Abstract
COVID-19 infection has been linked to ocular involvement, particularly retinal microvascular changes. Additionally, prolonged hypoxemia may affect retinal sublayers located within the retinal watershed zone. The aim of this study was to evaluate retinal and optic nerve OCT parameters in patients with COVID-19 illness of varying severity and compare them with controls. In this prospective case-control study, a total of 78 consecutive patients who had recently recovered from SARS-CoV-2 infection (29 outpatients, 32 ward-admitted patients, and 17 ICU-admitted patients) were included, along with 85 controls. All participants had no ocular disease or symptoms. Spectralis SD-OCT scans of the macula and optic nerve were obtained from all participants 6 weeks after initial diagnosis. The central subfield thickness of the macula (CSFT), peripapillary retinal nerve fiber layer thickness (pRNFL), and retinal sublayers’ volumes in the total 6-mm ETDRS zone were recorded and analyzed using the ANCOVA test, adjusting for age. The mean CSFT in controls was 271 μm, while in the outpatient, ward-admitted, and ICU-admitted groups, it was 251, 260, and 253 μm, respectively (P = 0.093). No differences were observed in pRNFL between the groups (P> 0.1). However, the outer plexiform layer (OPL) was the only retinal sublayer that demonstrated a significant difference in 6-mm volume across the groups, with volumes of 0.832 μm3 in controls, 0.822 μm³ in the outpatients, 0.814 μm³ in ward-admitted patients, and 0.785 μm³ in ICU-admitted cases (P = 0.006). Our findings suggest that patients with severe COVID-19 illness, especially those requiring respiratory support, may develop ischemia and atrophy of the OPL. This indicates that the OPL might be the most vulnerable retinal sublayer to systemic hypoxemia, a hypothesis that requires confirmation through future studies.
Similar content being viewed by others
Introduction
COVID-19, the most severe pandemic of the 21st century in terms of global mortality and morbidity, continues to cause significant illness in certain populations even five years after its onset1,2. Numerous studies have explored the ocular complications of this disease, particularly retinal vascular (or microvascular) occlusions and uveitis3,4,5. These complications are believed to arise from coagulopathy and vascular endothelial damage due to the cytokine storm, as well as virus-host antigen mimicry6.
Respiratory distress, a hallmark of SARS-CoV-2 infection, often leads to prolonged hypoxia in affected patients, with a subset requiring oxygen supplementation or even mechanical respiratory support1. In theory, such hypoxemia can induce retinal ischemia, potentially resulting in atrophy and thinning7,8. Given the unique anatomy of retinal circulation and perfusion, different retinal sublayers may exhibit varying levels of vulnerability to injury caused by hypoxemia.
Understanding these effects is crucial, as they may provide insights into the broader systemic impact of COVID-19 and its complications. Despite extensive research on COVID-19-associated retinal vascular changes, the effects of respiratory distress and prolonged hypoxemia on the retinal substructure remain underexplored.
This study aims to bridge that gap by assessing the OCT parameters of the fovea, peripapillary retinal nerve fiber layer (pRNFL), and macular sublayers in patients with systemic COVID-19 illness of varying severity. We hypothesize that prolonged hypoxemia from COVID-19-associated respiratory distress may result in measurable changes to retinal sublayer thickness, serving as a marker for previous ischemic damage. Highlighting these changes could advance our understanding of the interplay between systemic hypoxia and retinal health, emphasizing the importance of recognizing retinal alterations as a potential consequence of severe COVID-19 illness.
Materials and methods
Patients
In this prospective case-control study, consecutive patients were enrolled from a specialized COVID-19 screening and treatment center during a pandemic peak, between April and June 2021. This study design was selected due to its feasibility for implementation during the peak of SARS-CoV-2 infections and its effectiveness in detecting differences in outcome measures between cases with varying COVID-19 severity and controls.
