Abstract
Both triglyceride-glucose (TyG) index, as a surrogate marker of insulin resistance, and low-density lipoprotein cholesterol (LDL-C) are independent risk factors for long-term prognosis among patients with cardio-renal-metabolic (CRM) disease. However, the co-exposures of TyG index and LDL-C to mortality is unclear. The aim of this study is to investigate the joint effects and risk stratification of the TyG index and LDL-C on all-cause and cardiovascular mortality in CRM patients. We analyzed CRM patients from the National Health and Nutrition Examination Survey (NHANES) database (1999–2018), calculating TyG index as Ln[fasting triglyceride (mg/dL)×fasting glucose (mg/dL)/2] and using multivariable Cox regression models to assess the joint effects of TyG index and LDL-C on all-cause and cardiovascular mortality. The interaction between the TyG index and LDL-C to mortality was also evaluated. During a median follow-up of 7.6 years, 22.8% and 8.4% of patients died from all-cause and cardiovascular causes, respectively. Among patients with LDL-C < 2.6 mmol/L, no significant differences were observed in all-cause and cardiovascular mortality when comparing higher TyG index to the lowest tertile (T1). Specifically, the hazard ratio (HR) for all-cause mortality in the second (T2) and third tertiles (T3) were 0.81 (95% confidence interval(CI): 0.59–1.09) and 0.87 (95%CI: 0.62–1.22), respectively, with a P for trend of 0.468. For cardiovascular mortality, the HR for T2 and T3 compared to T1 were 0.80 (95%CI: 0.48–1.32) and 0.72 (95%CI: 0.45–1.15), respectively, with a P for trend of 0.173. However, elevated TyG index was related to markedly increased risk of all-cause and cardiovascular mortality in patients with LDL-C ≥ 2.6 mmol/L. Specifically, for all-cause mortality, HR for T2 and T3 compared to T1 were 1.01 (95%CI: 0.79–1.28) and 1.38 (95%CI: 1.07–1.79), respectively, with a P for trend of 0.009. For cardiovascular mortality, the HR was 1.09 (95% CI: 0.72–1.65) for T2 and 1.80 (95% CI: 1.18–2.75) for T3, with a P for trend of 0.005. Interactive analysis also demonstrated that a significant association of TyG index and LDL-C with the risk of all-cause (P for interaction = 0.011) and cardiovascular (P for interaction = 0.050) mortality was observed. The findings highlight that elevated TyG index can significantly increase the risk of all-cause and cardiovascular mortality only among CRM patients with LDL-C ≥ 2.6 mmol/L, but not among patients with LDL-C < 2.6 mmol/L.
Similar content being viewed by others
Correspondence: Heyuan People’s Hospital; Guangdong Provincial People’s Hospital, Heyuan Hospital, Heyuan, 517,001, China. Phone: (+ 86)3,185,020.
Background
Cardiac, renal, and metabolic (CRM) conditions remain a leading cause of morbidity and mortality in the United States, which is estimated to account for about a third of contemporary deaths1. Cardiovascular, kidney, and metabolic functions are closely linked2, and share common pathophysiological mechanisms and risk factors in the onset of these diseases3. The impairment in one system may promote and amplify dysfunction in the others, leading to subsequent morbidity and mortality4,5,6. Therefore, the joint management of risk factors for the risk assessment and improvement of the prognosis among CRM patients is crucial.
Insulin resistance (IR), characterized by reduced sensitivity or responsiveness to insulin metabolism, has been widely recognized as an independent risk factor for mortality among CRM patients3. The triglyceride–glucose index (TyG) has been considered a dependable and surrogate biomarker for reflecting IR7. Current research has established a strong association between the TyG index and mortality in the high-risk populations, such as those with acute myocardial infarction (AMI)8, acute heart failure (AHF)9, and patients in intensive care units10. In addition, previous studies have also shown that a 1-unit increment of the TyG index was associated with a significantly higher risk of adverse cardiac and cerebrovascular events (MACCE) in patients with acute coronary syndromes, with a hazard ratio (HR) of 1.18, and the highest quartile of TyG had a higher MACCE incidence compared to the lowest quartile, with an HR of 1.2911. However, the relationships between the TyG index and mortality in the general community population remain unclear. The prognostic potential of the TyG index is being increasingly explored among populations at moderate to low risk, including those with chronic kidney disease(CKD)12, diabetes (DM)13, and the general population14. This indicates that there is a growing body of researches highlighting the importance of the TyG index, the specific association with mortality in different risk groups, especially within the CRM population, requires further elucidation.
