Abstract
This study aims to explore the mechanism behind the influence of stress on gas adsorption by coal during deep mining and improve the accuracy of gas disaster prevention and control. To achieve this aim, thermodynamic analysis was conducted on the process of gas adsorption by loaded coal, and modified thermodynamic model proposed by previous scholars. It is found that stress plays an important role in gas adsorption by coal. In addition, isothermal adsorption experiments were performed on coal samples under different stress states with the aid of a coal rock triaxial adsorption and desorption experimental device. Based on the experimental results, the variations in adsorption capacities of coal samples under different stress loads were obtained. Finally, molecular dynamics simulation of gas adsorption was conducted by taking lignite as the research object. The microscopic mechanism of gas adsorption by loaded coal is elucidated from the perspectives of isothermal adsorption parameters of the loaded coal, isothermal heat of adsorption, and the changing law of the probability density distribution of adsorbed gas molecules. The research findings are anticipated to offer theoretical and practical guidance for the prevention and control of gas disasters in deep coal mines and the extraction of coalbed methane.
Similar content being viewed by others
Introduction
Recent years have seen an increasing coal mining intensity, a more concentrated production layout and a rising mining depth, which results from the surging demand for energy and the improved mechanization and intelligence in coal mining. During coal seam mining, the stress state of the coal seam changes constantly under the joint action of geological and tectonic stresses. Research on how stress loading influences the adsorption and desorption characteristics of coal is crucial for providing basic theoretical guidance for the prediction of coalbed methane resources under deep mining conditions and the prevention and control of coal mine gas disasters.
Scholars have explored the influence of stress loading on the adsorption and desorption characteristics of coal. Skoczylas et al.1 investigated the influences of confining pressure on coal adsorption characteristics and on CO2/CH4 exchange adsorption characteristics. The results demonstrate that a rising confining pressure weakens the CH4 adsorption capacity of coal. With granular coal as the research object, Hol et al.2 found that under uniaxial stress conditions, an effective stress of 35 MPa promotes the desorption behaviors of gas adsorbed by coal, with the desorbed gas accounting for 5–50% of the adsorbed gas. Besides, they also discovered that the effective stress plays a role in weakening the adsorption capacity of coal. Subsequently, Hol et al.3 developed a thermodynamic model based on the statistical mechanics theory to describe the adsorption equilibrium concentration of CO2 by loaded coal matrix materials. The model shows that the adsorption equilibrium concentration of coal varies with the chemical activity of the fluid and the availability of adsorption sites in the nanoporous solid matrix. Meanwhile, they simplified the model to a common Langmuir model, but the physical meaning of constants characterizing adsorption equilibrium in the model remained unclear. On the basis of the results of Hol et al.3, Liu et al.4 generalized the thermodynamic model, and adopted the adsorption model of coal under stress loading for predicting the in-situ methane content in deep coal seams, Tian et al.5 used quantitative analysis to study the relationship between gas adsorption and average effective stress in coal-bearing shale.
As the molecular simulation technology and the molecular dynamics calculation technology get upgraded, scholars are paying attention to the micro-structure of coal and the role of the molecular simulation technology in research on gas adsorption by coal. Through first-principle calculation based on the density functional theory, Li et al.6 investigated the microscopic mechanism of methane adsorption by coal at the atomic level using the Wiser model. On the basis of the established macro-molecular coal model, Ren et al.7 investigated the evolution characteristics and mechanisms of the pore structure of coals with different degrees of metamorphic deformation by comparing the pore structures of coal samples with different degrees of metamorphic deformation and low-order tectonic coals, Zhang et al.8 investigated the effects of biaxial compressive strain on the adsorption structure of bituminous coal matrix and on the diffusion and permeation characteristics of CH4/CO2/N2 by adopting the grand canonical Monte Carlo and molecular dynamics methods, based on the classical Fick diffusion model for homogeneous spherical coal particles, Ren et al.9 introduced the integrated fractal dimension of the full aperture and the time-dependent diffusion attenuation coefficient to form the fractal-time-dependent Fick diffusion model, Wang et al.10 explored the effect of compressive stress on the pore structure and adsorption performance of coal by the classical Monte Carlo and molecular dynamics methods, and discussed the coupling relationships among stress, strain, pore structure, and adsorption performance. Yan et al.11,12 used the molecular dynamics and Monte Carlo methods to analyze the adsorption properties of CH4 molecules in a model of coal macromolecular structure.
Scholars have studied the impact of stress loading on coal adsorption characteristics. However, with most mines now entering deep mining, a variety of stress-dominated composite disaster types have emerged, along with traditional gas hazards. As a result, the effect of stress loading on gas adsorption, desorption, and seepage characteristics in coal seams has become a highlighted concern. The relationship between microstructural changes of coal and gas adsorption and macroscopic phenomena under stress loading is unclear. Therefore, further molecular dynamics studies of gas adsorption in loaded coal should be conducted to elucidate the mechanism of stress loading on gas adsorption in coal.