Patients with a history of previous ocular trauma or surgery (other than uncomplicated cataract surgery), significant ocular diseases such as dense cataract, glaucoma, age-related macular degeneration (ARMD), presumed COVID-19-associated complications (such as retinal vein occlusion [RVO] or uveitis), refractive errors exceeding ± 3 diopters (D), or significant systemic conditions (e.g., diabetes, rheumatologic disorders, asthma, Alzheimer’s disease) were excluded. The inclusion and exclusion criteria were designed to eliminate cases with ocular or systemic conditions that could influence the magnitude or reliability of total retinal or retinal sublayer thickness measurements, as well as systemic diseases that could interfere with COVID-19 disease. Eligible patients were required to be able to attend the ophthalmology clinic six weeks after their initial referral to the COVID-19 emergency facility, cooperate with necessary imaging procedures, have no ophthalmic symptoms related to the recent SARS-CoV-2 infection, and exhibit a healthy-appearing macula as confirmed by fundoscopy and spectral-domain optical coherence tomography (SD-OCT) images.
There were two primary reasons for selecting 6-week interval. First, most SARS-CoV-2-infected patients were likely well enough to visit the ophthalmic imaging facility and cooperate with the imaging procedures. Second, retinal ischemia in the acute phase presents with hyperreflectivity and edema in the affected layer or sector. Typically by about six weeks, these changes progress to atrophy4,9. This interval was chosen to allow for the detection of chronic sequelae of the disease when it is likely to have stabilized.
All participants, or their guardians (in cases of severe illness), provided written informed consent, and the study adhered to the principles outlined in the Declaration of Helsinki. The study protocol was approved by the Ethics Committee at Shiraz University of Medical Sciences.
Based on SARS-CoV-2 PCR test results and clinical examinations, the patients were classified into four groups: Group 1 (controls; healthy individuals or those with a common cold, with negative PCR test results), Group 2 (outpatient SARS-CoV-2-positive cases), Group 3 (ward-admitted SARS-CoV-2-positive patients), and Group 4 (ICU-admitted SARS-CoV-2-positive patients). In this study, we did not collect data on the patients’ blood pressure or oxygen saturation.
Measurements
All patients underwent a comprehensive eye examination, which included measurement of best-corrected visual acuity (BCVA), slit-lamp biomicroscopy, and dilated fundus examination. OCT imaging was performed using the Spectralis OCT2 system (Heidelberg Engineering, Heidelberg, Germany). Imaging procedures were conducted by a trained examiner following the manufacturer’s recommendations. The device was calibrated prior to the study and at regular intervals throughout. For OCT imaging, a pRNFL scan of the optic nerve and a 30-degree macular volume scan were performed. The first successful scan with a quality score of at least 20 was recorded. Images with artifacts or uncorrectable segmentation errors were excluded. As cases with significant myopia or hyperopia were excluded from the study, the axial length scan depth setting on the OCT device was adjusted to “medium” (24.00 ± 2.50 mm).
The integrated HEYEX software was used to measure the total volume of each retinal sublayer within the 6-mm Early Treatment Diabetic Retinopathy Study (ETDRS) zone. The analyzed sublayers included the inner retinal layers (IRL; internal to the external limiting membrane), the outer retinal layers (ORL; external limiting membrane to the retinal pigment epithelium [RPE]), the retinal nerve fiber layer (RNFL), the ganglion cell layer (GCL), the inner plexiform layer (IPL), the inner nuclear layer (INL), the outer plexiform layer (OPL), the outer nuclear layer (ONL), and the RPE.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics software (version 26; SPSS Inc., Chicago, Illinois, USA). Data are presented as adjusted means ± standard error of the mean (SEM). To account for the differing age distribution among groups, retinal sublayer values obtained from OCT imaging were compared among the four groups (controls and SARS-CoV-2-positive groups: outpatient, ward-admitted, and ICU-admitted) using analysis of covariance (ANCOVA), with age included as a covariate. Pairwise comparisons were conducted with Bonferroni correction to adjust for multiple comparisons. ANCOVA assumptions were tested using the Shapiro-Wilk test to assess the normality of residuals, Levene’s test to evaluate homogeneity of variance, and interaction tests to verify the homogeneity of regression slopes, and it was ensured that the assumptions were met before proceeding with the analysis. Our analysis was conducted on only one eye of the patients (right eyes) to eliminate bias arising from interocular correlations.
