Abstract
Statistically, 30% of asthma deaths occur among asthmatics with insignificant symptoms, which creates a social burden. We aimed to analyze the role of erythrocyte distribution width (RDW) in the prognosis of asthma, especially in patients with insignificant symptoms. We included 3039 adult (≥ 20 years) asthma patients from the National Health and Nutrition Examination Survey (NHANES). Cox regression was used to assess the association between RDW and long-term mortality. We adjusted three models to reduce potential bias. Subgroup analysis is used to evaluate specific populations. In addition, receiver operating characteristic (ROC) curves were used to analyze the predictive effect of RDW on asthma mortality. After a mean follow-up of 130 months, we found a positive correlation between RDW and long-term mortality. After aliquoting RDW into thirds, the high RDW (RDW ≥ 13.0%) group had higher all-cause mortality (HR 1.66, 95% CI 1.18–2.34) and respiratory mortality (HR 8.69, 95% CI 2.03–37.3). There was a significant interaction of RDW in the male and wheezing subgroups for respiratory mortality. Combining RDW and wheezing, we found that patients with high RDW and wheezing had the most increased respiratory mortality, and patients with high RDW but no wheezing also had higher mortality. Furthermore, the area under the curve of the RDW in predicting respiratory death in asthmatics was greater than 80%. Our study showed an association between high RDW and poor prognosis in asthma patients. In combination with wheezing symptoms, RDW is expected to be a biomarker for asthma management.
Similar content being viewed by others
Introduction
Asthma is a common chronic inflammatory disease of the airways, affecting approximately 300 million people worldwide1. In recent years, despite significant advances in treatment, asthma hospitalization rates remain higher than expected and even end in tragic deaths2,3. GINA 2024 suggests that up to 30% of asthma-related deaths occur in patients who have infrequent asthma symptoms and whose physicians do not believe they need to be controlled. It is often related to inadequate recognition and management of the disease itself. Unlike many other chronic diseases, most management decisions in asthma are made in nonspecialized settings, such as general practitioners and emergency departments2. Our primary objective was to identify a biomarker that aids clinicians in assessing the severity and prognosis of asthma.
Red blood cell distribution width (RDW) is a cheap and readily available parameter used in the differential diagnosis of anemia4. Extensive research has revealed that elevated RDW levels are closely linked to inflammatory responses and act as an independent risk factor for cardiovascular, endocrine, and cancer-related conditions4,5,6,7. Additionally, there has been a notable surge in investigating RDW’s role in respiratory diseases. RDW is an independent risk factor for poor prognosis of pulmonary fibrosis, pulmonary embolism, and chronic obstructive pulmonary disease (COPD)8,9,10,11.
Few studies have been conducted on RDW and asthma prognosis, and asthma prognostic analyses incorporating wheezing symptoms are similarly sparse. We plan to use data from the National Health and Nutrition Examination Survey (NHANES) from 2003 to 2012. The primary focus of the study will be to investigate the association between RDW and long-term mortality in asthma patients, considering all-cause and respiratory mortality. We used a cohort study design to analyze whether RDW can be used as a prognostic marker for asthma.
Methods
Study population
NHANES is a study based on the entire U.S. population. Data collection included home screening, interviews, and physical examination12. To ensure the representation of the overall U.S. population, NHANES staff annually selected a sample of 15 counties, encompassing approximately 5000 individuals, and calculated sampling weights using complex sampling techniques. All information from the NHANES project is available on the official NHANES website13. The Ethics Committee of the National Centre for Health Statistics approved the NHANES survey study. All participants signed an informed consent form and data were de-identified, so no additional ethical waivers were required. This study was carried out in accordance with the Declaration of Helsinki.