In this study, the process of gas adsorption by loaded coal was theoretically analyzed first. Furthermore, gas adsorption experiments were performed on loaded coal samples with the aid of a coal rock triaxial adsorption desorption experimental device, and molecular dynamics simulation was conducted on methane adsorption by coal under different stresses using the Monte Carlo method. Based on the experimental and simulation results, the influence of stress loading on methane adsorption by coal was revealed, and the micro-mechanism of gas adsorption by loaded coal was demonstrated. The research findings are anticipated to provide theoretical and practical guidance for the prevention and control of gas disasters in deep coal mines and the extraction of coalbed methane.
Theory and methods
Theoretical analysis
To facilitate research on the gas adsorption characteristics of coal under different stress states, the fluid state in the coal pore medium is defined as the supercritical state of a single gas. The stress on coal is effective stress, which is the difference between the external load stress and the pore gas pressure, expressed as:
where \(\overline{\sigma }\) is the effective stress, MPa; \(\sigma_{ij}\) is the external load stress, MPa; P is the pore gas pressure, MPa; \(\delta_{ij}\) is the Kronecker symbol.
In this section, the surface coverage, adsorption equilibrium concentration and microscopic influencing factors of adsorbed gases on loaded coal matrices are analyzed by using the Langmuir kinetic equation for gas adsorption on coal and the distribution characteristics of the adsorption potential energy on the coal surface. This analysis elucidated the adsorption characteristics of the loaded coal matrix under hydrostatic pressure conditions.
Distribution characteristics of adsorption potential energy of adsorption sites on the coal surface
Each site inside the coal matrix has adsorption capacity and is composed of adsorption potential wells that can capture single gas molecules. According to previous researches2,3,4, the distribution characteristics of adsorption potential wells on the coal matrix surface when a single free-state gas molecule is adsorbed in an adsorption vacancy of the coal matrix under stress loading \(\sigma_{ij}\) are shown in Fig. 1.
According to Fig. 1, during adsorption, when a single gas molecule on the surface of the loaded coal matrix changes from a free state to an adsorbed state, the change in its potential energy is expressed as:
Microscopic influences on the substrate of loaded coal
Liu et al.4 modified the proposed thermodynamic model presented by Hol et al.3 to present expressions for the surface coverage of adsorbed gases on loaded coal substrates and their adsorption equilibrium concentrations. The applicability of these expressions to CH4 adsorption was verified.
As the fluid state in the coal pore medium is the supercritical state of a single gas, previous studies have shown that coal adsorption of supercritical gases can be used to adsorb the density of the adsorbed phase instead of saturated vapor pressure, the density of the gas phase instead of the gas pressure, which is a good solution to the problem of the saturated vapor pressure of the supercritical fluid has no physical significance13, therefore, by replacing ag with \(\rho_{a} + \rho_{g}\), Eqs. (3) and (4) can be corrected as:
where \(\Omega_{0}\) is the volume expansion deformation of a single gas molecule due to adsorption behaviours. The volume strain of the coal matrix caused by the adsorption of a single gas molecule can be decomposed into the positive strain component and the deviatoric strain component; \(D_{ij}\) is the ratio of the deviatoric strain component to the positive strain component; \(\sigma_{ij}^{\prime }\) is the deviatoric stress component; eg0 is the potential energy of a single free-state gas molecule in matrix pores and fractures at temperature T and pore pressure P0; ρa is the density of adsorbed-phase gas, and ρg is the density of gas under the adsorption equilibrium pressure, g/cm3; V0 is the partial molar volume of adsorbed gas; NA is the Avogadro constant, 6.02 × 1023/mol; R is a universal gas constant, generally R = 8.31 J/(K·mol); C is the adsorption equilibrium concentration of gas; Cs is the saturation adsorption concentration of gas molecules per unit mass of coal matrix, mol/kg; T is the ambient temperature; and K is a thermodynamic equilibrium constant. According to the traditional definition14, the thermodynamic equilibrium constant is only subject to temperature. Hence, the thermodynamic equilibrium constant of coal-methane adsorption is defined as:
From the equations of the surface coverage rate and adsorption equilibrium concentration of adsorbed gas in the loaded coal matrix, it can be seen that gas adsorption by the loaded coal matrix is mainly influenced by the following 5 microscopic factors:
-
(1)
The density of adsorbed gas and the density of gas under the adsorption equilibrium pressure;
-
(2)
The reference potential energy \(e_{s}^{{P_{0} }}\) (or \(e_{g0} - e_{s}^{{P_{0} }}\)) and the thermodynamic equilibrium constant K0;
-
(3)
The saturated adsorption concentration of adsorbable sites in the unit mass coal matrix Cs;
-
(4)
The partial molar volume of adsorbed gas V0;
-
(5)
The stress load on the coal matrix σij.
When adsorbent coal, type of adsorbate gas, adsorption pore pressure, and ambient temperature are fixed, the adsorption equilibrium concentration of loaded coal is only correlated with the stress load σij of the coal matrix, which plays an important role in the capacity of coal to adsorb gas. When external stress σij > Pδij or σ − P, the adsorption equilibrium concentration C is obviously lower than that in a stress-free state. This is mainly attributed to the joint effects of adsorption expansion effect, effective stress, and deviatoric stress \(\sigma_{ij}^{\prime }\) caused by the stress–strain of the coal matrix. Therefore, compared with stress-free coal, the loaded coal matrix under effective stress σ − P corresponds to a weakened adsorption capacity, and the weakening of adsorption capacity is positively correlated with the effective stress on the coal matrix.
