 100-watt tungsten bulb at various time intervals. The photocatalytic activity of different ZnONP types (α, β, γ, δ, ε, and η) was assessed by monitoring the degradation of CR dye under light irradiation (Fig. S4). Upon dissolution of ZnONPs in the CR solution, the absorbance spectra exhibited values ranging from 0.978 to 1.15. However, a gradual decrease in absorbance was observed throughout the irradiation process, accompanied by a progressive decolorization of the solution over 90 min. The percentage of CR dye photodegradation was determined by measuring the absorbance at different time points for various ZnONP types. Figure S4 illustrates the degradation of CR dye as a function of irradiation time. The results indicate that ZnONPs exhibit enhanced photocatalytic degradation compared to bare ZnONPs and the dye alone (Fig. S4a). Furthermore, a positive correlation was observed between irradiation time and the percentage of degradation. Specifically, the degradation percentage increased with irradiation time, reaching a maximum of 50.78% after 90 min. ZnONP-η demonstrated 39.85% photocatalytic degradation after 30 min of light exposure. Extending the irradiation time to 90 min resulted in a higher level of photocatalytic degradation, reaching 50.78% (Fig. S5b). To assess the influence of photocatalyst concentration on photocatalytic activity, various ZnO catalysts (ZnONP-α, β, γ, δ, ε, and η) were synthesized and employed in the photodegradation of Congo Red (CR) dye. As depicted in Fig. 5b, the percentage of photodegradation increased with increasing molarity of the catalyst. Furthermore, Figure S5a presents the rate constants (k) for CR dye degradation, determined to be 0.0010 min⁻¹, 0.0011 min⁻¹, 0.0020 min⁻¹, 0.0021 min⁻¹, 0.0025 min⁻¹, and 0.0031 min⁻¹ for ZnONP-α, β, γ, δ, ε, and η, respectively. Notably, ZnONP-η exhibited a degradation rate constant for CR that was 3.1 times higher than that of ZnONP-α, 2.8 times higher than ZnONP-β, 1.55 times higher than ZnONP-γ, 1.47 times higher than ZnONP-δ, and 1.24 times higher than ZnONP-ε. These findings indicate that ZnONP-η possesses a superior capacity for degrading CR dye compared to other ZnONP types. The observed increase in degradation rate with higher molarity ZnONPs may be attributed to a corresponding increase in active sites, providing a significant explanation for the relationship between NP type and degradation percentage.
Additionally, the kinetic analysis was conducted to establish the relationship between the natural logarithm of Ct/C0 and irradiation time (Eq. 1). Figures S5c and S5d depict the plots of relative concentration versus irradiation time for the synthesized ZnONPs (NP-α, NP-β, NP-γ, NP-δ, NP-ε, and NP-η). A decrease in the concentration of the CR solution was observed with increasing irradiation time for all NP types. The overall CR degradation capabilities of ZnONP-α, NP-β, NP-γ, NP-δ, NP-ε, and NP-η were approximately 19%, 20%, 34%, 38%, 39%, and 51%, respectively. ZnONP-η exhibited significantly higher photocatalytic activity in the degradation of the CR dye compared to other ZnONPs. This superior performance may be attributed to the high efficiency of the catalyst and its exceptional kinetic performance, as evidenced by the relationship between relative concentration and maximum irradiation time.
The explanation for the degrading efficiency of the ZnONPs (Fig. S5c) is based on the ratio Ct/C0. Fig. S5d illustrates the relationship between time and the natural logarithm of the concentration ratio Ct/C0, as a means of representing the first-order rate Eq.
Here, C0 denotes the initial concentration of the CR dye, while Ct represents the concentration at a specific time interval. The reaction rate constant, represented by k, signifies the photocatalytic effectiveness of the catalyst. Fig. S5d illustrates a strong correlation between the photocatalytic degradation processes and follows the first-order rate reaction.