Low-density lipoprotein cholesterol (LDL-C) can permeate arterial walls, accumulate within the intima, and promote the generation of atherosclerotic plaque, which can significantly constitute a major risk factor for long-term mortality of CRM patients15,16. The joint assessment of the TyG index with other risk factors is essential for risk stratification in the community population. Cui et al. found the joint assessment of TyG index and inflammation contributes to residual risk stratification and primary prevention of cardiovascular disease (CVD)17. Emerging evidence suggests that the TyG index is not only strongly correlated with dyslipidemia but also enhances risk stratification for long-term prognosis compared to traditional risk factors18,19. Therefore, there is potential for a significant interaction between the TyG index and LDL-C in relation to long-term mortality. However, the precise nature of this interaction is currently not well understood in the CRM population.
Therefore, the objective of this research was to investigate the joint effect and risk stratification of TyG index and LDL-C with all-cause and cardiovascular mortality among patients with CRM, based on a large-scale prospective cohort, which may be beneficial to the clinical management of CRM patients in clinical practice.
Method
Study design and population
Data were employed from the National Health and Nutrition Examination Survey (NHANES) database, a program administered by the Centers for Disease Control and Prevention and the National Centers for Health Statistics in the US. CRM patients are defined as individuals with at least one of the following conditions: CVD, CKD, or DM20, and patients with CRM disease were included in this research. CRM disease was selected based on self-reported medical histories and confirmed through diagnostic codes from the NHANES database. Participants were identified through a combination of medical interviews, physical examinations, and laboratory tests conducted as part of the NHANES survey. The Research Ethics Review Committee of the National Center for Health Statistics approved the NHANES study, and all participants provided written informed consent.
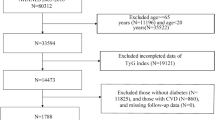
The cohort included a total of 21,604 patients with CRM during 1999–2018. Patients meeting the following criteria were excluded: (1) participants with age < 18; (2) receiving dialysis treatment or combining with renal failure (estimated glomerular filtration rate (eGFR)<15 ml/min/1.73m2) in the past year; (3) combined with malignant tumor; (4) participants experiencing pregnancy; (5) missing information on TyG index (fasting blood glucose [FBG] or triglyceride [TG]), or LDL-C; (6) missing information on mortality or survival time. Finally, a total of 6,010 eligible patients with CRM were enrolled in the study (Fig. 1).
Outcomes and exposure definitions
The main outcomes of this research were all-cause and cardiovascular mortality. Information about mortality status and the cause of death was gained from the NHANES Linked Mortality Files, which was created by the National Center for Health Statistics (NCHS) by linking the NHANES data to the National Death Index (NDI). In this research, short-term mortality was defined as death within 5-year, and long-term mortality was defined as post-5-year mortality. NHANES used an autoanalyzer to enzymatically measure plasma TG, FPG, and LDL-C levels from fasting blood samples. The TyG index was calculated by Ln[fasting triglyceride (mg/dL)×fasting glucose (mg/dL)/2]21.
Definition of covariates
Baseline variables, including demographic data (age, gender, educational levels, race, insurance, poverty index, and matrimony), lifestyle variables (smoking status, alcohol drinking, and physical activity), medical history(CVD, anemia, hypertension[HT], CKD, DM), drug history(glucose-lowering drugs, antihypertensive drugs, as well as lipid-lowering drugs), anthropometric measurements(height, weight, body mass index[BMI], systolic blood pressure[SBP], and diastolic blood pressure[DBP]), and laboratory variables (hemoglobin A1c [HbA1c], high-density lipoprotein cholesterol[HDL-C], neutrophil/lymphocyte ratio[NLR], total cholesterol, white blood cell[WBC], blood urea nitrogen[BUN], serum creatinine, serum uric acid(SUA), eGFR, and urinary albumin/creatinine ratio[uACR]) were selected. Blood samples were taken by the Mobile Examination Centers (MECs) during the medical examination component of the NHANES survey. Demographics, lifestyle variables, medical history, and drug history were collected through self-reported questionnaires. Anthropometric indicators and biochemical parameters were obtained through medical examinations and laboratory tests, respectively. Further details can be found at https://www.cdc.gov/nchs/nhanes/index.htm.
Variables including smoking (current smoker, former smoker, and never smoked), alcohol drinking (none, moderate, and heavy), education (below high school, high school, and college or above), matrimony (never married, divorced/separated/widowed, and married/living with partner) and physical activity (PA) (moderate, sedentary, and vigorous) were further defined. CVD is composed of heart attack, congestive heart failure, angina, CAD, and stroke22. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation23, and CKD was defined as eGFR ≤ 60 ml/min/1.73 m2 or uACR ≥ 30 mg/g. Diabetes was defined as fasting glucose ≥ 7.0 mmol/L or HbA1c(%) ≥ 6.5 or self-reported diagnosis history of diabetes or use of any hypoglycemic medication24. Anemia is defined as men with a hemoglobin level less than 130 g/L and women with a hemoglobin level less than 120 g/L according to World Health Organization standards25.