Results
Data from 78 cases with recent SARS-CoV-2 infection (29 outpatients, 32 ward-admitted, and 17 ICU-admitted) and 85 controls were collected and analyzed. Table 1 summarizes the baseline characteristics of the study participants. No significant differences were observed among the groups in terms of sex, spherical equivalent (SE) refraction, BCVA, or intraocular pressure (IOP). However, the age composition differed significantly between the groups. To address the potential influence of age on OCT variables, age was included as a covariate in all statistical analyses of OCT parameters.
The adjusted mean central subfield thickness (CSFT) ± SEM was 271 ± 3 μm in the control group, 251 ± 9 μm in the outpatient group, 260 ± 4 μm in ward-admitted patients, and 253 ± 5 μm in ICU-admitted cases (P = 0.093). Table 2 provides a detailed summary of the layer-specific retinal volume measurements across the four studied groups.
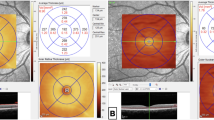
The differences in retinal sublayer volumes were statistically insignificant across the groups, except for the OPL, which demonstrated a declining trend with increasing disease severity (P = 0.006; pairwise comparison: p = 0.009 for ICU-admitted patients versus controls; Fig. 1).
The ANCOVA test offers the advantage of providing adjusted mean values based on a controlled parameter (age in our study), allowing for a clearer comparison of the parameter values between groups. In addition, it facilitates comparisons among the four categorical groups while adjusting for covariates. However, recognizing the limitations of this method and to account for the possibility of type I statistical error, we also performed a linear regression analysis to identify independent predictors of OPL volume. Variables included in the analysis were age, sex, SE refraction, and SARS-CoV-2 infection status (control vs. positive). Through stepwise multiple regression analysis, the SARS-CoV-2 group emerged as the only independent predictor of OPL volume (unstandardized coefficient: −0.025; standard error: 0.011; P = 0.026), further supporting the ANCOVA findings.
No significant differences in pRNFL thickness were observed among the groups (Table 3).
Discussion
Although COVID-19 illness has primarily presented as an acute respiratory syndrome, further reports reveal its complex pathophysiology, which involves an intricate immunological response, leading to coagulopathy and vasculopathy affecting multiple organ systems10. Ocular complications associated with SARS-CoV-2 infection are frequently reported. The posterior segment of the eye can be affected by conditions such as uveitis, central serous chorioretinopathy, RVO, and paracentral acute middle maculopathy (PAMM)3,4,5,11.
According to our findings, the OPL was the only retinal sublayer to show a significant difference in 6-mm volume among the groups. In the control group, the OPL volume was 0.832 μm3, while it measured 0.822, 0.814, and 0.785 μm³ in the outpatient, ward-admitted, and ICU-admitted SARS-CoV-2-positive patients, respectively (P= 0.006). This observation may be attributable to the unique anatomical location of the OPL, located in the watershed zone of the retina, between the retinal and choroidal circulation12,13,14 (Fig. 2). In addition, the deep capillary plexus of the retinal circulation, which contributes to OPL perfusion, has the lowest vascular density compared to the superficial and intermediate capillary plexuses14. In cases of systemic hypoxemia, oxygen tension easily drops in the watershed zone of the retina. The OPL, being one of the primary oxygen-consuming layers of the retina, is particularly vulnerable to such reductions in oxygen supply15,16.
An SD-OCT angiography B-scan of the macula shows the source of perfusion for different retinal sublayers. The outer plexiform layer (OPL) is located in the watershed zone between the retinal (central retinal artery, CRA) and choroidal circulation. This likely results in the lowest hydrostatic pressure (HP) and oxygen tension (O2) in the retina.