ZnONPs possess the ability to generate unpaired electrons (e-) and electron deficiencies (h+) on their surface, as indicated by Eq. 2, and 3. These entities then engage in interactions with the dye, resulting in the formation of superoxide (⋅O2−), which subsequently leads to the production of peroxide and hydroxyl molecules, it may responsible for the degradation of the dye, as represented by Eqs. 4, 5, and 6. In this context, the symbols R and R’ represent the cyclic group and phenyl group, respectively in Eq. 7. The following equations depict the dye degradation reaction mechanism:
In the context of catalytic mechanism of ZnONPs, previous studies37,38 have shown that violet light can excite electrons from the valence band (VB) to the conduction band (CB) of ZnO. This process generates electron-hole pairs (e⁻/h⁺) on the catalyst surface, which are essential for the degradation of CR dye. The diazo groups in the CR dye are cleaved due to the generation of hydroxyl radicals (•OH) on the photocatalyst surface. These radicals are formed via the oxidation of water molecules38,39. Furthermore, photoinduced electrons can reduce adsorbed oxygen molecules (O₂) to superoxide radicals (O2−). These superoxide radicals then react with water molecules to produce hydrogen peroxide (H₂O₂) (Eqs. 4 & 5). Subsequently, H₂O₂ undergoes further reduction by electrons, generating additional hydroxyl radicals (-OH) (Eq. 6). Through a series of oxidation reactions involving these reactive species, the CR dye is ultimately mineralized into carbon dioxide (CO₂), water (H₂O), and non-toxic molecules.
Discussion
The present study explored the production of zinc oxide nanoparticles (ZnONPs) utilising an extract from chia seeds (Salvia hispanica) and zinc nitrate. Research validated the effective synthesis of these nanoparticles with a range of assays, including UV-visible spectroscopy, FTIR, XRD, SEM, TEM, SAED, and EDX. These procedures yielded information on the dimensions, morphology, elemental composition, and crystallinity of the ZnONPs. The study demonstrates that phytochemicals, naturally occurring substances in plants, included in chia seed extract, were important in the synthesis of the nanoparticles. This finding is corroborated by a unique peak (at 350–358) nm in the UV spectrum and aligns with prior studies employing different plant extracts, including Mimosa pudica and tea leaves, for the synthesis of ZnONPs40,41. The primary phytochemical constituents of chia seed extract, including chlorogenic acid, quercetin, caffeic acid, and rosmarinic acid, possess active organic groups crucial for nanoparticle (NP) stabilization. These groups facilitate binding to NPs via electrostatic attraction between negatively charged moieties such as C-O, N-O, C-O, and -OH. Fourier-transform infrared (FTIR) spectroscopy confirmed the presence of O-H and C-O functional groups, as evidenced by characteristic stretching vibrations observed at 3450.38 cm⁻¹ and 1157.16 cm⁻¹, respectively. Notably, the presence of a hydroxyl (-OH) group at the ortho and para positions of the benzene ring in these phytochemicals results in the formation of electron-withdrawing methoxy and allyl groups, respectively. This suggests that the specific molecular structure and electronic properties of these phytochemicals contribute to their ability to stabilize NPs, potentially through enhanced electrostatic interactions and electron delocalization.
Previous research42,43,44,45 has demonstrated that the presence of an -OH group enables a molecule to release a proton, leading to the formation of an anionic species stabilized by resonance structures. Consequently, compounds such as caffeic acid can effectively donate two electrons simultaneously due to the inductive effect and the presence of stable resonating structures. Therefore, the aqueous extract of chia seed, containing chlorogenic acid, caffeic acid, quercetin, or rosmarinic acid, functions as a reducing agent, facilitating the oxidation of Zn(NO3)2 and the subsequent production of ZnONPs46,47. These phytochemicals remain adsorbed to the NP surface even after repeated washing. Furthermore, the observed absorption peaks could be attributed to the band-gap absorption of ZnO, arising from electron transitions between the valence band and the conduction band48. These NPs exhibit remarkable absorption properties, suggesting their potential for incorporation into medicinal applications such as sunscreen formulations or antibacterial ointments7,49. ZnONPs synthesized using zinc nitrate precursors exhibit a hexagonal morphology16 with sizes ranging from 30 to 60 nm50. Similar size ranges have been reported for ZnONPs synthesized using Azadirachta indica seed husk extract (25–60 nm)51. Energy-dispersive X-ray spectroscopy (EDX) analysis of the synthesized zinc oxide nanoparticles revealed higher mass percentages of oxygen and zinc in NP-η (Zn − 59.41%, O − 19.45%) compared to NP-δ (Zn − 39.31%, O − 19.07%) and NP-ε (Zn − 40.93%, O − 26.63%). These values are comparable to those reported for ZnONPs synthesized using other methods30. Recent publications have detailed the green synthesis of zinc oxide nanoparticles using leaf extracts of Koserat and highlighted their antibacterial activity against Gram-positive microbes, as well as their antidiabetic, antioxidant, and catalytic properties46,47,48. These findings provide valuable insights into the mechanisms underlying NP stabilization using natural plant extracts, highlighting the potential of green synthesis approaches in nanomaterial fabrication.