Statistical analysis
All statistical analyses were conducted using a complex, multistage probability sampling design. The study includes data from eight distinct survey cycles over a period of 18 years, starting with the initial phase from 1999 to 2002 and continuing with biennial cycles from 2003 to 2018. Fasting sample weights were applied in accordance with the NHANES Analytical Guidelines.
Baseline characteristics between groups were compared using weighted analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables. The mortality rates were calculated by dividing the number of deaths in each group by the total number of participants in that group. As our study used NHANES’ complex, weighted, multi-stage sampling design, the mortality rates presented are weighted outcomes, displayed as rates ± standard errors. NHANES study participants came from the community, and the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATPIII) guidelines recommend 2.6 mmol/L as an appropriate cutoff in a community cohort26. Restricted cubic splines (RCS) analysis was utilized to assess the dose-response relationships of the TyG index with all-cause and cardiovascular mortality in LDL-C < 2.6 mmol/L and ≥ 2.6 mmol/L, respectively. Kaplan-Meier method was applied to time-to-event endpoints, with a stratified log-rank test used to compare differences among groups defined by TyG and LDL-C levels. Cox regression analysis was used to evaluate the individual and joint effects of the TyG index and LDL-C on cardiovascular and all-cause mortality. To evaluate whether there is a linear trend in mortality risk across increasing exposure groups, the categorical exposure groups were transformed into a numerical scale. This transformation served as the primary predictor in our model. The estimated coefficient for this variable quantifies the trend in mortality risk corresponding to escalating levels of exposure. Potential confounding factors were corrected in the multifactor COX model, and the selection of these confounding covariates was based on prior literature, clinical guidelines, and the pathophysiology of CRM disease. We chose to conduct interaction analyses to explore the potential interaction between TyG index and LDL-C in relation to mortality. The significance of the interaction was assessed using a likelihood ratio test, comparing the fit of the model with and without the interaction term. This analysis is conducted on both an additive scale (using P for interaction) and a multiplicative scale (using hazard ratio and confidence intervals) to provide a comprehensive understanding of the interaction effect. The stratified analyses are to examine the relationship between the TyG index and LDL-C within different subgroups (CVD, CKD, and DM) of CRM patients, particularly based on LDL-C levels (< 2.6 mmol/L and ≥ 2.6 mmol/L). By stratifying our analyses, we can identify specific patients who may be at higher risk and potentially benefit from targeted interventions. Missing data on covariates were handled using multiple imputations, and a sensitivity analysis was performed. A list of missing data on covariates is provided in Supplementary Table 1.
The analyses were performed using R software (Version 4.2.3), and statistical significance was determined using two-tailed tests with a threshold of P < 0.05.
Results
Baseline characteristics
The mean age of the patients was 56.96 ± 0.31 years, with females accounting for approximately 52.97%. Table 1 presented the baseline characteristics according to LDL-C and TyG tertiles. Patients with elevated TyG index tended to be male and often had DM, but the proportions of both hypertension and anemia decreased. They also showed higher levels of BMI, SBP, DBP, WBC, HbA1c, and SUA. Moreover, these patients were more likely to receive glucose-lowering drugs, antihypertensive drugs, and lipid-lowering drugs, irrespective of their LDL-C levels being < 2.6 mmol/L or ≥ 2.6 mmol/L. In addition, patients with a higher TyG index were more likely to be drinking and to have combined HT in the LDL-C<2.6 mmol/L group, while those exhibited a higher level of uACR in the LDL-C ≥ 2.6 mmol/L group. Compared with patients with LDL-C<2.6 mmol/L, when the TyG index was at a high level, patients with LDL-C ≥ 2.6 mmol/L were younger, have a higher proportion of female, and had a lower proportion of complications, but there was no significant difference in BMI(Table 1).
With a median follow-up period was 7.6 years ([interquartile range: 4.1–11.9]), there were 1370 (22.8%) and 504 (8.4%) patients who died from all-cause and cardiovascular death, respectively. Participants with elevated TyG index had higher all-cause mortality (P for trend = 0.001) and cardiovascular mortality (P for trend = 0.008) among patients with LDL-C ≥ 2.6 mmol/L. However, an increase in long-term all-cause mortality (P for trend = 0.351) and cardiovascular mortality (P for trend = 0.540) with higher TyG index were not statistically significant for LDL-C < 2.6 mmol/L (Fig. 2). Further analysis revealed that among participants with LDL-C < 2.6 mmol/L, an increase in the TyG index was associated with an even decreasing trend in short-term mortality, including both all-cause mortality (P for trend = 0.010) and cardiovascular mortality (P for trend = 0.020) (Supplementary Fig. 1). Kaplan–Meier analysis consistently demonstrated that the association between the TyG index and the risk of all-cause (P = 0.003) and cardiovascular (P = 0.010) mortality varied according to LDL-C stratification (Supplementary Fig. 2).
