Abstract
Intensive care unit-acquired weakness (ICU-AW) is recognized as newly-acquired bilateral muscle weakness, which is a complication of critical illness in the ICU; however, there are no reports on the pathogenesis and early predictors of ICU-AW specifically associated with cardiogenic shock (CS). Therefore, this study aimed to investigate the clinical characteristics of ICU-AW in patients with CS requiring mechanical circulatory support (MCS). This study was a single-center, prospective, and observational study. Patients aged 16 years and older who underwent MCS for CS were included. ICU-AW was diagnosed based on Medical Research Council (MRC) score after awakening. The ICU-AW group included patients with the MRC score < 48 points, and the non-ICU-AW group included those with ≥ 48 points. Twenty-eight cases were enrolled on admission and MRC score was evaluated in 23 cases after awakening. Eleven patients were included in the non-ICU-AW group and 12 patients (52%) were in the ICU-AW group. The ICU-AW group showed a higher prevalence of extracorporeal membrane oxygenation and ventilator use. Creatine kinase, troponin T, interleukin (IL)-15 levels on admission were significantly higher, whereas hemoglobin and albumin levels were significantly lower in the ICU-AW group. A strong negative correlation was observed between the initial MRC scores and IL-15 levels. ICU-AW occurred 52% of patients with CS using MCS, indicating the significance of recognizing and managing this complication for those patients. In addition, IL-15 can be a potential biomarker for the early prediction of ICU-AW.
Similar content being viewed by others
Introduction
Cardiogenic shock (CS) is characterized by low cardiac output, primarily caused by cardiac dysfunction, leading to severe conditions such as severe peripheral organ hypoperfusion with tissue hypoxia and elevated lactate levels, often resulting in multiple organ failure and death1,2,3. In some patients with CS, rapid and severe muscle weakness is frequently observed4,5.
Intensive care unit-acquired weakness (ICU-AW) is now recognized as a prevalent complication among critically ill patients in the ICUs6. Reports have been published on the rapid loss of muscle strength in patients receiving ventilation and in septic patients7,8,9,10,11,12. These conditions are categorized as critical illness polyneuropathy (CIP), critical illness myopathy (CIM), or combined critical illness neuromyopathy (CINM). Stevens et al. proposed the term “ICU-AW” to describe patients exhibiting clinically noticeable weakness without any apparent cause other than critical illness8.
The precise mechanism by which ICU-AW is initiated remains uncertain. However, the development of this condition is thought to be influenced by a number of factors, including systemic inflammation-induced multiple organ failure, immobilization, hyperglycemia, and the use of glucocorticoids and neuromuscular blocking agents9,11,13,14. These factors likely lead to type II muscle fiber atrophy, selective loss of myosin, and hypercatabolism owing to decreased myofascial excitability, resulting in progressive muscle wasting15,16,17. The incidence of ICU-AW is notably high, affecting up to 80% of patients undergoing extracorporeal membrane oxygenation (ECMO)18 and 25–75% in those with severe sepsis19,20,21,22. ICU-AW has mainly been reported in patients with severe infectious diseases, particularly systemic inflammatory conditions such as sepsis. Some studies include patients with CS, such as acute myocardial infarction; however, there are currently no reports on the clinical characteristics of ICU-AW in patients with non-septic CS. Patients with CS who receive mechanical circulatory support (MCS) are considered to be at risk of developing ICU-AW due to therapeutic sedation, immobilization, as well as peripheral organ hypoperfusion and microvascular hypoperfusion-induced inflammatory activation3. In our experience, there are cases of significant muscle weakness in such patients with CS; however, the clinical characteristics, as well as early diagnostic markers of these patients remain unclear. In previous reports on ICU-AW biomarkers, neurofilaments and growth differentiation factor-15 (GDF-15) levels have been reported to be elevated in patients with ICU-AW. However, neurofilament levels are not elevated on admission, and GDF-15 levels correlate with MRC scores on day 7 but not elevated in early stage23. Early-stage biomarkers of ICU-AW, which can predict the development of ICU-AW in patients with CS, is highly desirable.