This study investigated the biological activity of synthesized NPs, focusing on their potential applications in various fields, including antibacterial, antioxidant, antidiabetic, and catalytic activities. The antidiabetic potential of ZnO NPs was assessed by evaluating their inhibitory effects on the key digestive enzymes α-amylase and α-glucosidase. Type-2 diabetes, Diabetic mellitus, is a metabolic disorder characterised by persistent hyperglycaemia. This condition arises due to reduced insulin synthesis or the resistance of cells to already produced insulin. The inhibition of postprandial hyperglycemia can be achieved by preventing the activity of these two digestive enzymes (α-amylase and α-glucosidase), which are responsible for carbohydrate hydrolysis in the human body52. The particles may offer a therapeutic method for the treatment of diabetes. Furthermore, in context of antioxidant property, an analysis revealed the presence of polyphenols and flavonoids in the seed extract of S. hispanica. These two categories of natural chemicals have been extensively documented as having antioxidant properties53. The presence of these functional groups could potentially account for the antioxidant characteristic (Table 2). The DPPH-FRSA method was employed to measure the antioxidant activity of particles by quantifying the radical scavengers that were attached to its surface. The results were consistent with previous studies that utilized Geranium wallichianum to synthesize ZnONPs53,54. The ABTS assay illustrates the ability of antioxidants to eliminate ABTS and generate ABTS•+. The nanoparticles possess antioxidant activity, which refers to their ability to diminish the colour efficiency of ABTS•+ either by directly interacting with the preformed radical cation or by inhibiting early oxidation and ABTS•+ formation18,20. Consequently, there is an increasing demand to harness these biological resources for the production of advanced materials.
In addition, the synthesized NPs exhibited potent antibacterial abilities, with the greatest inhibitory effect seen in ZnONP-α compared to NP-β, NP-γ, NP-δ, NP-ε, and NP-η, it indicates that the smaller sized NPs have much efficiency towards antibacterial activity. It was found that green synthesized ZnONPs have strong bactericidal characteristics, which cause harmful effects on many bacterial cellular functions, including enzymatic and metabolic processes. Consequently, these effects ultimately result in the death of bacterial cells55,56. The mechanism behind the cell death may cause the production of reactive oxygen species (ROS), including superoxides, hydroxyl radicals, and hydrogen peroxide. The inability of superoxide and hydroxide ions to penetrate the cell can be attributed to their negative charge56. Consequently, these ions accumulate on the bacterial cell walls, leading to detrimental effects on their structural integrity. This process has the potential to induce cellular wall damage, leading to the subsequent release of intracellular components and ultimately resulting in cell death. The cell wall of bacteria was found to be adversely affected by ZnONPs due to the presence of electrostatic forces, as well as the rough surface of the nanoparticles. This interaction resulted in an enhanced permeability of the cell membranes to Zn ions, ultimately leading to the manifestation of bacterial toxicity35,57. There exist multiple pathways that collectively induce the death of bacterial cells; nevertheless, the precise mechanisms by which these biogenic nanoparticles exert their antimicrobial effects remain uncertain. The findings substantiated prior studies on the antibacterial action of ZnONPs were influenced by linking reactive oxygen species (ROS) to the impairment of cell membranes58. Collectively, the nonspecific mechanical action of green synthesized ZnONPs against pathogenic bacteria offers an environmentally friendly and clean path for the production of antimicrobial drugs.
The NPs exhibited a monodisperse characteristic, which result the significantly increased surface area for the photocatalyst. When the nanoparticles absorb light, then electron moves from the valence band transitions to the conduction band, it resulting in the formation of an electron-hole. The hydroxide free radicals are generated via the conduction band, where they react with dissolved oxygen to produce superoxide. Subsequently, the superoxide further generates the hydroxide free radicals. The hydroxide radical (-OH) generated within the solution exhibited significant reactivity and had the potential to induce degradation of CR. Consequently, the efficiency of dye degradation by ZnONPs was enhanced as a consequence of the pronounced separation of electron-hole pairs, leading to a substantial generation of OHo, HOOo, and Ho radicals, which facilitated the breakdown of dye molecules (Eq. 8 and 9). The synthesized ZnO nanoparticles demonstrate superior catalytic capabilities in the degradation of dyes, which can be attributed to their significant surface area-to-volume ratio, cost-effectiveness, lack of toxicity, and ability to degrade substances. These attributes render them well-suited for use in wastewater treatment and the biomedical industry. Comprehensive examinations of ZnONPs, both in-vitro and in-vivo, are necessary for comprehending their potential utility in the pharmaceutical industry. It is crucial to evaluate their potential in the realms of biomedical and environmental applications.