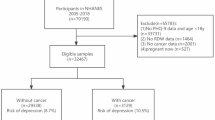
Our study population was patients with asthma ≥ 20 years old. We chose this age group specifically to ensure the availability of follow-up data for the study participants. The presence of asthma was determined by the subject’s affirmative answers to the following questions, “Has a doctor or other health professional ever told you that you had asthma?“. Non-responders with no history of smoking, chronic bronchitis or emphysema but on anti-asthma medications were also categorized as asthmatics14. Since RDW is a relevant indicator of anemia, we excluded anemic populations from our analysis. Anemia was hemoglobin < 130 g/L in men, < 120 g/L in non-pregnant women, and < 110 g/L in pregnant women. Figure 1 illustrates the detailed inclusion and exclusion criteria.
RDW measurement
RDW levels (%) are measured by the Coulter Analyzer in the Mobile Inspection Center and have a standard range of 11.5–14.5%15,16. To elucidate the relationship between RDW and mortality in patients with asthma, we did mean tertile groups based on the values of RDW (T1: 10.8–12.3%, T2: 12.4–12.9%, T3: ≥ 13.0%).
Clinical mortality
The study’s primary outcome was all-cause and respiratory mortality in patients with asthma. We included asthma-related deaths, defining respiratory mortality as deaths due to chronic lower respiratory disease (J40–J47)17. We determined 3-year, 5-year, and 7-year mortality rates from all causes based on the duration and outcome of follow-up.
Study covariates
We included demographic data to reduce potential bias, including age, sex, race, smoking status, body mass index (BMI), eosinophils, hemoglobin, and Inhaled corticosteroids (ICS). The ICS mainly includes beclomethasone, fluticasone, and budesonide. The SABA mainly includes albuterol. Drug information was extracted from the NHANES formulary (RXQ_RX). The race is divided into Non-Hispanic White, Mexican American, Non-Hispanic Black, and other races. Smoking status was categorized as never (smoking < 100 cigarettes), former (smokes > 100 cigarettes but has quit), and current (still smoking).
Our study considered the associations between RDW and several medical conditions, including cardiovascular disease (CVD), COPD, hypertension, and diabetes4,18. CVD is any of the diseases reported by the patient (heart attack, coronary artery disease, congestive heart failure, stroke). COPD was defined as any of the following: (a) Individuals reporting emphysema, (b) Individuals with FEV1/FVC < 70% after inhalation of bronchodilators, and (c) Individuals reporting chronic bronchitis who were on medications to treat COPD and excluded asthma19. The presence of one of the following conditions was defined as diabetes (fasting blood glucose ≥ 7.0 mmol/L, glycated hemoglobin ≥ 6.5%, glucose tolerance test ≥ 11.1 mmol/L, taking glucose-lowering medication, diagnosed by an internist)20. Hypertension is defined as compliance with either condition (systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg, taking antihypertensive medication, diagnosed by a physician)21.
We used the past year’s management of asthma patients as covariates, including wheezing, asthma emergency room visits, and overnight hospitalizations. The presence of wheezing was defined by the subject’s affirmative response to the following question, “In the past 12 months, have you wheezed or whistled in your chest?“. An asthma emergency is when a patient must go to an emergency room or urgent care center within the past 12 months because of asthma. Overnight hospitalizations are hospital stays in the past 12 months, excluding emergency room visits.
Statistical analysis
Continuous data that conformed to normal distribution were expressed as mean (standard deviation, SD), and count data were expressed as N (%). Comparisons between variables were made using one-way ANOVA and chi-square tests. The number of missing values for all covariates was less than 0.3%. Due to the small number of missing variables, we treated these missing values as blank variables when analyzing the data.
We applied Cox regression to analyze the association of RDW with all-cause and respiratory mortality. We adjusted the three models to reduce potential factors’ bias. Model 1 was adjusted for sex, age and race. Model 2 adjusted for age, race, smoking history, education, COPD, CVD, hypertension, diabetes, ICS use, SABA use, wheezing, and overnight hospitalizations (variables with P < 0.05 in univariate COX regression analysis). Model 3 was adjusted for sex, age, race, smoking history, BMI, education, COPD, hypertension, cardiovascular disease, diabetes, eosinophils, ICS use, SABA use, hemoglobin, wheezing, asthma emergency, and overnight hospitalizations.