Therefore, this study aimed to elucidate the clinical characteristics of ICU-AW in patients with CS requiring MCS treatment and identify early-stage biomarkers that can predict the development of ICU-AW.
Results
Study population
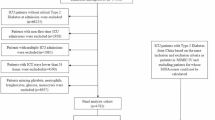
A total of 47 patients with CS who underwent MCS between April 2020 and June 2022 were enrolled. Nineteen patients were excluded based on the predefined exclusion criteria, and 28 patients were eligible for participation. Physical function assessment was conducted in 23 patients, excluding one patient who died after consent was obtained, and 4 patients who had cerebral complications before awakening. Among them, 12 patients (52%) met the ICU-AW criteria, with an initial MRC score < 48 points and 11 patients were included in the non-ICU-AW group (Fig. 1). For long-term evaluation, cases with stroke (n = 1) and death (n = 1) in the ICU-AW group and dropout (n = 1) in the non-ICU-AW group were excluded; thus, 10 patients in the ICU-AW group and 10 patients in the non-ICU-AW group were analyzed. Table 1 presents the patient characteristics and treatment details, indicating a significantly higher rate of fulminant myocarditis, greater utilization of veno-arterial ECMO (VA-ECMO) and ventilators, and longer periods of deep sedation in the ICU-AW group than in the non-ICU-AW group. However, no significant differences existed in age or sex between groups in the current study. The areas of the psoas major muscle on computed tomography were comparable between the groups (Supplementary Fig. 1).
Comparison of blood biochemistry and cytokine data between the ICU-AW group and the non-ICU-AW group
Table 2 shows the comparison of blood biochemistry and cytokine data between the non-ICU-AW and ICU-AW groups on admission. Creatine kinase and troponin T levels were significantly higher in the ICU-AW group (p < 0.05), while hemoglobin and albumin levels were significantly lower in the ICU-AW group than the non-ICU-AW group. Furthermore, the ICU-AW group showed significantly elevated IL-15, indicating multiple organ damage, compared to the non-ICU-AW group.
Changes in physical function over time
Figure 2 shows the change of MRC score, grip strength, and %grip strength over time. Among patients in the ICU-AW group, 7 (58%) showed improvement in their MRC scores to ≥ 48 points at 1 to 2 weeks after their initial MRC scores evaluation, while 3 (25%) patients still showed scores < 48 points after 4 weeks and experienced prolonged muscle weakness. Notably, these 3 patients exhibited low initial MRC scores (median, 22 points) and long MCS duration (median, 56 days), and one patient died of multiple organ failure during follow-up. In the ICU-AW group, grip strength improved slowly (Fig. 2B and C).
All 20 patients in both groups who were available for evaluation at 6 months reached the MRC scores ≥ 48 points, and no patient met the criteria for ICU-AW. However, the median %grip strength were 75% and 77% in the ICU-AW and non-ICU groups, respectively. Both groups showed lower grip strength than adults of similar age, although no significant differences were observed between the groups. %Peak VO2 at 6 months were also low but comparable between the groups (54% in the ICU-AW group vs. 50% in the non-ICU-AW group, p = 0.53, Table 3). The SF-36 showed no significant difference in QOL between the groups; however, both groups had lower values in physical function and role function (physical) than the Japanese population average of 50 points (Supplementary Fig. 2).
Prediction of the MRC score using biomarkers on admission
Supplementary Table 2 presents the association between various clinical data on admission and the initial MRC scores evaluation, with a median of day 6 for the non-ICU-AW group and day 11 for the ICU-AW group. Among the various biomarkers, serum IL-15 level on day 1 demonstrated a strong negative correlation (r=− 0.75, p < 0.001) with the initial MRC score (Supplementary Tables 2 and Fig. 3A). IL-15 exhibited an even stronger correlation (r=− 0.81, p < 0.001) with the lowest MRC score during the study (Supplementary Tables 3 and Fig. 3B). IL-15 levels were significantly higher in the ICU-AW group on days 1, 3, and 7, with no significant between-group difference (p = 0.48) at discharge (Fig. 4).