Conclusion
This study elucidates a sustainable and eco-friendly methodology for the synthesis of zinc oxide nanoparticles (ZnONPs) utilizing Salvia hispanica (chia) seed extract as a bio-reducing and capping agent. This approach circumvents the use of hazardous chemicals and offers a rapid, energy-efficient, and scalable alternative to conventional methods. By meticulously modulating the molarity of the zinc nitrate precursor, ZnONPs exhibiting a hexagonal morphology with a controlled size range of 40–70 nm were successfully synthesized. Comprehensive characterization techniques, including UV-Vis spectroscopy, X-ray diffraction (XRD), and transmission electron microscopy (TEM), were employed to ascertain the optical properties, crystalline structure, and morphology of the synthesized ZnONPs. Remarkably, these ZnONPs exhibited pronounced antibacterial activity against S. aureus, potent antioxidant activity through the effective scavenging of DPPH free radicals, and a notable inhibitory effect on α-amylase activity, suggestive of potential antidiabetic properties. Furthermore, the ZnONPs demonstrated exceptional photocatalytic activity, effectively degrading Congo Red (CR) dye under UV irradiation, highlighting their potential for wastewater remediation. The observed correlation between nanoparticle size and precursor molarity underscores the profound influence of synthesis parameters on the physicochemical and biological properties of ZnONPs, emphasizing the importance of controlled synthesis for altering their functionalities towards specific applications. The study provides a compelling foundation for future investigations aimed at optimizing synthesis conditions, exploring the efficacy of diverse plant extracts, and elucidating the intricate mechanisms underlying the observed biological activities, ultimately paving the way for the development of advanced therapeutic agents and innovative biomedical applications.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Naghizadeh, A. et al. Biogenic and eco-benign Synthesis of Silver Nanoparticles Using Jujube core Extract and its Performance in Catalytic and Pharmaceutical Applications: Removal of Industrial Contaminants and in-vitro Antibacterial and Anticancer Activities. Environ. Technol. Innov. 23101560 (2021).
Chandak, P. G. et al. Nanoparticles in Endodontics-A Review. J. Evol. Med. Dent. Sci. 10, 976–983 (2021).
Ebrahimzadeh, M. A. et al. Enhanced catalytic and antibacterial efficiency of biosynthesized Convolvulus fruticosus extract capped gold nanoparticles (CFE@ AuNPs). J. Photochem. Photobiol., B. 209, 111949 (2020).
Ferro, C., Florindo, H. F. & Santos, H. A. Selenium nanoparticles for biomedical applications: from development and characterization to therapeutics. Adv. Healthc. Mater. 10 (16), 2100598 (2021).
Tortella, G. et al. Bactericidal and virucidal activities of biogenic metal-based nanoparticles: advances and perspectives. Antibiotics 10 (7), 783 (2021).
Yassen, D. A., Othman, F. M. & Hamead, A. A. A. Preparation of CuO/ZnO Nano-particles using Sol-Gel technique and studying the characterization. Eng. Technol. J. 40 (06), 862–868 (2022).
Selim, Y. A., Azb, M. A., Ragab, I., Abd El-Azim, H. M. & M Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Sci. Rep. 10 (1), 3445 (2020).
Komarov, V. V. A review of radio frequency and microwave sustainability-oriented technologies. Sustainable Mater. Technol. 28, e00234 (2021).
Rahman, T. U., Anwar, M. R., Zeb, M. A. & Liaqat, W. Green synthesis, characterization, antibacterial activity of metal nanoparticles and composite oxides using leaves extract of Ocimum basilicum L. Microsc. Res. Tech. 85 (8), 2857–2865 (2022).
Chen, H. et al. Recent developments of electrospun zein nanofibres: strategies, fabrication and therapeutic applications. Mater. Today Adv. 16, 100307 (2022).