The typical OCT presentation of retinal ischemia, as observed in conditions such as central retinal artery occlusion (CRAO), branch retinal artery occlusion (BRAO), PAMM, and acute macular neuroretinopathy (AMN), includes hyper-reflectivity and thickening of the inner retinal layers during the acute phase, followed by inner retinal atrophy in the subsequent weeks17,18. Bayram et al.19 reported OCT findings in severe cases of COVID-19 illness and found an increase in OPL thickness during the acute phase of the disease. Their results (indicating acute OPL ischemia) are consistent with our study, which showed OPL thinning six weeks after SARS-CoV-2 infection (chronic sequelae of OPL ischemia).
Although we proposed prolonged systemic hypoxemia as the most probable cause of OPL thinning in cases with SARS-CoV-2 infection, other COVID-19-associated immune-mediated mechanisms may also contribute. These mechanisms include microvasculopathy and coagulopathy6, which can affect various capillary beds and may have a detectable impact on the most vulnerable retinal vascular plexus (the deep capillary plexus) and sublayer (the OPL).
The OPL of the retina plays a crucial role in intraretinal visual processing, acting as the primary site for synaptic connections between photoreceptors (rods and cones), bipolar cells, and horizontal cells. This layer is essential for the transfer and integration of visual signals, contributing to key processes such as spatial resolution, contrast enhancement, and color discrimination. Damage to the OPL disrupts these critical synaptic interactions, impairing the transmission of visual information. As a result, fine visual tasks such as edge detection, color vision, contrast sensitivity, and pattern recognition may be adversely affected20,21,22. Such impairments may compromise daily living activities that require precise visual performance.
The OPL thinning associated with COVID-19 disease, as documented in our study, may have significant long-term implications for individuals with a history of severe SARS-CoV-2 infection. This thinning could potentially impact patients’ quality of life by reducing their ability to perform fine visual tasks. To better understand the functional consequences of this finding, further studies are required to explore its impact on visual performance and quality of life of such patients.
The differences in CSFT observed between the four groups in our study were marginally significant (P = 0.093). Although the difference between the control group and the overall COVID-19 patient cohort was 10–20 μm—potentially clinically relevant—there was no consistent trend within the SARS-CoV-2-infected subgroups (CSFT values in ICU-admitted patients were comparable to those in outpatients, but both were lower than in ward-admitted patients). Therefore, this study does not confirm a definitive impact of SARS-CoV-2 infection on CSFT, but the topic warrants further investigation. In addition, the lack of significant findings in this study regarding other retinal sublayers does not necessarily rule out their potential involvement in COVID-19. While subtle changes may exist beyond the detection capabilities of our study’s power, the adjusted mean values reported did not exhibit any consistent pattern. Therefore, the likelihood of a Type II error in this context is low.
The pRNFL thickness is a valuable biomarker for assessing the optic nerve head. Both thinning (as observed in conditions like glaucoma or chronic optic neuritis/ischemia) and thickening (as seen in acute ischemia or papilledema) can be indicative of early pathological changes in the optic disc. In our study, we included pRNFL analysis to evaluate the impact of COVID-19 disease and its associated respiratory distress on optic nerve head health, as could be detected through pRNFL measurements. This study did not find any clinically or statistically significant differences in pRNFL thickness between the groups (see Table 3). The existing literature on this topic presents contradictory findings. While some previous studies have reported a reduction in pRNFL thickness in specific sectors23,24,25, others have documented increased thickness19,26. These discrepancies may be attributed to statistical limitations, such as small sample sizes and methodological inconsistencies, as well as variations in the severity or duration of COVID illness among the populations evaluated in different studies. Further research is required to further elucidate this issue.
Powers and limitations
The present study involved the prospective enrollment of consecutive patients, which enhanced the reliability of its findings. The overall sample size was adequate to evaluate the primary outcomes; however, the modest sample size within the SARS-CoV-2 positive subgroups introduces the possibility of a type II statistical error. As such, the potential effects of COVID-19 disease on other retinal sublayers cannot be excluded, highlighting the need for future studies with larger sample size.