The correlation of IL-15 with laboratory data on admission and course of treatment is shown in Supplementary Table 4. IL-15 was associated with blood urea nitrogen (r = 0.71, p < 0.01), creatine kinase (r = 0.69, p < 0.01), the sequential organ failure assessment score (r = 0.60, p < 0.01), albumin (r = − 0.55, p < 0 0.01), and troponin T (r = 0.54, p < 0.01), suggesting that IL-15 is a marker reflecting the severity of CS.
Discussion
To our best knowledge, this is the first investigation identifying clinical presentation and predictive biomarkers of ICU-AW development in patients with CS requiring MCS. We found that 52% of enrolled patients were diagnosed as ICU-AW. Muscle strength of the patients with ICU-AW showed a trend toward improvement over time; however, some patients experienced prolonged muscle weakness. We also found a strong negative correlation between the initial MRC scores and serum IL-15 levels on admission, suggesting that IL-15 can be a useful early-stage biomarker to predict ICU-AW development.
Incidence of ICU-AW in patients with CS
The incidence of ICU-AW in patients with CS requiring MCS was 52% in our study, which is similar to the range reported in patients with sepsis and those on long-term ventilator management18,19,20,21,22. CS is characterized by low cardiac output and peripheral organ hypoperfusion due to cardiac dysfunction. Peripheral organ hypoperfusion induces systemic inflammatory activation triggered by various inflammatory cascades, including cytokine release, complement formation, and free radical production, contributes to multiple organ damage in CS3. Additionally, the rapid development of skeletal muscle atrophy and dysfunction has been observed in the early stages of the primary disease due to increased catabolism of muscle proteins in the presence of inflammation24,25,26. Although the exact mechanisms underlying the development of ICU-AW remain unclear, the systemic inflammation appear to be a strong contributing factor. In the present study, the ICU-AW group exhibited a higher rate of fulminant myocarditis, which may account for the higher CK and Troponin T levels. In addition, a high rate of VA-ECMO usage and low hemoglobin and albumin levels were observed in the ICU-AW group. These findings suggest that ICU-AW can develop not only in patients with sepsis, but also in patients with severe CS requiring MCS, and that it is associated with severe systemic inflammatory response.
Nutritional support is known to have a negative correlation with ICU-AW27. In this study, the time to start enteral nutrition was significantly longer in the ICU-AW group (Table 1), suggesting the correlation with the development of ICU-AW.
Recovery from ICU-AW
The MRC scores of patients in the ICU-AW group improved over time, with all patients achieving MRC scores ≥ 48 points and meeting the criteria for recovery from ICU-AW at 6 months. However, the recovery process differed among patients, with some demonstrating early improvement at approximately 2 weeks, and others experiencing prolonged muscle weakness for 4 weeks or longer, scoring < 48 points. Patients with prolonged muscle weakness had significantly lower initial MRC scores, significant muscle weakness immediately after awakening, and a longer MCS period, potentially contributing to delayed recovery. Recovery from CIP is reportedly delayed than that from CIM, and cases of further delay or no recovery have been reported in CINM22. Since electrophysical examinations and/or muscle biopsies were not performed in this study, it was difficult to differentiate between these conditions; however, it was possible that the delayed recovery cases may have been CIP or CINM.
A previous report shows that residual muscle weakness prolonged even at 6 months after discharge in 7–15% of the patients with ICU-AW28. Even after the normalization of the MRC scores, activity of daily living and QOL remained significantly lower, suggesting long-term physical and QOL impairments29. In our study, at 6 months after onset, grip strength were approximately 75% of that of age-matched peers and were comparable between the groups. These observations are likely attributable to the inclusion of patients with CS and the fact that cardiac function was not fully normalized in some patients at 6 months. Exercise capacity and QOL were also low at 6 months, and were comparable between the groups. Although limited data exist on long-term physical function and QOL in patients with CS, a few reports have indicated a prolonged decline in QOL in some part of patients30. Therefore, a possibility exists that there were residual effects of CS on physical function and QOL at 6 months in the patients.