Knez Hrnčič, M., Ivanovski, M., Cör, D. & Knez, Ž. Chia seeds (Salvia hispanica L.): an overview—phytochemical profile, isolation methods, and application. Molecules 25 (1), 11 (2020).
Motyka, S., Kusznierewicz, B., Ekiert, H., Korona-Głowniak, I. & Szopa, A. Comparative analysis of metabolic variations, antioxidant profiles and antimicrobial activity of Salvia hispanica (Chia) seed, sprout, leaf, flower, root and herb extracts. Molecules 28 (6), 2728 (2023).
De Falco, B., Amato, M. & Lanzotti, V. Chia seeds products: an overview. Phytochem. Rev. 16, 745–760 (2017).
Al-Qasmi, N. Facial eco-friendly synthesis of copper oxide nanoparticles using Chia seeds extract and evaluation of its electrochemical activity. Processes 9 (11), 2027 (2021).
El-Beltagi, H. S. et al. Biosynthesis of zinc oxide nanoparticles via neem extract and their anticancer and antibacterial activities. PeerJ, 12, e17588. (2024).
Sharma, R. et al. Advancements in biogenic synthesis of zinc oxide nanoparticles for superior water decontamination and antibacterial efficacy. Ionics 30 (10), 6509–6530 (2024).
Gupta, S. et al. Metallic ion-based graphene oxide functionalized silk fibroin-based dressing promotes wound healing via improved bactericidal outcomes and faster re-epithelization. Biomed. Mater. 17 (3), 035010 (2022).
Malik, M. N. et al. Bioprospecting Dodonaea viscosa Jacq.; a traditional medicinal plant for antioxidant, cytotoxic, antidiabetic and antimicrobial potential. Arab. J. Chem. 15 (3), 103688 (2022).
Vinotha, V. et al. Synthesis of ZnO nanoparticles using insulin-rich leaf extract: anti-diabetic, antibiofilm and anti-oxidant properties. J. Photochem. Photobiol., B. 197, 111541 (2019).
Faisal, S. et al. Edible mushroom (Flammulina Velutipes) as biosource for silver nanoparticles: from synthesis to diverse biomedical and environmental applications. Nanotechnology 32 (6), 065101 (2020).
Hussain, A. et al. Biogenesis of ZnO nanoparticles using Pandanus odorifer leaf extract: anticancer and antimicrobial activities. RSC Adv. 9 (27), 15357–15369 (2019).
Brudzynski, K., Abubaker, K. & Miotto, D. Unraveling a mechanism of honey antibacterial action: Polyphenol/H2O2-induced oxidative effect on bacterial cell growth and on DNA degradation. Food Chem. 133 (2), 329–336 (2012).
Jan, H. et al. Plant-based synthesis of zinc oxide nanoparticles (ZnO-NPs) using aqueous leaf extract of aquilegia pubiflora: Their anti-proliferative activity against HepG2 cells inducing reactive oxygen species and other in vitro properties. Oxidative Medicine and Cellular Longevity, 17, 2021:4786227. (2021).
Khyrun, S. F. et al. Environmental and biomedical applications in the synthesis and structural, optical, elemental characterizations of mg doped ZnO nanoparticles using Coleus aromaticus leaf extract. South. Afr. J. Bot. 151, 290–300 (2022).
Hitkari, G., Chowdhary, P., Kumar, V., Singh, S. & Motghare, A. Potential of copper-zinc oxide nanocomposite for photocatalytic degradation of Congo red dye. Clean. Chem. Eng. 1, 100003 (2022).
Abdelbaky, A. S., El-Mageed, A., Babalghith, T. A., Selim, A. O., Mohamed, S. & A. M Green synthesis and characterization of ZnO nanoparticles using Pelargonium odoratissimum (L.) aqueous leaf extract and their antioxidant, antibacterial and anti-inflammatory activities. Antioxidants 11 (8), 1444 (2022).
Liang, Y., Wicker, S., Wang, X., Erichsen, E. S. & Fu, F. Organozinc precursor-derived crystalline ZnO nanoparticles: synthesis, characterization and their spectroscopic properties. Nanomaterials 8 (1), 22 (2018).
