Abstract
Ethylene Oxide (EO), a volatile organic compound, has garnered considerable attention for its potential impact on human health. Yet, the ramifications of EO exposure on the cognitive functionality of the elderly remain unclear. The aim of this study is to determine whether EO exposure in the elderly correlates with cognitive function. In this cross-sectional study, an analysis was conducted on 471 participants from the 2013 to 2014 United States National Health and Nutrition Examination Survey (NHANES). T1, T2, and T3 was used to represent the low, moderate, and high tertiles of log10-transformed HbEO levels, respectively. Weighted linear regression analysis, weighted logistic regression analysis, and restricted cubic spline models were employed to assess the association between HbEO and standardized z-scores of four cognitive tests. Firstly, analysis of variable characteristics across the different log10-transformed HbEO groups revealed that HbEO was higher in males, non-Hispanic whites, and smokers and that Z scores for Delayed Recall Test (DRT), Animal Fluency Test (AFT), and Digit Symbol Substitution Test (DSST) decreased as HbEO increased (p < 0.05). After adjusting for confounding factors, the log10-transformed HbEO levels were found to be negatively correlated with DRT-Z scores (T3 vs. T1 in Model 3: β (95% CI) = − 0.35 (− 0.54, − 0.15), p = 0.002, p for trend = 0.002). In addition, Stratified analyses showed that the four cognitive scores were negatively correlated with HbEO levels in those under 80 years of age. And men had worse AFT scores compared to women. Overall, the four Z-scores roughly trended downward as log10-transformed HbEO rose. Based on the findings of this research, EO exposure may be associated with adverse performance in the DRT among elderly individuals in the United States.
Similar content being viewed by others
Introduction
As the world’s elderly population grows rapidly, so does the visibility of age-related cognitive health problems1,2. It is estimated that by 2060, the number of Alzheimer’s disease patients in the U.S. will double to over six million3. The life quality of patients is greatly affected by dementia, imposing a considerable financial strain on both families and society4. It’s anticipated that by 2030, worldwide economic damages due to dementia might escalate to $25.4 trillion, a significant increase from the $95.756 trillion in 20155. However, currently, there exist no feasible solutions to slow the progression of dementia or improve its clinical course6. A notable aspect of dementia is reduced cognitive function, which seems to precede the onset of other symptoms7. Consequently, pinpointing risk elements for cognitive deterioration and executing strategies could lower the occurrence of dementia. The decline in mental abilities stems from a complex mix of genetic, environmental, lifestyle, and dietary influences8,9,10,11.
Lately, the recognition of cognitive abilities in older adults has become increasingly worrying, attributed to various environmental pollution issues. A range of elements including tobacco smoke, particulate matter, polycyclic aromatic hydrocarbons, per- and polyfluoroalkyl substances, and phthalates, among others, are known to lead to cognitive deterioration12,13,14,15. Therefore, it’s vital to investigate and minimize contact with environmental chemicals to avert cognitive deterioration in older adults. Ethylene oxide (EO), a crucial chemical in industry, finds extensive application in producing antifreeze, cleaning agents, fabrics, pesticides, sterilizing medical devices, and fumigation in food and beauty products16,17. Furthermore, public exposure to EO occurs through inhaling polluted air, smoking tobacco, or inhaling vehicle exhaust18,19. As an alkylating agent, EO can interact with hemoglobin, resulting in the formation of hemoglobin adducts of EO (N-(2-hydroxyethyl) valine) (HbEO), a key and effective marker for assessing EO exposure20. Various researches suggest EO’s possible neurotoxic effects, resulting in diverse neurological alterations including sensory-motor neuropathy, weakened muscles, reduced sensation, and degeneration of demyelinating fibers21,22. Dating back to the 1990s, instances were documented where healthcare workers exposed to EO in their jobs showed cognitive deficits, yet there’s a deficit of solid clinical research to verify this link22. Additionally, increasing research indicates that EO may trigger inflammatory responses linked to multiple illnesses23,24,25. Considering the profound effect of inflammatory responses on the onset and advancement of cognitive deterioration, our theory proposes that EO may contribute to the emergence of cognitive deficits in the elderly.
Therefore, this study aims to assess the link between cognitive skills in people aged 60 and above and their exposure to EO, using information from the National Health and Nutrition Examination Survey (NHANES) in the United States.