The association of TyG index, and LDL-C with all-cause and cardiovascular mortality
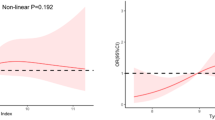
After adjusting for confounders, neither individual TyG index nor LDL-C were independently associated with all-cause or cardiovascular mortality (Supplementary Table 2). However, the combined analysis revealed significant associations between categories of LDL-C and TyG index and long-term mortality. Among patients with LDL-C ≥ 2.6 mmol/L, increasing TyG index was linked to higher risks of all-cause and cardiovascular mortality. Specifically, compared to the first tertile (T1), the HR for the second (T2) and third tertiles (T3) were 1.01 (95% (confidence interval)CI: 0.79–1.28) and 1.38 (95%CI: 1.07–1.79) for all-cause mortality, and 1.09 (95%CI: 0.72–1.65) and 1.80 (95%CI: 1.18–2.75) for cardiovascular mortality, respectively, with a P for trend of 0.009 for all-cause mortality and 0.005 for cardiovascular mortality. However, there was no statistically significant association between TyG index and all-cause or cardiovascular mortality in patients with LDL-C < 2.6 mmol/L. Specifically, the HR for all-cause mortality for T2 and T3 compared to T1 were 0.81 (95%CI: 0.59–1.09) and 0.87 (95%CI: 0.62–1.22), respectively, with a P for trend of 0.468. For cardiovascular mortality, the HR was 0.80 (95%CI: 0.48–1.32) and 0.72 (95%CI: 0.45–1.15), respectively, with a P for trend of 0.173. Interactive analyses further revealed significant interactions between the TyG index and LDL-C in predicting risks for all-cause (P for interaction = 0.011) and cardiovascular mortality (P for interaction = 0.050) (Fig. 3). Further analysis also found that compared with patients with LDL-C ≥ 2.6 mmol/L and TyG in T1, those with LDL-C ≥ 2.6 mmol/L and TyG in T3 showed increased risks of all-cause mortality (HR: 1.47, 95%CI: 1.15–1.88, P = 0.002) and cardiovascular mortality (HR: 1.65, 95%CI: 1.09–2.49, P = 0.018). Similarly, patients with LDL-C < 2.6 mmol/L and TyG in T1 also exhibited elevated risks for all-cause mortality (HR: 1.59, 95%CI: 1.22–2.08, P < 0.001) and cardiovascular mortality (HR: 1.64, 95% CI: 1.04–2.58, P = 0.032)(Table 2). Therefore, the combined effects of TyG index and LDL-C on mortality were synergistic, with the impact of a high TyG index on mortality risk was significantly amplified in the presence of elevated LDL-C levels. Further subgroup analysis also indicated that elevated TyG index was associated with a higher risk of all-cause and cardiovascular mortality among patients with CKD, CAD, and diabetes in the LDL-C ≥ 2.6 mmol/L group, but not in LDL-C < 2.6 mmol/L group (Fig. 4). The results also remained consistent in analyses conducted on datasets after the imputation of missing data (Supplementary Tables 3 and Supplementary Fig. 3). Restricted cubic spline analyses also demonstrated a nearly linear relationship between the continuous scale TyG and both all-cause mortality (nonlinear P = 0.182) and cardiovascular mortality (nonlinear P = 0.244) in patients with LDL-C ≥ 2.6 mmol/L, Conversely, no significant relationship was observed between TyG index and either all-cause or cardiovascular mortality among patients with LDL-C<2.6 mmol/L(Fig. 5).
The joint association between TyG index and LDL-C for all-cause and cardiovascular mortality. Model adjusted for age, gender, race, alcohol drinking, smoking, physical activity, poverty index, education, matrimony, insurance, body mass index, hypertension, diabetes, chronic kidney disease, cardiovascular disease, anemia, neutrophil/lymphocyte ratio, high-density lipoprotein cholesterol, serum uric acid, blood urea nitrogen, glucose-lowering drug, antihypertensive drug, and lipid-lowering drug.
The subgroups analyses of the joint association between TyG index and LDL-C for all-cause and cardiovascular mortality. Due to the limited sample size, the subgroups analyses model adjusted for age, gender, race, alcohol drinking, smoking, physical activity, poverty index, education, matrimony, insurance, and body mass index.
Hazard ratio for the all-cause, and cardiovascular death based on restricted cubic spine function for the TyG index and LDL-C. All restricted cubic spline analyses were adjusted for age, gender, race, alcohol drinking, smoking, physical activity, poverty index, education, matrimony, insurance, body mass index, hypertension, diabetes, chronic kidney disease, cardiovascular disease, anemia, neutrophil/lymphocyte ratio, high-density lipoprotein cholesterol, serum uric acid, blood urea nitrogen, glucose-lowering drug, antihypertensive drug, and lipid-lowering drug.