Khan, M. S., Dhavan, P. P., Ratna, D. & Shimpi, N. G. Ultrasonic-assisted biosynthesis of ZnO nanoparticles using Sonneratia alba leaf extract and investigation of its photocatalytic and biological activities. J. Cluster Sci., 1–17. (2022).
Velsankar, K., Sudhahar, S., Parvathy, G. & Kaliammal, R. Effect of cytotoxicity and antibacterial activity of biosynthesis of ZnO hexagonal shaped nanoparticles by Echinochloa frumentacea grains extract as a reducing agent. Mater. Chem. Phys. 239, 121976 (2020).
Arumugam, M. et al. Green Synthesis of zinc Oxide Nanoparticles (ZnO NPs) Using Syzygium cumini: Potential Multifaceted Applications on Antioxidants, Cytotoxic and as Nanonutrient for the Growth of Sesamum indicum. Environ. Technol. Innov. 23101653 (2021).
Ali, J. et al. Synthesis and characterization of phytochemical fabricated zinc oxide nanoparticles with enhanced antibacterial and catalytic applications. J. Photochem. Photobiol., B. 183, 349–356 (2018).
Ihsan, J. et al. Synthesis, characterization, and biological screening of metal nanoparticles loaded gum acacia microgels. Microsc. Res. Tech. 84, 1673–1684 (2021).
Mohammadi-Aloucheh, R. et al. Antiproliferative Activity of zinc oxide-silver Nanocomposite Interlinked with Vaccinium Arctostaphylos L. Fruit Extract against cancer Cells and bacteria. Chem. pap. 1–11 (2022).
Mendes, C. R. et al. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 12 (1), 2658 (2022).
Hoseinzadeh, E. et al. A review on nano-antimicrobials: metal nanoparticles, methods and mechanisms. Curr. Drug Metab. 18 (2), 120–128 (2017).
Sujatha, V., Venkatesan, A., Sancharan, A. & Thirunavukkarasu, C. Biogenic zinc oxide nanoparticles attenuate Acute Lymphoblastic Leukemia Cell Proliferation through oxidative stress and DNA damage. Pharm. Sci. 30 (2), 262–273 (2024).
Lanjwani, M. F., Tuzen, M., Khuhawar, M. Y. & Saleh, T. A. Trends in photocatalytic degradation of organic dye pollutants using nanoparticles: a review. Inorg. Chem. Commun. 159, 111613 (2024).
Kadhim, M. J. et al. The most important parameters that affect the photocatalytic activity of ZnO nanostructures against organic dyes: a review. Iran. J. Catal., 13(1). (2023).
Saad, I., Ralha, N., Abukhadra, M. R., Zoubi, A., Ko, W. & Y. G Recent advances in photocatalytic oxidation techniques for decontamination of water. J. Water Process. Eng. 52, 103572 (2023).
Balogun, S. W., James, O. O., Sanusi, Y. K. & Olayinka, O. H. Green synthesis and characterization of zinc oxide nanoparticles using bashful (Mimosa pudica), leaf extract: a precursor for organic electronics applications. Sn Appl. Sci. 2, 1–8 (2020).
Bahari, N. et al. Role of Honey as a Bifunctional reducing and Capping/Stabilizing Agent: application for silver and zinc oxide nanoparticles. Nanomaterials 13 (7), 1244 (2023).
Álvarez-Chávez, L. M., Valdivia-López, M. D. L. A., Aburto-Juarez, M. D. L. & Tecante, A. Chemical characterization of the lipid fraction of Mexican Chia seed (Salvia hispanica L). Int. J. Food Prop. 11 (3), 687–697 (2008).
Reyes-Caudillo, E., Tecante, A. & Valdivia-Lopez, M. A. Dietary fibre content and antioxidant activity of phenolic compounds present in Mexican Chia (Salvia hispanica L.) seeds. Food Chem. 107 (2), 656–663 (2008).
Ixtaina, V. Y. et al. Characterization of Chia seed oils obtained by pressing and solvent extraction. J. Food Compos. Anal. 24 (2), 166–174 (2011).
Capitani, M. I., Spotorno, V., Nolasco, S. M. & Tomás, M. C. Physicochemical and functional characterization of byproducts from Chia (Salvia hispanica L.) seeds of Argentina. LWT Food Sci. Technol. 45, 94–102 (2012).
Abomuti, M. A., Danish, E. Y., Firoz, A., Hasan, N. & Malik, M. A. Green synthesis of zinc oxide nanoparticles using Salvia officinalis leaf extract and their photocatalytic and antifungal activities. Biology 10 (11), 1075 (2021).