One important limitation of the study was that the groups were not age-matched, a factor inherent to the methodology of enrolling consecutive patients. Given the higher prevalence and severity of SARS-CoV-2 infection in older individuals, the average age of patients with severe disease was higher than that of those with mild disease or controls. However, age is a known biomarker affecting retinal OCT measurements27. To address this limitation, an ANCOVA approach was employed to control for age across all measurements. The adjusted mean values presented in this article were derived from the ANCOVA analysis. In addition, the observed positive finding of OPL volume changes between groups was reassessed using multiple linear regression. This further validation aimed to strengthen the reliability of the results and reduce the likelihood of a Type I statistical error.
In this study, our control group comprised individuals who either had exposure to COVID-19 patients or experienced upper respiratory tract symptoms that warranted a PCR test for SARS-CoV-2 infection (hence, the term “healthy controls” may be somewhat misleading). This inclusion strategy resulted from our prospective and consecutive sample collection approach from a COVID-19 screening facility. Nonetheless, we applied stringent criteria to ensure that participants with significant diseases affecting the outcome measures were excluded from the control group. Moreover, the likelihood of bias in control group selection is further diminished by the observed meaningful trend of OPL thinning in patients with more severe forms of COVID-19 illness.
Lastly, we did not collect data on mean blood pressure, oxygen saturation, or OCT angiography. As a result, our conclusion that the OPL may be the most vulnerable retinal sublayer to systemic hypoxemia remains speculative and warrants further investigation.
Conclusions
This study demonstrates that patients with severe SARS-CoV-2 infection, suffering from respiratory distress and prolonged hypoxia, may experience ischemia and atrophy of the OPL. These findings also suggest the potential vulnerability of the OPL to systemic hypoxemia, likely due to its anatomical location in the retina-choroid watershed zone and its high oxygen demand. However, this notion is hypothetical and need verification through future studies. The observed OPL atrophy may have clinical implications, including potential impacts on fine visual tasks, such as contrast sensitivity, color vision, and edge detection. These aspects were beyond the scope of the current study, and warrant further investigation to better understand the functional consequences of OPL changes in the context of COVID-19 illness.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
Collaborators, G. D. Global age-sex-specific mortality, life expectancy, and population estimates in 204 countries and territories and 811 subnational locations, 1950–2021, and the impact of the COVID-19 pandemic: a comprehensive demographic analysis for the global burden of Disease Study 2021. Lancet 403, 1989–2056 (2024).
Fang, Z., Ahrnsbrak, R. & Rekito, A. Evidence mounts that about 7% of US adults have had Long COVID. Jama 332, 5–6 (2024).
Wang, K., Li, J., Guo, K. & Zhang, X. New-onset or relapse of uveitis after rapid spreading of COVID-19 infection in China and risk factor analysis for relapse. BMC Ophthalmol. 24, 196 (2024).
Premi, E. et al. Clinical and diagnostic findings of Acute Macular Neuroretinopathy and Paracentral Acute Middle Maculopathy in the COVID-19 era. Ophthalmologica 246, 181–191 (2023).
Ashkenazy, N. et al. Hemi- and Central Retinal Vein Occlusion Associated with COVID-19 infection in young patients without known risk factors. Ophthalmol. Retina. 6, 520–530 (2022).
Teo, K. Y., Invernizzi, A., Staurenghi, G. & Cheung, C. M. G. COVID-19-Related retinal micro-vasculopathy - A review of current evidence. Am. J. Ophthalmol. 235, 98–110 (2022).
Bhavsar, K. V. et al. Acute macular neuroretinopathy: a comprehensive review of the literature. Surv. Ophthalmol. 61, 538–565 (2016).
Moura-Coelho, N., Gaspar, T., Ferreira, J. T., Dutra-Medeiros, M. & Cunha, J. P. Paracentral acute middle maculopathy-review of the literature. Graefes Arch. Clin. Exp. Ophthalmol. 258, 2583–2596 (2020).
Bernal-Morales, C. et al. Paracentral acute middle maculopathy after uneventful ocular surgery with local anaesthetic blocks. Eye (Lond). 36, 219–227 (2022).
Wolf, A. et al. The mechanistic basis linking cytokine storm to thrombosis in COVID-19. Thromb. Update. 8, 100110 (2022).