Discussion
To our knowledge, this is the first prospective study that evaluated the joint association of TyG index and LDL-C levels with the subsequent risk of all-cause and cardiovascular mortality among CRM patients. Our research found that the associations of the TyG index with all-cause and cardiovascular mortality were modified by LDL-C levels. Elevated TyG index can significantly increase the risk of all-cause and cardiovascular mortality only among CRM patients with LDL-C ≥ 2.6 mmol/L, but not among patients with LDL-C < 2.6 mmol/L. Further analysis also observed a significant interaction. Therefore, joint assessment of TyG index and LDL-C should be emphasized for risk stratification and improvement of long-term prognosis among CRM patients in the community.
Previous research has found that individual TyG index is significantly associated with the risk of mortality in emergency and critically ill populations. A higher baseline TyG index significantly increases the risk of mortality among critically ill patients27,28,29. Zheng et al. also found that baseline TyG is related to the prognosis of cardiac arrest30. Further research also found the dynamic changes in TyG during hospitalization were related to one-year all-cause death among critically ill patients with CAD31. Therefore, the TyG index has important prognostic significance among the critically ill population in clinical practice. However, in the general or community population, the effect of individual TyG index on long-term prognosis was weak, even without statistical difference. Chen et al. demonstrated that after full adjustment, a significant relationship between the TyG stratification and all-cause and cardiovascular death in the general population was not observed32. Ghazaal et al. consistently found that after adjusting for DM, the effects of TyG index on all-cause and cardiovascular death disappeared, and TyG index was not associated with the risk of non-cardiovascular death in the general population33. Our study also found that individual TyG index was not associated with all-cause and cardiovascular death among CRM subjects in the community cohort. Therefore, further studies are needed to explore risk stratification in the general community population. Previous research showed that TyG index was positively related to lipid-related markers by Pearson correlation analysis, including LDL-C (r = 0.238, P < 0.001) in AMI patients without DM31. However, there has been no research to explore the joint association of TyG index and LDL-C with the risk of long-term mortality among CRM patients in the community. Our study found that there is a significant joint association of TyG index and LDL-C with all-cause and cardiovascular mortality among CRM patients, which shows no difference between all-cause and cardiovascular mortality on an elevated TyG index in patients with LDL-C < 2.6 mmol/L, while higher TyG index was related to significantly increased risk of all-cause and cardiovascular mortality among patients with LDL-C ≥ 2.6 mmol/L.
The underlying mechanisms of the joint effect of TyG index and LDL-C remain unknown. The TyG index is significantly associated with long-term prognosis and has been recognized as a reliable and newfound biomarker for gauging IR7. Pathophysiological research has indicated IR can induce low-grade inflammation, dyslipidemia, and chronic hyperglycemia, which may serve as important mechanisms for the increased risk of death34,35,36. Moreover, IR induces dyslipidemia, mainly manifested as an increase in serum total cholesterol, LDL-C, or triglycerides, together increasing the mortality of all-cause and cardiovascular death37. Furthermore, the accumulation of triglyceride and LDL-C in hepatocytes will further promote IR through inflammation, oxidative stress, and endothelial dysfunction38. Therefore, the joint effect of IR with dyslipidemia may significantly increase the risk of all-cause and cardiovascular mortality.
Interestingly, findings from our studies suggested an association of low LDL-C levels with a higher risk of all-cause and cardiovascular death among CRM patients, and subgroup analysis also showed a consistent trend. A growing body of research has also shown a similar relationship. Previous research indicated a paradoxical relationship between low LDL-C levels and a higher risk of death among patients with acute coronary syndrome39,40. In addition, a recent meta-analysis demonstrated that the benefits of lowering LDL-C did not exist when the LDL-C < 2.6 mmol/L41. Consistently, Wu et al., also found low LDL-C levels increased the risk of all-cause and cause-specific death42. A plausible cause for the lack of a significant correlation between LDL-C and long-term prognosis is that CRM subjects with low LDL-C levels.
were in poor condition and had more complications. Our research also showed that patients with low LDL-C levels had a higher prevalence of CVD-related diseases, as well as other complications related to poor prognosis, including anemia (12.94% vs. 7.04%), HT (59.99% vs. 51.48%), and poor renal function. Similarly, Kovesdy et al. also found that the paradoxical association of low LDL-C levels with a higher risk of mortality attenuated as the adjusted variables increased. After further correcting for confounders, low LDL-C levels were no longer independently associated with an increased risk of death43. Therefore, the higher all-cause and cardiovascular death among participants with low LDL-C levels may be caused by underlying disease to some extent.