Kuznetcova, D. V. et al. Nanoliposomes and nanoemulsions based on Chia seed lipids: Preparation and characterization. Int. J. Mol. Sci. 21 (23), 9079 (2020).
Zak, A. K. et al. Sonochemical synthesis of hierarchical ZnO nanostructures. Ultrason. Sonochem. 20 (1), 395–400 (2013).
Kumar, D., Bhatkalkar, S. G., Sachar, S. & Ali, A. Studies on the antiglycating potential of zinc oxide nanoparticle and its interaction with BSA. J. Biomol. Struct. Dynamics. 39 (18), 6918–6925 (2021).
Dallatu, Y. A., Shallangwa, G. A. & Africa, S. N. Synthesis and growth of spherical ZnO nanoparticles using different amount of plant extract: characterization and morphology of structures. J. Appl. Sci. Environ. Manage. 24 (12), 2147–2151 (2020).
El-Belely, E. F. et al. Green synthesis of zinc oxide nanoparticles (ZnO-NPs) using Arthrospira platensis (class: Cyanophyceae) and evaluation of their biomedical activities. Nanomaterials 11 (1), 95 (2021).
Santos, C. M. et al. Inhibition of the carbohydrate-hydrolyzing enzymes α-amylase and α-glucosidase by hydroxylated xanthones. Food Funct. 13 (14), 7930–7941 (2022).
Iqbal, J. et al. Green synthesis of zinc oxide nanoparticles using Elaeagnus angustifolia L. leaf extracts and their multiple in vitro biological applications. Sci. Rep. 11 (1), 20988 (2021).
Manimaran, K., Balasubramani, G., Ragavendran, C., Natarajan, D. & Murugesan, S. Biological applications of synthesized ZnO nanoparticles using Pleurotus djamor against mosquito larvicidal, histopathology, antibacterial, antioxidant and anticancer effect. J. Cluster Sci. 32, 1635–1647 (2021).
Sánchez-López, E. et al. Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials 10 (2), 292 (2020).
Muhammad, A. et al. Dietary exposure of copper and zinc oxides nanoparticles affect the fitness, enzyme activity, and microbial community of the model insect, silkworm Bombyx mori. Sci. Total Environ. 813, 152608 (2022).
Guan, G. et al. Antibacterial properties and mechanism of biopolymer-based films functionalized by CuO/ZnO nanoparticles against Escherichia coli and Staphylococcus aureus. J. Hazard. Mater. 402, 123542 (2021).
Mammari, N., Lamouroux, E., Boudier, A. & Duval, R. E. Current knowledge on the oxidative-stress-mediated antimicrobial properties of metal-based nanoparticles. Microorganisms 10 (2), 437 (2022).
Author information
Authors and Affiliations
Contributions
Kiran Singh: Conceptualization, Experiment designing, Investigation, Formal analysis, Molecular docking analysis, Writing – original draft; Shweta Yadav: Conceptualization, Su¬pervision, Validation, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Singh, K., Yadav, S. Biosynthesis of a range of ZnO nanoparticles utilising Salvia hispanica L. seed extract and evaluation of their bioactivity. Sci Rep 15, 4043 (2025). https://doi.org/10.1038/s41598-025-87355-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87355-3
Keywords
This article is cited by
-
Visible-light photocatalytic mineralization of 4-Chlorophenol over ZnO-loaded sulfonated carbonaceous bentonite: kinetic analysis, pathway elucidation, and catalyst reusability
Scientific Reports (2026)
-
Sustainable Development of Plant-derived Nanomaterials as Emerging Antibacterial Agents
Chemistry Africa (2026)
-
Synergistic antifungal activity of lepidium sativum ZnO nanoparticles and nystatin against resistant candida species
Scientific Reports (2025)
-
Solar-Light Powered Photocatalytic Disintegration of Rose Bengal Dye Using Zn1-xMgxO1-δ–CuO–NiO Nanocomposites: Preparation, Functional Analysis and Optical Evaluation
Journal of Inorganic and Organometallic Polymers and Materials (2025)
-
Synthesis of zinc oxide nanoparticles using Syzygium malaccense leaf extract: photocatalytic decomposition of ciprofloxacin and antimicrobial studies
Nanotechnology for Environmental Engineering (2025)