To further explore the predictive effect of RDW on various causes of mortality, we applied time-dependent subject work characteristics (ROC) to assess the predictive impact of RDW on 3-year, 5-year, and 7-year mortality22. We plan to use subgroup analyses to explore the relationship between RDW and mortality in each group, which will be presented as a forest plot. Kaplan-Meier survival curves were plotted to examine the relationship between RDW and mortality. We also use fitted curves to analyze the relationship between RDW and mortality.
Sensitivity analysis
All analyses were conducted using complex sampling weights (wtmec4 year). In addition, we performed four sensitivity analyses to ensure the stability of the results. First, the inclusion of the anemic population in the study was analyzed to avoid potential bias due to the its exclusion. Second, we excluded those who died at 2 years of follow-up to exclude potential reverse causality. Third, respiratory mortality was analyzed using competing risk models to determine their consistency with Kaplan–Meier survival curve results.
The study analysis used R v4.2.2 (http://www.R-project.org, The R Foundation).
Results
Baseline characteristics
A total of 3039 participants were enrolled in our study. These 3039 participants can be weighted to reflect 26,381,207 individuals across the United States (Table S1). The mean follow-up was 130 months (range 4–205 months). The average age of subjects was 46.3 years; 57.3% were female, and 53.7% were white (Table 1). The high RDW group (Tertile 3: RDW ≥ 13.0%) had higher age, BMI, smoking history, and comorbidities (COPD, CVD, hypertension, diabetes). In terms of asthma-related aspects, the high RDW group had higher ICS and SABA rates, were more likely to see a physician for asthma, and had higher all-cause and respiratory mortality rates. Univariate Cox analysis was performed to analyze the relationship between covariates and mortality. The results (Table S2) found that age, race, smoking history, education, COPD, CVD, hypertension, diabetes, ICS use, SABA use, wheezing, and overnight hospitalizations were associated with all-cause and respiratory mortality in patients with asthma.
Association between RDW and outcomes
As shown in Table 2, RDW was significantly associated with mortality in all four models. After adjustment for Model 3, each unit increase in RDW was associated with a 15% increase in the risk of all-cause mortality and a 17% increase in the risk of respiratory mortality. In addition, there was a significantly higher all-cause mortality (HR 1.66, 95% CI 1.18–2.34, P = 0.004) and respiratory mortality (HR 8.69, 95% CI 2.03–37.3, P = 0.004) in the high-RDW (Tertile 3: RDW ≥ 13%) group compared with the low-RDW (Tertile 1: RDW ≤ 12.3%). This result remained stable across the sensitivity analysis (Table S3–S4). Figure S1 illustrates the fitted curves for RDW versus all-cause and respiratory mortality. Kaplan–Meier survival curves (Fig. S2) and competing risk models (Fig. S3) also suggest that higher RDW is associated with poorer long-term prognosis (all-cause and respiratory mortality) in asthma patients.
Figure 2 shows the results of the time-dependent ROC curves. As shown, RDW was excellent in predicting respiratory-related deaths in asthma patients, with an area under the curve (AUC) of 0.802 (3 years), 0.834 (5 years), and 0.767 (7 years), respectively. The area under the curve (AUC) of RDW for all-cause mortality in patients with asthma was 0.700 (3 years),0.691 (5 years), and 0.674 (7 years), respectively.
We drew forest plots (Fig. 3) after stratifying the analysis by age, race, smoking status, body mass index, CVD, COPD, wheezing, asthma emergencies, and overnight hospitalizations. The relationship between RDW and all-cause mortality remained stable across subgroups (P for interaction > 0.05). For respiratory mortality, there was a significant interaction of RDW in the gender and wheezing subgroups (P for interaction < 0.05). The association between RDW and respiratory mortality was more pronounced in men and among asthmatics without wheezing symptoms.