In addition, previous studies have also shown that it is of great significance to jointly assess the relationship between uric acid and IR in patients with cardiometabolic diseases. Froylan et al. found that SUA concentration is notably correlated with IR and impaired insulin secretion, but not with beta-cell dysfunction, among patients at risk for developing diabetes44. Alessandro et al. also revealed that SUA does not track with changes over time in insulin sensitivity, or glycemia in women with recent gestational diabetes, providing evidence against causality in its association with diabetes45. Meanwhile, Gou et al. also demonstrated that TyG was related to a higher risk of hyperuricemia in Chinese and American populations46. These studies underscore the potential for TyG index and SUA to serve as a therapeutic target in the management of cardiometabolic diseases.
Our results suggest that it is important to control IR through lifestyle changes and medications and to control elevated LDL-C levels with lipid-lowering medications. Furthermore, understanding the joint associations of TyG index and LDL-C levels with all-cause and cardiovascular death and their predictive values in long-term prognosis could help primary care physicians identify patients at high risk of poor prognosis, particularly focusing on those with LDL-C ≥ 2.6 mmol/L where the TyG index significantly elevates mortality risks. Hence, the joint evaluation of TyG and LDL-C in routine clinical practice is essential to improve prognosis among patients with CRM. Besides, our results highlighted the importance of regular detection of TyG index and LDL-C and the interventions that target both insulin resistance and dyslipidemia may reduce the risk of all-cause and cardiovascular death. In addition, excessively low LDL-C levels in the assessment of the risk of long-term death may serve as a newfound indicator in clinical practice, and if necessary, screen for related comorbidities that may affect death. Ultimately, further high-quality prospective research for the joint effect between TyG index and LDL-C in CRM patients should be conducted to evaluate the appropriate management strategy.
Strengths and limitations
The strengths of our study include its prospective design, which allows for a more accurate assessment of the causal relationship between the TyG index, LDL-C and mortality risks among CRM patients. Our research is the first to evaluate the joint association of these two indicators, providing novel insights into risk stratification for CRM patients. Additionally, the large sample size and long follow-up period enhance the statistical power and generalizability of our findings. However, it is important to consider several limitations. Firstly, despite adjusting for potential confounders, there are still residual confounding effects of indefinite factors that may contribute to the increased risk of death. Second, this study only explored the baseline TyG index, and did not evaluate how the temporal variation of this index affects its association with death. Tian et al. also have shown that different periods of cumulative TyG lead to different risks of mortality47. Thirdly, due to sample size limitations, we did not further evaluate the relationship between TyG index and mortality, based on the cutoff of LDL-C < 1.8 mmol/L. However, the NCEP-ATPIII guidelines recommend 2.6 mmol/L as an appropriate cutoff in a community cohort26. Finally, the NHANES study was conducted only in the United States, and it is unclear whether our findings are applicable to other regions. Therefore, further high-quality studies in different populations or international cohorts are needed to verify our findings, especially in non-US populations.
Conclusions
Our research found the relationship between TyG index and all-cause and cardiovascular mortality was modified by LDL-C levels. Elevated TyG index can significantly increase the risk of all-cause and cardiovascular mortality only among CRM patients with LDL-C ≥ 2.6 mmol/L, but not among patients with LDL-C < 2.6 mmol/L. Joint assessment of TyG index and LDL-C levels should be emphasized for risk stratification and improvement of long-term prognosis among CRM patients.
Data availability
All data are available as publicly accessible datasets through NHANES. It is open and publicly accessible through the following link: https://wwwn.cdc.gov/nchs/nhanes/.
Abbreviations
- TyG:
-
triglyceride-glucose
- LDL-C:
-
low-density lipoprotein cholesterol
- SBP:
-
systolic blood pressure
- DBP:
-
diastolic blood pressure
- BMI:
-
body mass index
- HbA1c:
-
glucated hemoglobin
- NLR:
-
neutrophil/lymphocyte ratio
- SUA:
-
serum uric acid
- WBC:
-
white blood cell
- HDL-C:
-
high-density lipoprotein cholesterol
- BUN:
-
blood urea nitrogen
- uACR:
-
urea albumin creatinine ratio
- eGFR:
-
estimated glomerular filtration rate
- CVD:
-
cardiovascular disease
- HT:
-
hypertension
- CKD:
-
chronic kidney disease
- DM:
-
diabetes mellitus
- T:
-
tristile
References
Ahmad, F. B. & Anderson, R. N. The leading causes of death in the US for 2020. Jama 325 (18), 1829–1830 (2021).
Sarafidis, P. et al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol. Dial Transpl. 34 (2), 208–230 (2019).
Danaei, G. et al. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol. 2 (8), 634–647 (2014).