Methods
Study population
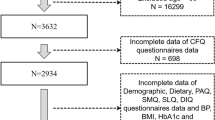
NHANES constitutes a cross-sectional study aimed at assessing the health and nutritional status of the United States outpatient population. The study protocol was approved by the National Center for Health Statistics (NCHS) Ethics Committee, and all methods were performed in accordance with the Declaration of Helsinki. The participants provided written informed consent to participate in the study. Participants who were aged ≥ 60 years were considered starting from the NHANES 2013–2014 cycle, with a total of 8334 individuals (n = 8334). Demographic, physical examination, and laboratory examination data were collected during household interviews and examination center visits (MEC). The interviewer observed the personal prescription medications to gather information about medication usage. We removed participants who had incomplete information for HbEO (n = 1084), body mass index (BMI), and alcohol consumption (n = 18), as well as adults who had incomplete data for the Immediate Recall Test (IRT), Delayed Recall Test (DRT), Animal Fluency Test (AFT), and Digit Symbol Substitution Test (DSST) (n = 108). Overall, 471 participants were analyzed (Fig. 1).
Blood ethylene oxide measurement
The reliability of HbEO has been confirmed as a biomarker for evaluating levels of EO exposure. The concentration of HbEO was measured using an improved Edman reaction via HPLC-MS technology. The measured outcomes were normalized to hemoglobin and expressed as picomoles of adduct per gram of hemoglobin. The threshold of detection (LLOD) for HbEO is 12.9 pmol/g Hb, and any value below this is estimated as LLOD/√2. In this study, the rate of detecting HbEO (N% > LOQ) was 95.9%.
Cognitive function assessment
In order to assess cognitive function more comprehensively and rationally, we used four methods of assessment: IRT, DRT, AFT, and DSST. Large-scale screenings and clinical studies have used these tests extensively26. The AFT, a scale that measures executive function, has scores that can be used to differentiate the severity of cognitive dysfunction27. The DSST is a component of the Wechsler Adult Intelligence Scale that evaluates processing speed, sustained attention, and working memory28. The DRT was administered after completion of the IRT, AFT, and DSST. Further analysis of the overall cognitive performance on the four tests was performed by averaging the Z-scores of the four tests. The formula for calculating Z-scores is Z =(χ−µ)/ σ, where χ represents the test score for each participant, and µ and σ denote the mean and standard deviation of test scores for all participants, respectively.
Covariates
In our study, covariates encompass a spectrum of factors previously established or considered to be associated with HbEO or cognitive function. The factors encompass various sociodemographic attributes (like age, gender, race, marital status, education level, and household poverty income ratio)29, body mass index30, lifestyle factors (physical activity, smoking status, alcohol consumption31, and medical history (hypertension, diabetes mellitus, cardiovascular disease)32. Hypertension and diabetes mellitus were determined through measurements of relevant indices, medication usage, and self-reported diagnoses, while cardiovascular disease was self-reported.
Statistical analysis
Considering the complexity of the NHANES sampling design, appropriate weighting was applied. For continuous variables, weighted means (standard deviation) were used for analysis, while categorical variables were assessed using sample size (weighted percentages). Weighted ANOVA was used for analyses of differences in weighted means for continuous variables, and the Rao-Scott χ2 test was used for comparisons of differences in weighted percentages for categorical variables. To explore the relationship between HbEO and IRT, DRT, AFT, and DSST z scores, weighted linear regression analyses were used. Three regression models were developed to validate the relationship between HbEO and cognitive function. The original model did not adjust for any potential confounders. Model 1 adjusted for age, sex, race, marital status, and poverty-income ratio. A further adjustment was made in Model 2 for BMI, smoking, alcohol consumption, and physical activity. Model 3 was additionally adjusted for diabetes mellitus, cardiovascular disease, and hypertension. Individuals below the lowest quartile of each Z-score were defined as cognitively impaired. In addition, stratified and interaction analyses were performed to assess the relationship between HbEO levels and age and sex. The association between HbEO and cognitive performance was further validated using the restricted cubic splines (RCS). To ensure the reliability of the findings, sensitivity analyses were conducted to exclude individuals with other health conditions (e.g., hypertension, cardiovascular disease, diabetes, and cancer). Statistical analyses for this study were performed using R version 4.2.3. Statistical significance was defined as a two-sided P value of less than 0.05.