Given the interaction between wheezing and RDW, we further divided the study population into four groups (No wheezing, RDW < 13.0%; wheezing, RDW < 13.0%; No wheezing, RDW ≥ 13.0%; wheezing, RDW ≥ 13.0%) to perform multivariate COX regression. Tertile 1 and Tertile 2 in the RDW were combined into one group because the number of respiratory deaths in both groups was small after grouping by wheezing. Table 3 shows that patients with symptoms of wheezing and elevated RDW had an elevated risk of all-cause mortality (wheezing, RDW ≥ 13.0%: HR 1.81, 95% CI 1.22–2.67, P = 0.003) compared with the group without wheezing and with a low to moderate RDW; and in terms of respiratory mortality, the other three groups had an elevated risk of respiratory mortality when compared with the former (wheezing, RDW < 13.0%: HR 3.95, 95% CI 1.11-14.0, P = 0.033; No wheezing, RDW ≥ 13.0%: HR 5.66, 95% CI 1.42–22.5, P = 0.014; wheezing, RDW ≥ 13.0%: HR 9.79, 95% CI 2.55–37.6, P < 0.001).
Discussion
Few previous studies have examined the relationship between RDW and the prognosis of asthma patients. Our findings shed light on the crucial role of RDW as a significant prognostic indicator in this population. Asthma patients with high RDW had higher long-term mortality (all-cause and respiratory) than controls. Combining RDW and wheezing, we found that patients with high RDW and wheezing had the most increased respiratory mortality, and patients with high RDW but no wheezing also had higher mortality. This association remained stable in the three models adjusted for different covariates. In addition, the results of the sensitivity analyses were similar to the main results.
Asthma is a chronic airway disease that requires long-term treatment, and even asthmatics with mild symptoms can have life-threatening attacks23,24. As articulated in GINA 2024, the definition of severe asthma is widely accepted and used. However, the utility and relevance of mild asthma’s definition is unclear. Patients and clinicians often interpret “mild asthma” as not being at risk and not requiring controlled treatment. Nevertheless, it is disheartening to note that up to 30% of asthma-related deaths occur in patients with infrequent asthma symptoms. Our study found that asthmatics with wheezing symptoms in the past year had a higher risk of respiratory death, regardless of RDW. Poorer asthma management increases the risk of death in asthmatics, which is in line with the perception of the disease. On the other hand, we found that patients without wheezing symptoms but with elevated RDW (RDW ≥ 13.0%) also exhibited a higher risk of respiratory death. Our study confirms that RDW holds promise as a biomarker for managing prognostic risk in asthma patients.
As RDW was significantly associated with mortality in asthmatic patients, we further performed a time-dependent ROC analysis of RDW and mortality from all causes. The area under the curve (AUC) of RDW for predicting respiratory-related mortality in asthmatic patients was 0.802 (3 years), 0.834 (5 years), and 0.767 (7 years), respectively. The 80% area under the curve and the long-term predictive effect make RDW a promising biomarker for assessing the prognosis of asthma patients. Subgroup analyses also showed that male asthmatics with high RDW were at higher risk of respiratory mortality (P for interaction < 0.05). Another study on heart failure confirmed the higher correlation of RDW in men with poor prognoses25. We should pay more attention to the high RDW in men with asthma.
Several studies have suggested that elevated RDW may indicate an increased inflammatory response and oxidative stress5,26,27. Inflammatory cytokines have been observed to hinder the maturation of erythrocytes induced by erythropoietin, leading to an increase in RDW28. Additionally, oxidative stress and lower oxygen partial pressure can contribute to RDW elevation by causing damage to red blood cells and shortening the life span29,30. The diagnosis of intermittent hypoxaemia is often overlooked in clinical work. However, this condition can trigger various compensatory reactions and contributes to adverse events. This finding helps to explore the pathophysiological mechanisms that may influence asthma outcomes and to make targeted interventions.