Biomarkers for early prediction of ICU-AW
As no current drug therapy is available for ICU-AW, its management mainly involves early mobilization, appropriate medication, and nutritional and glycemic control8,11. However, if blood tests performed on admission could effectively predict the onset of ICU-AW, it would enable clinicians to promptly implement more aggressive interventions, such as neuroelectrical stimulation and early mobilization.
Our findings established a strong negative correlation between serum IL-15 levels on admission and the MRC scores at awakening as well as the lowest MRC scores during treatment. IL-15 levels were elevated from day 1 and were significantly higher on days 3 and 7 in the ICU-AW group than in the non-ICU-AW group. IL-15 can be a useful and easily measurable biomarker for identifying patients at risk of developing ICU-AW, especially those undergoing MCS.
IL-15 is a ~ 15 kDa glycoprotein belonging to the 4 α-helix bundle cytokine family31. Unlike other cytokines, IL-15 is widely expressed in many cell types, including monocytes, macrophages, dendritic cells, fibroblasts, epithelial cells, and skeletal muscle cells. In humans, IL-15 is widely distributed in various tissues, including the heart, lungs, liver, thymus, and kidneys and plays a critical role as a mediator of inflammation. Treatment with IL-15 has been reported to modify the clinical course, hemodynamics, and histopathology of murine myocarditis32. IL-15 promotes the survival of cardiomyocytes in ischemia-reperfusion33.
IL-15 has a protective effect on skeletal muscle, as evidenced by its ability to induce hypertrophy of myotubes in C2C12 myoblasts in mice34. Therefore, it is unlikely that IL-15 promotes muscle atrophy or hypercatabolism. Instead, the elevated IL-15 levels observed in our study likely reflected the severity of the disease, particularly the inflammatory response, rather than a direct negative effect on muscle function.
Limitations
Our study has several limitations. First, this was a single-center study with a relatively small number of patients, which may have led to potential bias and limited the generalizability of the findings. Due to the small sample size, it is difficult to compare between the groups in the same severity. Therefore, it is difficult to identify risk factors and propose possible mechanisms of ICU-AW other than disease severity. Especially, further investigation is required to ascertain why patients with myocarditis are disproportionately represented in the ICU-AW group. The small sample size could also have resulted in an underestimation of the true incidence of ICU-AW because our study was limited to young patients with CS. Future multicenter studies with larger cohorts are required to validate our findings. Second, since we followed up only for 6 months for the patients, longer durations would better gauge long-term prognosis after CS with MCS.
Conclusions
Our study provides insights into the characteristics of ICU-AW in patients with CS requiring MCS. ICU-AW frequently occurred in patients with CS using MCS, indicating the significance of recognizing and managing this complication for those patients. While the MRC score improved over 6 months, there was evidence of a prolonged decline in physical function-related QOL and exercise capacity, indicating the need for long-term monitoring and interventions to address functional impairments.
Moreover, we indicates that serum IL-15 may be a valuable predictive biomarker of ICU-AW, which may be useful for early identification of ICU-AW in patients with CS using MCS.
Methods
Study design and data collection
This study was a single-center, prospective, and observational study. Patients aged 16 years and older admitted to Kyushu University Hospital between April 2020 and June 2022 and received MCS for CS were included. Notably, we included patients requiring VA-ECMO, who had high mortality and mobidity. The exclusion criteria were older age (> 70 years), suspected central nervous system disorder on admission, short-term MCS use in the perioperative period, impaired motor function before admission, and immunosuppressive drug use before admission.
Protocols for the management of MCS patients
Daily sedation targets were generally aimed at a Ramsay sedation score (RASS) between − 1 and + 1. Patients with circulatory or respiratory unstable conditions often required deep sedation, defined as RASS − 4 or − 5.