Results
Baseline characteristics of the study population based on HbEO tertiles
Among the 471 participants, the baseline characteristics of individuals categorized by HbEO tertiles are shown in Table 1. Females accounted for 52.99% of the participants, with an average age of 68.94 ± 0.48 years. The majority of participants were non-Hispanic Americans, comprising 80.71%. Significant differences in gender, race, smoking, DRT z-score, and AFT z-score were observed across different levels of HbEO, with p-values < 0.05. Regarding the field of demographic sociology, an increase in HbEO levels was linked to a greater percentage of men, individuals who are not of Hispanic-white ethnicity, and individuals who smoke. Regarding cognitive function, higher HbEO levels were associated with lower DRT z-scores and AFT z-scores.
Association between HbEO and the z-score of IRT, DRT, AFT, and DSST
As shown in Table 2, across the four different weighted linear regression models, T3 of HbEO exposure, compared to T1, was negatively correlated with various cognitive Z-scores. In other words, higher HbEO levels were associated with lower cognitive Z-scores. This negative association was statistically significant for DRT cognitive Z-scores in the crude model, Model 2, and Model 3, indicating a significant dose-dependent decrease in cognitive performance with increasing concentrations of HbEO exposure (P < 0.05).
Sensitivity analysis, weighted logistic regression between HbEO and cognitive impairment as defined by z-score
Among the four distinct logistic regression models with varying weights, there was a positive correlation between T3 of HbEO exposure and the occurrence of cognitive impairment as defined by DRT cognitive Z-scores, when compared to the T1. To put it differently, an increased occurrence of cognitive impairment, as determined by DRT, was markedly associated (P < 0.05) with elevated HbEO levels. The discovery aligns with the findings showcased in Table 3. However, no statistically significant connection was found to exist between HbEO levels and cognitive impairment as defined by other cognitive Z-scores.
Stratified analysis on sex and age
The stratified analysis forest plot was generated using weighted linear regression analysis, and the interaction effect was assessed using multiplicative interaction analysis (Fig. 2). In the forest plot, the negative correlation between log10-transformed HbEO and IRT-Z scores was observed in the 70–80 age group. Additionally, log10-transformed HbEO showed a negative correlation with DRT-Z scores in the 70–80 age group, a negative correlation with AFT-Z scores in males and the 60–70 and 70–80 age groups, and a negative correlation with DSST-Z scores in the 60–70 age group. However, the multiplicative interaction effects between log10-transformed HbEO and the four different Z scores were not statistically significant, with all P for interaction > 0.05.
Restricted cubic spline analysis
The restricted cubic spline analysis illustrates a general decreasing trend in cognitive scores across all four different Z scores as log10-transformed HbEO levels rise. This suggests that with increasing exposure to epoxyethane, cognitive scores decline, indicating a decrease in cognitive abilities (Fig. 3).
Discussion
This cross-sectional study of 471 older Americans examined the effects of varying levels of ethylene oxide exposure on cognitive performance. Cognitive performance was assessed through four different lenses: IRT, DRT, AFT, and DSST. Our findings showed an overall decreasing trend in Z-scores for the four different cognitive assessments as exposure to ethylene oxide increased, suggesting a decline in cognitive performance. After all confounders were excluded, we observed relatively stable positive associations. In the fully adjusted model, the highest tertile T3 was positively associated with cognitive impairment as defined by the Z score of the DRT, i.e., the higher the level of HbEO, the higher the prevalence of cognitive impairment represented by the DRT. No interaction was found in its interaction analyses with age and gender, suggesting that the relationship between DRT scores and HbEO exposure can remain relatively stable between groups.
In performing subgroup analyses we found that age played an important role in ethylene oxide-induced cognitive impairment. In patients under 80 years of age, all four cognitive scores were negatively correlated with HbEO levels, demonstrating a stronger association between cognitive impairment and exposure. This may suggest that early intervention to reduce ethylene oxide exposure may have a greater benefit on cognitive function. In the subgroup analysis of gender, we can see that male patients have lower Z scores for AFT compared to females. AFT represents executive functioning and verbal fluency, which may be more impaired in males than females with increased exposure to ethylene oxide. No correlation between cognitive scores and ethylene oxide was seen in women. This result is consistent with analyses of population characteristics with different HbEO levels, with men, non-Hispanic whites, and smokers having higher levels of HbEO.