RDW has also been used as a proxy indicator for iron deficiency8,31. Perturbations in systemic and pulmonary iron levels have been linked to pulmonary inflammation in various diseases, including asthma32. Ali found that the free iron level in bronchoalveolar lavage (BAL) supernatant was reduced in asthmatic patients and associated with lower forced expiratory volume in 1 s (FEV1)33. Another study also confirmed that high serum ferritin levels were associated with a reduced chance of asthma34. Decreased circulating and pulmonary iron levels impair erythropoiesis, leading to elevated RDW35. In addition to the above mechanisms, recent studies have identified multiple proteins of the senescence-associated secretory phenotype involved in the association between RDW and mortality36. Cellular senescence may be an important reason why high RDW is associated with high mortality in asthma patients.
This study has a relatively large asthma population and applies complex sampling weights to reduce sampling error. Undeniably, there are some limitations to our research. First, because the diagnosis of asthma, COPD, and CVD is obtained through self-report, it may lead to potential bias. Due to the limitations of retrospective studies, some COPD patients may be misdiagnosed as having asthma. Secondly because it was a retrospective study, asthma severity classifications and more detailed causes of death due to asthma (J-45) were difficult to obtain. Third, the data from the survey of asthmatics came from a decade ago, and guidelines and medication application changes may have caused some bias. Fourth, because the included population was asthmatics aged 20 years and older, our results cannot be generalized to asthmatics under 20 years of age. Finally, this study was a single-database study, and we hope to consolidate our results in the future by applying multicenter data especially including a similar asthma-free control group.
Conclusions
Our study shows an association between elevated RDW and poor prognosis in asthma patients. Asthmatics without wheezing symptoms but with elevated RDW have a higher risk of respiratory death. Future studies need to focus on the predictive role of dynamic changes in RDW in asthma control. In addition, because asthma behaves differently in children than adults, revealing the relationship between RDW and childhood asthma may provide valuable insights into the disease early development of the disease.
Data availability
The research data is available directly from the official NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm).
Abbreviations
- RDW:
-
Red blood cell distribution width
- COPD:
-
Chronic obstructive pulmonary disease
- NHANES:
-
National Health and Nutrition Examination Survey
- BMI:
-
Body mass index
- ICS:
-
Inhaled corticosteroids
- SABA:
-
Short-acting Beta2 agonist
- ROC:
-
Receiver operating characteristic
- BAL:
-
Bronchoalveolar lavage
- FEV1 :
-
Forced expiratory volume in 1 s
- CVD:
-
Cardiovascular disease
References
Cloutier, M. M. et al. Managing asthma in adolescents and adults: 2020 Asthma Guideline Update from the National Asthma Education and Prevention Program. JAMA 324 (22), 2301–2317. https://doi.org/10.1001/jama.2020.21974 (2020).
Shaw, D. E. et al. Balancing the needs of the many and the few: where next for adult asthma guidelines? Lancet Respir. Med. 9 (7), 786–794. https://doi.org/10.1016/S2213-2600(21)00021-7 (2021).
O’Byrne, P. et al. Asthma progression and mortality: the role of inhaled corticosteroids. Eur. Respir. J. 54 (1), 1900491. https://doi.org/10.1183/13993003.00491-2019 (2019).
Salvagno, G. L., Sanchis-Gomar, F., Picanza, A. & Lippi, G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 52 (2), 86–105. https://doi.org/10.3109/10408363.2014.992064 (2015).
Förhécz, Z. et al. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am. Heart J. 158 (4), 659–666. https://doi.org/10.1016/j.ahj.2009.07.024 (2009).
Lippi, G. & Plebani, M. Red blood cell distribution width (RDW) and human pathology. One size fits all. Clin. Chem. Lab. Med. 52 (9), 1247–1249. https://doi.org/10.1515/cclm-2014-0585 (2014).