After hemodynamic and respiratory stabilization, weaning from MCS or mechanical ventilation were frequently discussed in our multidisciplinary team. Our weaning protocol from VA-ECMO was based on the Guidelines35.
Our standard rehabilitation program for patients on MCS was initiated as early as possible after the commencement of MCS, when patients met the RASS target of − 1 to + 1. Rehabilitation began with resistance and active range of motion exercises for the upper and lower limbs, progressing to sitting, standing, and ultimately ambulation with MCS.
We started enteral nutrition when the patients were hemodynamically stabilized with MCS. Before starting enteral nutrition, we administer parenteral nutrition according to the patients’ condition.
ICU-AW assessment
ICU-AW was diagnosed using current diagnostic reference standards8. Patients underwent limb muscle strength assessment using the Medical Research Council (MRC) score after awakening (Richmond Agitation-Sedation Scale from − 1 to + 1) and when they were able to respond to 5 specific questions (“Open [close] your eyes”, “Look at me”, “Open your mouth and put out your tongue”, “Nod your head” and “Raise your eyebrows when I have counted up to 5”). These evaluations were conducted twice at intervals of at least 24 hours by the same physical therapist, and the maximum value was considered the initial MRC score. When it was impossible to measure muscle strength owing to cannulation or other reasons, contralateral side’s muscle strength was estimated and used. The ICU-AW group included patients with the MRC score < 48 points, and patients with the MRC score of 48 points or higher were categorized in the non-ICU-AW group8.
Grip strength was measured twice on each side in the sitting position with the shoulder joint in mid-position, the elbow joint in 90° flexion, and the wrist joint in mid-position. The maximum values were recorded. The grip strength percentage (%grip strength) for each patient was calculated based on the Japanese Generational Grip Strength Reference (Supplementary Table 1).
The MRC score and grip strength were measured at the initial assessment, weekly, at the time of discharge, and at 6 months after onset.
The areas of psoas major muscle were measured by computed tomography imaging at the time close to the MRC measurement. Using a software, measurements were made at the L4/5 intervertebral lumbar disc level in the axial plane.
Inflammatory marker assays
Blood samples were collected on days 1, 3, and 7 and at hospital discharge. Serum was separated from the blood collected into ethylenediamine tetraacetic acid tubes via centrifugation at 3500 rpm for 10 min. Samples were stored at − 80 °C until further processing. The levels of the following cytokines were measured: interleukin (IL)-1β, IL-6, IL-10, IL-15, tumor necrosis factor alpha, interferon gamma, monocyte chemotactic protein-1, and GDF-15.
GDF-15 plasma concentration was determined using an enzyme-linked immunosorbent assay with a test kit purchased from R&D SYSTEMS (Minneapolis, MN, USA). Other cytokines were measured using V-PLEX Plus Chemokine Panel 1 (human), V-PLEX Plus Cytokine Panel 1 (human), and V-PLEX Plus Proinflammatory Panel 1 (human) (Meso Scale Discovery, USA).
Long-term course of recovery
Physical function, cardiac function, and prognosis were monitored and assessed for up to 6 months after MCS initiation. In addition, a cardiopulmonary exercise test (CPX) was performed to measure peak oxygen uptake (VO2), anaerobic metabolic threshold (AT), and ventilatory efficiency. Then, quality of life (QOL) was assessed using the Short Form-36 (SF-36) and EuroQol 5-dimensions 5-levels.
Statistical analysis
Data are presented as median (interquartile range, IQR) for continuous variables and number (%) for categorical variables. Chi-squared test or Wilcoxon’s rank-sum test was used for between-group comparison. Mixed-effect models were used to analyze changes over time. The association between the initial MRC score and laboratory data on admission was assessed using Pearson’s product-rate correlation coefficient. Variables that were not normally distributed were log-transformed and analyzed. Furthermore, the association between the extracted biomarkers, data on admission, and course of treatment was analyzed using Pearson’s correlation coefficients. Statistical significance was defined as p < 0.05. All analyses were performed using JMP, Version pro 16 (SAS Institute Inc., Cary, NC, USA).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Tehrani, B. N. et al. A standardized and comprehensive approach to the management of cardiogenic shock. JACC Heart Fail. 8, 879–891 (2020).