Yahalomi, T. et al. Acute Central Serous Chorioretinopathy Outbreak during the COVID-19 pandemic: a pilot study. Med. (Kaunas) 60 (2024).
Rahimy, E. & Sarraf, D. Paracentral acute middle maculopathy spectral-domain optical coherence tomography feature of deep capillary ischemia. Curr. Opin. Ophthalmol. 25, 207–212 (2014).
Sarraf, D. et al. Paracentral acute middle maculopathy: a new variant of acute macular neuroretinopathy associated with retinal capillary ischemia. JAMA Ophthalmol. 131, 1275–1287 (2013).
Lavia, C. et al. Vessel density of superficial, intermediate, and deep capillary plexuses using optical coherence tomography angiography. Retina 39, 247–258 (2019).
Yu, D. Y., Cringle, S. J., Yu, P. K. & Su, E. N. Intraretinal oxygen distribution and consumption during retinal artery occlusion and graded hyperoxic ventilation in the rat. Invest. Ophthalmol. Vis. Sci. 48, 2290–2296 (2007).
Yu, D. Y. & Cringle, S. J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 20, 175–208 (2001).
Feucht, N. et al. Multimodal imaging in acute retinal ischemia: spectral domain OCT, OCT-angiography and fundus autofluorescence. Int. J. Ophthalmol. 11, 1521–1527 (2018).
Yu, S. et al. The spectrum of superficial and deep capillary ischemia in retinal artery occlusion. Am. J. Ophthalmol. 159, 53–63e51 (2015).
Bayram, N., Gundogan, M., Ozsaygılı, C. & Adelman, R. A. Posterior ocular structural and vascular alterations in severe COVID-19 patients. Graefes Arch. Clin. Exp. Ophthalmol. 260, 993–1004 (2022).
Haley, T. L. et al. Light-dependent changes in the outer plexiform layer of the mouse retina. Front. Ophthalmol. 3 (2023).
Rajalakshmi, T. & Prince, S. Physiological modeling for detecting degree of perception of a color-deficient person. Proc. Inst. Mech. Eng. H. 231, 276–285 (2017).
Sjöstrand, F. S. Color vision at low light intensity, dark adaptation, Purkinje shift, critical flicker frequency and the deterioration of vision at low illumination. Neurophysiology at the nanometer range of neural structure. J. Submicrosc Cytol. Pathol. 35, 117–127 (2003).
Dağ Şeker, E. & Erbahçeci Timur, İ. E. Assessment of early and long-COVID related retinal neurodegeneration with optical coherence tomography. Int. Ophthalmol. 43, 2073–2081 (2023).
Sumer, F. & Subasi, S. Effects of COVID-19 on Retinal and Choroidal Thickness by Optical Coherence Tomography. J. Glaucoma. 32, 569–574 (2023).
Dag Seker, E. & Erbahceci Timur, I. E. COVID-19: more than a respiratory virus, an optical coherence tomography study. Int. Ophthalmol. 41, 3815–3824 (2021).
Burgos-Blasco, B. et al. Optic nerve and macular optical coherence tomography in recovered COVID-19 patients. Eur. J. Ophthalmol. 32, 628–636 (2022).
Najeeb, S., Ganne, P., Damagatla, M., Chaitanya, G. & Krishnappa, N. C. Mapping the thickness of retinal layers using Spectralis spectral domain optical coherence tomography in Indian eyes. Indian J. Ophthalmol. 70, 2990–2997 (2022).
Funding
The research received no specific grant from any funding agency in the public,
Author information
Authors and Affiliations
Contributions
Conception: MHN, MRT; Design: MHN, MRT; Data acquisition: MRB, HS; Data interpretation: MRB, HS; Data analysis: MHN; Drafting: All authors; Critical reviewing: MHN, MRT; Final approval: All authors; Agreement to be accountable for all aspects of the work: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Talebnejad, M.R., Badie, M.R., Shahriari, H. et al. Analysis of retinal sublayers in patients with systemic COVID-19 illness with varying degrees of severity. Sci Rep 15, 2877 (2025). https://doi.org/10.1038/s41598-025-87446-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87446-1