Je, M., Mb, M. & Vvb, R. Three neglected numbers in the CBC: the RDW, MPV, and NRBC count. Cleve. Clin. J. Med. 86 (3). https://doi.org/10.3949/ccjm.86a.18072 (2019).
Ulrich, A. et al. Mendelian randomisation analysis of red cell distribution width in pulmonary arterial hypertension. Eur. Respir. J. 55 (2), 1901486. https://doi.org/10.1183/13993003.01486-2019 (2020).
Zhou, X. Y., Chen, H. L. & Ni, S. S. Red cell distribution width in predicting 30-day mortality in patients with pulmonary embolism. J. Crit. Care. 37, 197–201. https://doi.org/10.1016/j.jcrc.2016.09.024 (2017).
Karampitsakos, T. et al. Increased monocyte count and red cell distribution width as prognostic biomarkers in patients with idiopathic pulmonary fibrosis. Respir. Res. 22 (1), 140. https://doi.org/10.1186/s12931-021-01725-9 (2021).
Kalemci, S. et al. The relationship between hematological parameters and the severity level of chronic obstructive lung disease. Pol. Arch. Intern. Med. 128 (3), 171–177. https://doi.org/10.20452/pamw.4198 (2018).
Curtin, L. R. et al. National Health and Nutrition Examination Survey: sample design, 2007–2010. Vital Health Stat. 2. 160, 1–23 (2013).
Cavicchia, P. P. et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J. Nutr. 139 (12), 2365–2372. https://doi.org/10.3945/jn.109.114025 (2009).
Cheng, W. et al. Higher systemic immune-inflammation index and systemic inflammation response index levels are associated with stroke prevalence in the asthmatic population: a cross-sectional analysis of the NHANES 1999–2018. Front. Immunol. 14, 1191130. https://doi.org/10.3389/fimmu.2023.1191130 (2023).
Zhou, G. et al. Association between Red Blood cell distribution width and thyroid function. Front. Endocrinol. (Lausanne). 12, 807482. https://doi.org/10.3389/fendo.2021.807482 (2021).
Gunter, E. W. & McQuillan, G. Quality control in planning and operating the laboratory component for the Third National Health and Nutrition Examination Survey. J. Nutr. 120 (Suppl 11), 1451–1454. https://doi.org/10.1093/jn/120.suppl_11.1451 (1990).
NCHS Data Linkage - Mortality Data. https://www.cdc.gov/nchs/data-linkage/mortality.htm (accessed 19 June 19).
Patel, K. V. et al. Red cell distribution width and mortality in older adults: a meta-analysis. J. Gerontol. Biol. Sci. Med. Sci. 65 (3), 258–265. https://doi.org/10.1093/gerona/glp163 (2010).
Zhu, M. & Chen, A. Epidemiological characteristics of asthma-COPD overlap, its association with all-cause mortality, and the mediating role of depressive symptoms: evidence from NHANES 2005–2018. BMC Public. Health. 24 (1), 1423. https://doi.org/10.1186/s12889-024-18911-1 (2024).
Palmer, M. K. & Toth, P. P. Trends in lipids, obesity, metabolic syndrome, and diabetes Mellitus in the United States: an NHANES Analysis (2003–2004 to 2013–2014). Obes. (Silver Spring). 27 (2), 309–314. https://doi.org/10.1002/oby.22370 (2019).
Zhang, Y. & Moran, A. E. Trends in the prevalence, awareness, treatment, and control of hypertension among young adults in the United States, 1999 to 2014. Hypertension 70 (4), 736–742. https://doi.org/10.1161/HYPERTENSIONAHA.117.09801 (2017).
Kamarudin, A. N., Cox, T. & Kolamunnage-Dona, R. Time-dependent ROC curve analysis in medical research: current methods and applications. BMC Med. Res. Methodol. 17 (1), 53. https://doi.org/10.1186/s12874-017-0332-6 (2017).
Pauwels, R. A. et al. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet 361 (9363), 1071–1076. https://doi.org/10.1016/S0140-6736(03)12891-7 (2003).