Shah, M. et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin. Res. Cardiol. 107, 287–303 (2018).
Krychtiuk, K. A., Vrints, C., Wojta, J., Huber, K. & Speidl, W. S. Basic mechanisms in cardiogenic shock: part 1-definition and pathophysiology. Eur. Heart J. Acute Cardiovasc. Care 11, 356–365 (2022).
Hayes, K., Holland, A. E., Pellegrino, V. A., Mathur, S. & Hodgson, C. L. Acute skeletal muscle wasting and relation to physical function in patients requiring extracorporeal membrane oxygenation (ECMO). J. Crit. Care 48, 1–8 (2018).
Sasanuma, N. et al. A five-year follow-up of a patient with fulminant myocarditis who underwent a stepwise and goal-oriented individualized comprehensive cardiac rehabilitation program. J. Cardiol. Cases. 11, 160–163 (2015).
Osler, W. The principles and practice of medicine. Designed for the use of practitioners and students of medicine. Am. J. Med. Sci. 131, 114–118 (1906).
Schweickert, W. D. & Hall, J. ICU-acquired weakness. Chest 131, 1541–1549 (2007).
Stevens, R. D. et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit. Care Med. 37, s299–308 (2009).
Jolley, S. E., Bunnell, A. E. & Hough, C. L. ICU-acquired weakness. Chest 150, 1129–1140 (2016).
Vanhorebeek, I., Latronico, N. & Van den Berghe, G. ICU-acquired weakness. Intens. Care Med. 46, 637–653 (2020).
Kress, J. P. & Hall, J. B. ICU-acquired weakness and recovery from critical illness. N. Engl. J. Med. 370, 1626–1635 (2014).
Needham, D. M. et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit. Care Med. 40, 502–509 (2012).
Schefold, J. C., Bierbrauer, J. & Weber-Carstens, S. Intensive care unit-acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J. Cachexia Sarcopenia Muscle 1, 147–157 (2010).
Yang, Z., Wang, X., Wang, F., Peng, Z. & Fan, Y. A systematic review and meta-analysis of risk factors for intensive care unit acquired weakness. Medicine (United States) 101, e31405 (2022).
Derde, S. et al. Muscle atrophy and preferential loss of myosin in prolonged critically ill patients. Crit. Care Med. 40, 79–89 (2012).
Fazzini, B. et al. The rate and assessment of muscle wasting during critical illness: a systematic review and meta-analysis. Crit. Care 27, 1–26 (2023).
Dos Santos, C. et al. Mechanisms of chronic muscle wasting and dysfunction after an intensive care unit stay: a pilot study. Am. J. Respir. Crit. Care Med. 194, 821–830 (2016).
Chen, X., Lei, X., Xu, X., Zhou, Y. & Huang, M. Intensive care unit-acquired weakness in patients with extracorporeal membrane oxygenation support: frequency and clinical characteristics. Front. Med. (Lausanne) 9, 792201 (2022).
Ali, N. A. et al. Acquired weakness, handgrip strength, and mortality in critically III patients. Am. J. Respir. Crit. Care Med. 178, 261–268 (2008).
Sharshar, T. et al. Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit. Care Med. 37, 3047–3053 (2009).
Garnacho-Montero, J. et al. Critical illness polyneuropathy: risk factors and clinical consequences. A cohort study in septic patients. Intens. Care Med. 27, 1288–1296 (2001).
Hermans, G., De Jonghe, B., Bruyninckx, F. & Van den Berghe, G. Clinical review: critical illness polyneuropathy and myopathy. Crit. Care 12, 238 (2008).
Wieske, L. et al. Neurofilaments as a plasma biomarker for ICU-acquired weakness: an observational pilot study. Crit. Care 18, R18 (2014).