A multitude of research indicates that the deterioration of cognitive abilities could stem from the interplay of environmental factors, genetic predispositions, and lifestyle aspects33,34. Mounting research establishes a connection between cognitive impairments and the presence of harmful particles, gas pollutants, and various other environmental factors. Consequently, recognizing novel environmental elements is vital to avert the deterioration of cognitive abilities in older adults35,36. EO is recognized as an extensively reactive organic substance and a frequent contaminant in the environment17,37. Human health has been subjected to considerable scrutiny due to the adverse impacts of EO exposure. Research on animals suggests a direct link between prolonged exposure to EO and an increased likelihood of developing malignant tumors38,39. Furthermore, exposure to high doses of EO can harm various systems, encompassing the respiratory, circulatory, and nervous systems. Recent findings from NHANES indicate that exposure to EO influences how long individuals sleep40. Growing studies suggest a strong link between the length of sleep and cognitive skills. Implementing sleep interventions may play a vital role in averting cognitive deterioration41. The research by Patch and colleagues revealed that EO exposure significantly impacts the cognitive, neural, and behavioral capabilities of individuals, suggesting a link between EO and cognitive abilities42. Nonetheless, the majority of research on EO exposure has concentrated on work-related exposure, with scant attention to the broader population. This research pioneers in exploring the link between exposure to ethylene oxide and the likelihood of cognitive deterioration in the broader populace. Our findings indicate a correlation between cognitive abilities and EO exposure, implying a decline in cognitive scores as EO exposure escalates, resulting in reduced cognitive capacities. This might act as a guideline for subsequent studies.
The fundamental biological processes connecting exposure to EO to cognitive deterioration are still not well understood. Numerous research findings indicate that EO and its by-products may enhance oxidative stress and provoke inflammatory reactions. Lynch and colleagues dating back to 198443. Established that extended exposure to EO could result in inflammatory lesions affecting multiple organs in rodents. Within the adult population of the U.S., a rise in biomarkers linked to inflammation is associated with higher levels of EO exposure23. Additionally, numerous animal studies indicate that exposure to EO leads to heightened internal GSH intake and increased liver lipid peroxidation, both intensifying oxidative harm in the body44,45,46. Presently, there’s a broad consensus that a central role for inflammation and oxidative stress in causing cognitive impairment47,48. EO could also have a direct impact on peripheral nerves via skin absorption and affect the central nervous system through breathing in and blood vessel diffusion42. Further studies are required to delve deeper into the mechanisms and mechanisms to clarify the precise biological processes.
It’s essential to take into account various constraints present in this research. Initially, the study utilized a limited sample size owing to limitations in data. To accurately assess EO’s effect on cognitive abilities, a more extensive analysis of the sample size is required. Additionally, the research’s use of a cross-sectional approach precludes investigating the causal links between EO levels and the deterioration of cognitive functions. Furthermore, the outcomes of the study might be affected by factors that have not been quantified.
Conclusion
Our findings suggest a relatively stable negative association between EO exposure and DRT scores, with significant differences in both unadjusted and fully adjusted models. Stratified analyses by age showed that all four scores were significantly correlated with the degree of EO exposure, and this correlation was stronger in those aged < 80 years, which may indicate that early reduction of EO exposure may have greater benefits on cognitive function. Future and detailed research is essential to clarify the precise biological processes by which EO affects cognitive abilities in older adults.
Data availability
This study analyzed publicly available datasets at https://www.cdc.gov/nchs/nhanes/.
References
Dong, X., Li, S., Sun, J., Li, Y. & Zhang, D. Association of coffee, decaffeinated coffee and caffeine intake from coffee with cognitive performance in older adults: National Health and Nutrition Examination Survey (NHANES) 2011–2014. Nutrients 12(3) (2020).
Huang, Y. et al. Self- and interviewer-reported cognitive problems in relation to cognitive decline and dementia: Results from two prospective studies. BMC Med. 22(1), 23 (2024).
Rajan, K. B. et al. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement. 17(12), 1966–1975 (2021).
Kalaria, R. et al. The 2022 symposium on dementia and brain aging in low- and middle-income countries: Highlights on research, diagnosis, care, and impact. Alzheimers Dement 20(6):4290–4314 (2024).
Jia, J. et al. The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimers Dement. 14(4), 483–491 (2018).
Kluckert, C. & Hüll, M. Dementia Prevention. Fortschr. Neurol. Psychiatr. 92(3), 90–106 (2024).
Jean, K. R. & Dotson, V. M. Dementia: Common syndromes and modifiable risk and protective factors. Neurol. Clin. 42(4), 793–807 (2024).
Park, H. R. P. et al. Heritability of cognitive and emotion processing during functional MRI in a twin sample. Hum. Brain Mapp. 45(1), e26557 (2024).
Mohd Sahini, S. N., Mohd Nor Hazalin, N. A., Srikumar, B. N., Jayasingh Chellammal, H. S. & Surindar Singh, G. K. Environmental enrichment improves cognitive function, learning, memory and anxiety-related behaviours in rodent models of dementia: Implications for future study. Neurobiol. Learn. Mem. 208, 107880 (2024).