Reddel, H. K. et al. Should recommendations about starting inhaled corticosteroid treatment for mild asthma be based on symptom frequency: a post-hoc efficacy analysis of the START study. Lancet 389 (10065), 157–166. https://doi.org/10.1016/S0140-6736(16)31399-X (2017).
Zhang, X. et al. Red cell distribution width is associated with short-term mortality in critically ill patients with heart failure. ESC Heart Fail.. 29 https://doi.org/10.1002/ehf2.14023 (2022).
Yčas, J. W. Toward a blood-borne biomarker of chronic hypoxemia: red cell distribution width and respiratory disease. Adv. Clin. Chem. 82, 105–197. https://doi.org/10.1016/bs.acc.2017.06.002 (2017).
Lippi, G. et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch. Pathol. Lab. Med. 133 (4), 628–632. https://doi.org/10.5858/133.4.628 (2009).
Pierce, C. N. & Larson, D. F. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion 20 (2), 83–90. https://doi.org/10.1191/0267659105pf793oa (2005).
Ghaffari, S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid. Redox Signal. 10 (11), 1923–1940. https://doi.org/10.1089/ars.2008.2142 (2008).
Yčas, J. W., Horrow, J. C. & Horne, B. D. Persistent increase in red cell size distribution width after acute diseases: a biomarker of hypoxemia? Clin. Chim. Acta. 448, 107–117. https://doi.org/10.1016/j.cca.2015.05.021 (2015).
Emans, M. E. et al. Determinants of red cell distribution width (RDW) in cardiorenal patients: RDW is not related to erythropoietin resistance. J. Card. Fail. 17 (8), 626–633. https://doi.org/10.1016/j.cardfail.2011.04.009 (2011).
Ali, M. K. et al. Role of iron in the pathogenesis of respiratory disease. Int. J. Biochem. Cell. Biol. 88, 181–195. https://doi.org/10.1016/j.biocel.2017.05.003 (2017).
Ali, M. K. et al. Crucial role for lung iron level and regulation in the pathogenesis and severity of asthma. Eur. Respir. J. 55 (4), 1901340. https://doi.org/10.1183/13993003.01340-2019 (2020).
Brigham, E. P., McCormack, M. C., Takemoto, C. M. & Matsui, E. C. Iron status is associated with asthma and lung function in US women. PLoS One. 10 (2), e0117545. https://doi.org/10.1371/journal.pone.0117545 (2015).
Cherayil, B. J. Pathophysiology of iron homeostasis during inflammatory states. J. Pediatr. 167 (4 Suppl), S15–19. https://doi.org/10.1016/j.jpeds.2015.07.015 (2015).
Osawa, Y. et al. Proteins in the pathway from high red blood cell width distribution to all-cause mortality. EBioMedicine 76, 103816. https://doi.org/10.1016/j.ebiom.2022.103816 (2022).
Funding
This research was supported by the National Natural Science Foundation of China (82074422), Science and technology Innovation Project of China Academy of Chinese Medical Sciences (CI2021A01101), and National Public Hospital Reform and High-quality Development Demonstration Project (Haidian District, Beijing) (11010822T000001740154).
Author information
Authors and Affiliations
Contributions
Z.R. and D.L. participated in research design, result analysis and manuscript writing. Y.W., Y.F. and B.X. participated in study design and article revision. X.C. and S.Y. participated in data collection and statistical analysis. Q.M. participated in study design, manuscript editing, and funding acquisition. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of the National Centre for Health Statistics approved the NHANES survey study. All participants signed an informed consent form and data were de-identified, so no additional ethical waivers were required.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ruan, Z., Wang, Y., Fan, Y. et al. The relationship between red blood cell distribution width and long-term prognosis of asthma: a population-based study. Sci Rep 15, 6487 (2025). https://doi.org/10.1038/s41598-025-87469-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87469-8