Morel, J. et al. Regulation of Akt-mTOR, ubiquitin-proteasome and autophagy-lysosome pathways in locomotor and respiratory muscles during experimental sepsis in mice. Sci. Rep. 7, 1–12 (2017).
Kozlov, A. V. et al. Intensive care unit-acquired weakness: a review of recent progress with a look toward the future. Front. Med. (Lausanne) 7, 559789 (2020).
Bonetto, A. et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am. J. Physiol. Endocrinol. Metab. 303, 410–421 (2012).
Oshima, T. & Hatakeyama, J. Nutritional therapy for the prevention of post-intensive care syndrome. J. Intens. Care 12, 29 (2024).
Fan, E. et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit. Care Med. 42, 849–859 (2014).
Sidiras, G. et al. Long term follow-up of quality of life and functional ability in patients with ICU acquired weakness—A post hoc analysis. J. Crit. Care 53, 223–230 (2019).
Delmas, C. et al. Early prediction of 3-month survival of patients in refractory cardiogenic shock and cardiac arrest on extracorporeal life support. Indian J. Crit. Care Med. 21, 138–145 (2017).
Patidar, M., Yadav, N. & Dalai, S. K. Interleukin 15: A key cytokine for immunotherapy. Cytokine Growth Factor Rev. 31, 149–159 (2016).
Bigalke, B., Schwimmbeck, P. L., Haas, C. S. & Lindemann, S. Effect of interleukin-15 on the course of myocarditis in Coxsackievirus B3-infected BALB/c mice. Can. J. Cardiol. 25, e248–e254 (2009).
Yeghiazarians, Y. et al. IL-15: a novel pro-survival signaling pathway in cardiomyocytes. J. Cardiovasc. Pharmacol. 63, 406–411 (2014).
Quinn, L. S., Haugk, K. L. & Grabstein, K. H. Interleukin-15: a novel anabolic cytokine for skeletal muscle. Endocrinology 136, 3669–3672 (1995).
Lorusso, R. et al. ELSO interim guidelines for venoarterial extracorporeal membrane oxygenation in adult cardiac patients. ASAIO J. 67, 827–844 (2021).
Acknowledgements
This paper is dedicated to Tomomi Ide, who passed away on May 17, 2024. We would like to gratefully acknowledge her inspirational leadership. The authors thank Yukari Tanaka and Fumika Yamashita for their technical support of this study.
Funding
This work was supported by JSPS KAKENHI Grant Numbers JP24K22274 and JP22K08103, and Japan Research Foundation for Clinical Pharmacology.
Author information
Authors and Affiliations
Contributions
TH, IT and TF contributed to the conception, design, acquisition and analysis, interpretation of data, and writing of the manuscript. TT and MT contributed to the conception, design of the work, analysis of data, interpretation of data, and revision of the manuscript. YN and TN contributed to the design, acquisition, analysis of data and revision of the manuscript. TH, SM, KS, MN, TI, TA, TU, AS, SK, HT and KA contributed to analysis of data, interpretation of data, and revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Takeo Fujino is a member of the endowment department supported by NIPRO, Medtronic Japan and Abbott Medical Japan. The other authors declare no competing interests.
Ethical approval and consent to participate
It was conducted in accordance with the Declaration of Helsinki, and we obtained written informed consent after fully explaining the study, risks, and freedom to participate to patients or their families. We obtained the approval of the Ethics Review Committee for Clinical Research of the Kyushu University Medical School District Department (license number: 2019–585, approval date: 2019-2-24, study title: A Study of ICU-AW Occurrence and Improvement of Exercise Capacity in Critical Cardiac Disease).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Higuchi, T., Ide, T., Fujino, T. et al. Clinical characteristics and predictive biomarkers of intensive care unit-acquired weakness in patients with cardiogenic shock requiring mechanical circulatory support. Sci Rep 15, 3535 (2025). https://doi.org/10.1038/s41598-025-87381-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87381-1
Keywords
This article is cited by
-
Biomarkers for intensive care unit-acquired weakness: a systematic review for prediction, diagnosis and prognosis
Annals of Intensive Care (2025)