Schneider, E., O’Riordan, K. J., Clarke, G. & Cryan, J. F. Feeding gut microbes to nourish the brain: Unravelling the diet-microbiota-gut-brain axis. Nat. Metab. 6(8), 1454–1478 (2024).
Arenaza-Urquijo, E. M. et al. Sex and gender differences in cognitive resilience to aging and Alzheimer’s disease. Alzheimers Dement. 20(8), 5695–5719 (2024).
Aloizou, A. M. et al. Pesticides, cognitive functions and dementia: A review. Toxicol. Lett. 326, 31–51 (2020).
Weng, X. et al. Association between mixed exposure of phthalates and cognitive function among the U.S. elderly from NHANES 2011–2014: Three statistical models. Sci. Total Environ. 828, 154362 (2022).
Shi, Y. et al. Association between exposure to phenols and parabens and cognitive function in older adults in the United States: A cross-sectional study. Sci. Total Environ. 858(Pt 3), 160129 (2023).
Salinas-Rodríguez, A. et al. Exposure to ambient PM(2.5) concentrations and cognitive function among older Mexican adults. Environ. Int. 117, 1–9 (2018).
Jones, R. R. et al. Ethylene oxide emissions and incident breast cancer and non-hodgkin lymphoma in a US cohort. J. Natl. Cancer Inst. 115(4), 405–412 (2023).
Lynch, H. N. et al. Systematic review of the scientific evidence on ethylene oxide as a human carcinogen. Chem. Biol. Interact. 364, 110031 (2022).
Kirman, C. R. et al. Ethylene oxide review: Characterization of total exposure via endogenous and exogenous pathways and their implications to risk assessment and risk management. J. Toxicol. Environ. Health B Crit. Rev. 24(1), 1–29 (2021).
Jain, R. B. Associations between observed concentrations of ethylene oxide in whole blood and smoking, exposure to environmental tobacco smoke, and cancers including breast cancer: Data for US children, adolescents, and adults. Environ. Sci. Pollut Res. Int. 27(17), 20912–20919 (2020).
Zhou, C. et al. Positive association between blood ethylene oxide levels and metabolic syndrome: NHANES 2013–2020. Front. Endocrinol. (Lausanne). 15, 1365658 (2024).
Wang, H. et al. Association between urinary 2-hydroxyethyl mercapturic acid and dyslexia among school-aged children. Environ. Sci. Pollut Res. Int. 30(45), 101091–101098 (2023).
Brashear, A. et al. Ethylene oxide neurotoxicity: A cluster of 12 nurses with peripheral and central nervous system toxicity. Neurology 46(4), 992–998 (1996).
Zhu, X. et al. Blood ethylene oxide, systemic inflammation, and serum lipid profiles: Results from NHANES 2013–2016. Chemosphere 299, 134336 (2022).
Guo, J., Wan, Z., Cui, G., Pan, A. & Liu, G. Association of exposure to ethylene oxide with risk of diabetes mellitus: Results from NHANES 2013–2016. Environ. Sci. Pollut Res. Int. 28(48), 68551–68559 (2021).
Huang, Q., Li, S., Wan, J., Nan, W. & He, B. Association between ethylene oxide exposure and prevalence of COPD: Evidence from NHANES 2013–2016. Sci. Total Environ. 885, 163871 (2023).
Chu, N. M. et al. Chronic kidney disease, physical activity and cognitive function in older adults-results from the National Health and Nutrition Examination Survey (2011–2014). Nephrol. Dial Transpl. 37(11), 2180–2189 (2022).
Clark, L. J. et al. Longitudinal verbal fluency in normal aging, preclinical, and prevalent Alzheimer’s disease. Am. J. Alzheimers Dis. Other Dementias 24(6), 461–468 (2009).
Salthouse, T. A. What do adult age differences in the digit symbol substitution test reflect? J. Gerontol. 47(3), P121–128 (1992).
Mejia-Arango, S., Garcia-Cifuentes, E., Samper-Ternent, R., Borda, M. G. & Cano-Gutierrez, C. A. Socioeconomic disparities and gender inequalities in dementia: A Community-Dwelling Population Study from a Middle-Income Country. J. Cross Cult. Gerontol. 36(1), 105–118 (2021).
Kim, G., Choi, S. & Lyu, J. Body mass index and trajectories of cognitive decline among older Korean adults. Aging Ment. Health 24(5), 758–764 (2020).