Cherney, D. Z. I. et al. Impact of Cardio-Renal-Metabolic comorbidities on Cardiovascular outcomes and Mortality in type 2 diabetes Mellitus. Am. J. Nephrol. 51 (1), 74–82 (2020).
Forman, D. E. et al. Multimorbidity in older adults with Cardiovascular Disease. J. Am. Coll. Cardiol. 71 (19), 2149–2161 (2018).
Thomas, G. et al. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 6 (10), 2364–2373 (2011).
Tahapary, D. L. et al. Challenges in the diagnosis of insulin resistance: focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab. Syndr. 16 (8), 102581 (2022).
Drwiła, D. et al. Prognostic value of the triglyceride-glucose index among non-diabetic patients with acute myocardial infarction at one-year follow-up. Kardiol Pol. 79 (10), 1116–1123 (2021).
Cheng, H. et al. The triglyceride glucose index predicts short-term mortality in non-diabetic patients with acute heart failure. Adv. Clin. Exp. Med. 33 (2), 103–110 (2024).
Liao, Y. et al. Triglyceride-glucose index linked to all-cause mortality in critically ill patients: a cohort of 3026 patients. Cardiovasc. Diabetol. 21 (1), 128 (2022).
Khalaji, A. et al. Triglyceride-glucose index as a predictor of cardiac adverse events in acute coronary syndrome patients undergoing percutaneous coronary intervention: role of diabetes. BMC Cardiovasc. Disord. 24 (1), 514 (2024).
Qin, Y. et al. Triglyceride-glucose index and mortality risk in individuals with or without chronic kidney disease: insights from a national survey of United States adults, 1999–2018. Nutr. Metab. Cardiovasc. Dis. 34 (8), 1994–2001 (2024).
Liu, C. et al. Association between the triglyceride-glucose index and all-cause and CVD mortality in the young population with diabetes. Cardiovasc. Diabetol. 23 (1), 171 (2024).
Chen, J. et al. Association of triglyceride glucose index with all-cause and cardiovascular mortality in the general population. Cardiovasc. Diabetol. 22 (1), 320 (2023).
Valensi, P. et al. The impact of diabetes on heart failure development: the cardio-renal-metabolic connection. Diabetes Res. Clin. Pract. 175, 108831 (2021).
Whaley-Connell, A. & Sowers, J. R. Basic science: pathophysiology: the cardiorenal metabolic syndrome. J. Am. Soc. Hypertens. 8 (8), 604–606 (2014).
Cui, C. et al. Joint association of TyG index and high sensitivity C-reactive protein with cardiovascular disease: a national cohort study. Cardiovasc. Diabetol. 23 (1), 156 (2024).
Wen, J. et al. Association of triglyceride-glucose index with atherosclerotic cardiovascular disease and mortality among familial hypercholesterolemia patients. Diabetol. Metab. Syndr. 15 (1), 39 (2023).
Zhao, K. et al. Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio predict the prognosis in patients with type B aortic dissection receiving thoracic endovascular aortic repair. J. Thorac. Dis. 16 (3), 1971–1983 (2024).
Ostrominski, J. W. et al. Prevalence and overlap of Cardiac, Renal, and metabolic conditions in US adults, 1999–2020. JAMA Cardiol. 8 (11), 1050–1060 (2023).
Guerrero-Romero, F. et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 95 (7), 3347–3351 (2010).
Zhang, Y. et al. Exposure to acrylamide and the risk of cardiovascular diseases in the National Health and Nutrition Examination Survey 2003–2006. Environ. Int. 117, 154–163 (2018).
Levey, A. S. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 130 (6), 461–470 (1999).
ElSayed, N. A. et al. 2. Diagnosis and classification of diabetes: standards of Care in Diabetes-2024. Diabetes Care. 47 (Suppl 1), S20–s42 (2024).
Seitz, A. E., Eberhardt, M. S. & Lukacs, S. L. Anemia Prevalence and Trends in Adults Aged 65 and Older: U.S. National Health and Nutrition Examination Survey: 2001–2004 to J Am Geriatr Soc, 2018. 66(12): pp. 2431–2432. (2013).
Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Jama, 285(19): pp. 2486–2497. (2001).
Cai, W. et al. Association between triglyceride-glucose index and all-cause mortality in critically ill patients with ischemic stroke: analysis of the MIMIC-IV database. Cardiovasc. Diabetol. 22 (1), 138 (2023).
Zhang, R. et al. Independent effects of the triglyceride-glucose index on all-cause mortality in critically ill patients with coronary heart disease: analysis of the MIMIC-III database. Cardiovasc. Diabetol. 22 (1), 10 (2023).
Zheng, R. et al. Association between triglyceride-glucose index and in-hospital mortality in critically ill patients with sepsis: analysis of the MIMIC-IV database. Cardiovasc. Diabetol. 22 (1), 307 (2023).