Visontay, R., Rao, R. T. & Mewton, L. Alcohol use and dementia: New research directions. Curr. Opin. Psychiatry 34(2), 165–170 (2021).
Walker, K. A., Power, M. C. & Gottesman, R. F. Defining the relationship between hypertension, cognitive decline, and dementia: A review. Curr. Hypertens. Rep. 19(3), 24 (2017).
Sando, S. B. et al. Risk-reducing effect of education in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 23(11), 1156–1162 (2008).
Vagelatos, N. T. & Eslick, G. D. Type 2 diabetes as a risk factor for Alzheimer’s disease: The confounders, interactions, and neuropathology associated with this relationship. Epidemiol. Rev. 35, 152–160 (2013).
Sasaki, N. & Carpenter, D. O. Associations between metal exposures and cognitive function in American older adults. Int. J. Environ. Res. Public. Health 19(4) (2022).
Hsiao, C. C., Yang, A. M., Wang, C. & Lin, C. Y. Association between glyphosate exposure and cognitive function, depression, and neurological diseases in a representative sample of US adults: NHANES 2013–2014 analysis. Environ. Res. 237(Pt 1), 116860 (2023).
Vincent, M. J. et al. Ethylene oxide: Cancer evidence integration and dose-response implications. Dose Response 17(4), 1559325819888317 (2019).
Dunkelberg, H. Carcinogenicity of ethylene oxide and 1,2-propylene oxide upon intragastric administration to rats. Br. J. Cancer 46(6), 924–933 (1982).
Houle, C. D. et al. Frequent p53 and H-ras mutations in benzene- and ethylene oxide-induced mammary gland carcinomas from B6C3F1 mice. Toxicol. Pathol. 34(6), 752–762 (2006).
Han, L. & Wang, Q. Association between hemoglobin adducts of ethylene oxide levels and the risk of short sleep duration in the general population: An analysis based on the National Health and Nutrition Examination Survey. Environ. Sci. Pollut Res. Int. 30(31), 76761–76768 (2023).
Ma, Y. et al. Association between sleep duration and cognitive decline. JAMA Netw. Open 3(9), e2013573 (2020).
Patch, P. C. & Hartlage, L. C. Neurological and emotional sequelae of exposure to ethylene oxide. Int. J. Neurosci. 106(1–2), 101–107 (2001).
Lynch, D. W. et al. Carcinogenic and toxicologic effects of inhaled ethylene oxide and propylene oxide in F344 rats. Toxicol. Appl. Pharmacol. 76(1), 69–84 (1984).
Kuiper, H. C., Miranda, C. L., Sowell, J. D. & Stevens, J. F. Mercapturic acid conjugates of 4-hydroxy-2-nonenal and 4-oxo-2-nonenal metabolites are in vivo markers of oxidative stress. J. Biol. Chem. 283(25), 17131–17138 (2008).
Katoh, T., Higashi, K., Inoue, N. & Tanaka, I. Effects of chronic inhalation of ethylene oxide on lipid peroxidation and glutathione redox cycle in rat liver. Res. Commun. Chem. Pathol. Pharmacol. 61(2), 281–284 (1988).
Katoh, T., Higashi, K., Inoue, N. & Tanaka, I. Lipid peroxidation and the metabolism of glutathione in rat liver and brain following ethylene oxide inhalation. Toxicology 58(1), 1–9 (1989).
SekharRV GlyNAC supplementation improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, aging Hallmarks, metabolic defects, muscle strength, cognitive decline, and body composition: Implications for healthy aging. J. Nutr. 151(12), 3606–3616 (2021).
Tan, B. L. & Norhaizan, M. E. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients 11(11) (2019).
Funding
None.
Author information
Authors and Affiliations
Contributions
Meng Sun: Conceptualization, Formal analysis, Methodology, Project administration, Writing – original draft, Writing – review & editing. Meng Cai: Conceptualization, Data curation, Formal analysis, Software, Writing – original draft, Writing – review & editing. Sisi Sun: Data curation, Formal analysis. Huan Liu: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software. Guo Chen: Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The studies involving human participants were reviewed and approved by the NCHS Ethics Review Board and all participants have provided written informed consent.
Clinical trial number
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, M., Cai, M., Sun, S. et al. Association between ethylene oxide exposure and cognitive function in older adults from NHANES data. Sci Rep 15, 3472 (2025). https://doi.org/10.1038/s41598-025-87384-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87384-y