Boshen, Y. et al. Triglyceride-glucose index is associated with the occurrence and prognosis of cardiac arrest: a multicenter retrospective observational study. Cardiovasc. Diabetol. 22 (1), 190 (2023).
Guo, J. et al. The triglycerides-glucose index and the triglycerides to high-density lipoprotein cholesterol ratio are both effective predictors of in-hospital death in non-diabetic patients with AMI. PeerJ 10, e14346 (2022).
Cheng, L. et al. Association of dynamic change of triglyceride-glucose index during hospital stay with all-cause mortality in critically ill patients: a retrospective cohort study from MIMIC IV2.0. Cardiovasc. Diabetol. 22 (1), 142 (2023).
Alavi Tabatabaei, G. et al. Association of the triglyceride glucose index with all-cause and cardiovascular mortality in a general population of Iranian adults. Cardiovasc. Diabetol. 23 (1), 66 (2024).
Laakso, M. & Kuusisto, J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat. Rev. Endocrinol. 10 (5), 293–302 (2014).
Ormazabal, V. et al. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 17 (1), 122 (2018).
Shulman, G. I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl. J. Med. 371 (23), 2237–2238 (2014).
Hedayatnia, M. et al. Dyslipidemia and cardiovascular disease risk among the MASHAD study population. Lipids Health Dis. 19 (1), 42 (2020).
Li, M. et al. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal. Transduct. Target. Ther. 7 (1), 216 (2022).
Cho, K. H. et al. Low-density lipoprotein cholesterol level in patients with acute myocardial infarction having percutaneous coronary intervention (the cholesterol paradox). Am. J. Cardiol. 106 (8), 1061–1068 (2010).
Reddy, V. S. et al. Relationship between serum low-density lipoprotein cholesterol and in-hospital mortality following acute myocardial infarction (the lipid paradox). Am. J. Cardiol. 115 (5), 557–562 (2015).
Navarese, E. P. et al. Association between Baseline LDL-C Level and Total and Cardiovascular Mortality after LDL-C lowering: a systematic review and Meta-analysis. Jama 319 (15), 1566–1579 (2018).
Wu, M. et al. Association of low-density lipoprotein-cholesterol with all-cause and cause-specific mortality. Diabetes Metab. Syndr. 17 (6), 102784 (2023).
Kovesdy, C. P., Anderson, J. E. & Kalantar-Zadeh, K. Inverse association between lipid levels and mortality in men with chronic kidney disease who are not yet on dialysis: effects of case mix and the malnutrition-inflammation-cachexia syndrome. J. Am. Soc. Nephrol. 18 (1), 304–311 (2007).
Martínez-Sánchez, F. D. et al. Serum uric acid concentration is associated with insulin resistance and impaired insulin secretion in adults at risk for type 2 diabetes. Prim. Care Diabetes. 15 (2), 293–299 (2021).
Volpe, A. et al. Changes over time in uric acid in relation to changes in insulin sensitivity, Beta-cell function, and Glycemia. J. Clin. Endocrinol. Metab. 105 (3), e651–e659 (2020).
Gou, R. et al. Association between triglyceride glucose index and hyperuricemia: a new evidence from China and the United States. Front. Endocrinol. (Lausanne). 15, 1403858 (2024).
Tian, X. et al. Time course of the triglyceride glucose index accumulation with the risk of cardiovascular disease and all-cause mortality. Cardiovasc. Diabetol. 21 (1), 183 (2022).
Acknowledgements
We extend our heartfelt gratitude to all participants and investigators involved in the NHANES study. Your invaluable contributions have been instrumental to our research endeavors.
Funding
This research was funded and supported by Guangdong Medical Research Foundation (A2024142).
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows—(I) Research idea and study design: Wenguang Lai, Yucui Lin. (II) Statistical analysis: Wenguang Lai, Zhidong Huang; (III) Supervision and mentorship: Tingting Zhang; (IV) revised the manuscript: Zhiyong Gao. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lai, W., Lin, Y., Gao, Z. et al. Joint association of TyG index and LDL-C with all-cause and cardiovascular mortality among patients with cardio-renal-metabolic disease. Sci Rep 15, 5854 (2025). https://doi.org/10.1038/s41598-025-87416-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87416-7
Keywords
This article is cited by
-
Comparison of the triglyceride-glucose index and its modified indices for predicting mortality in advanced CLKM syndrome
Journal of Health, Population and Nutrition (2025)
-
Associations of six insulin resistance-related indices with the risk and progression of cardio-renal-metabolic multimorbidity: evidence from the UK biobank
Cardiovascular Diabetology (2025)
-
Development and validation of a novel nomogram to predict coronary artery calcification: a single center study
European Journal of Medical Research (2025)