Adsorption characteristics under hydrostatic pressure
With the gradual increase of the depth of coal seam mining, the coal seam mining will enter the hydrostatic pressure state, which leads to the diversity and severity of disasters15,16. For the special hydrostatic pressure state, when the coal matrix is loaded by triaxial stress, σ > P, at which time \(\overline{\sigma } = \sigma\) and the bias stress component disappears. (\(\sigma_{ij}^{\prime } = 0\)). At this time, the adsorption concentration of hydrostatic pressure loaded coal matrix can be expressed as:
It can be seen in Eq. (8) that under hydrostatic pressure conditions, the swelling behavior of the coal matrix has no effect on the adsorption concentration of the coal matrix, regardless of whether the swelling behavior of the coal matrix is isotropic or anisotropic, and it is only related to the hydrostatic pressure σ.
Methods
Experimental method
Experimental device
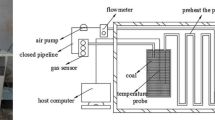
This study adopts the coal rock triaxial adsorption and desorption experimental device from the Key Laboratory of Gas Geology and Gas Management in Henan Polytechnic University for gas adsorption experiments on loaded coal (Fig. 2).
Coal sample preparation
Experimental coal samples were from the 1303 working face of the 3# coal seam of Dananhu No.1 Mine in Hami mining area, Xinjiang, China. This coal seam has a simple structure and a vitrinite reflectance of 0.32%. The coal samples are classified as low-rank lignite whose microscopic coal type belongs to dark-dark bright coal.
The collected coal samples were processed into standard samples (Φ 50 mm × 100 mm) by means of liquid nitrogen frozen core drilling, and dried in a drying oven at 80 °C for 48 h to remove moisture. After cooling to room temperature, they were sealed and stored in a dryer for later use.
Experimental method
According to the adsorption capacity determination method specified in the Chinese national standard “High Pressure Isothermal Adsorption Test Method for Coal-Capacity Method”, axial stresses of 0 MPa, 5 MPa, 10 MPa, 15 MPa, 20 MPa, 25 MPa, and 30 MPa were applied to the experimental samples, respectively, under a confining pressure of 3 MPa. The gas adsorption capacities of coal samples under different stress loads and an adsorption equilibrium pressure of 2.0 MPa were measured.
Molecular dynamics simulation method
Structure model and optimization of coal molecules
The typical Wiser model was selected as the object for simulating the gas adsorption characteristics of lignite17,18,19, and a 3D lignite structure composed of 5 molecules was established. Considering the properties of coal molecules and massive simulation results10,20, the COMPASS force field whose parameters are from quantum mechanical empirical parameters calculated from scratch was selected21. To optimize the coal structure, annealing kinetics optimization was performed on the 5-molecule lignite macro-molecular structure model at 300–600 K by the COMPASS force field. The finally obtained stable structure is depicted in Fig. 3.
In the process of annealing kinetics optimization, the density fluctuates around 1.25 g/cm3, extremely close to the measured density of coal samples. Additionally, the system energy varies with density and reaches its minimum (a mark of the most stable structure) at a density of 1.25 g/cm3 (Fig. 4).
Molecular dynamics optimization
With Barostat in the built-in Forcite Dynamics module of MS software, hydrostatic pressure was added to the established 5-molecule lignite macromolecular structure model by the Souza Martins function. The hydrostatic pressure was set to 4 levels, i.e., stress-free state (0 MPa), 10 MPa, 20 MPa, and 30 MPa, and the simulation temperature was set to 303.15 K. Under the above settings, 200 Ps molecular dynamics optimization was applied to the coal molecular model first. The finally obtained stable structure is depicted in Fig. 5.
Simulation method and parameter setting
The kinetics simulation on gas adsorption by coal was conducted using the Monte Carlo method with the μVT ensemble, whose molecular chemical potential in the system is constant and changes in particle concentration are allowed22,23,24,25. The adsorption capacity of coal to gas molecules was calculated by the Sorption module in Materials Studio software.
It should be clarified when performing the calculation of adsorption amount that the adsorption amount measured in the physical experiment is the excess adsorption amount of the coal sample (\(m_{excess}^{gas}\)), while the adsorption amount calculated during the molecular simulation is the absolute adsorption amount (\(m_{absolute}^{gas}\)), and the relationship between the two is as follows:
where \(\rho^{gas} \left( {P,T} \right)\) is the density of the gas at a pore pressure of P and a temperature of T, which can be found using the Peng–Robinson equation of state26; Vp is the free volume of the molecular analog crystal.
In addition, the absolute adsorption capacity obtained by molecular simulation is the sum of the product of gas density and crystal volume under a certain temperature and pressure and the excess adsorption capacity measured in the physical experiment. When the temperature and pore pressure of molecular simulation are determined, \(\rho^{gas} \left( {P,T} \right) \cdot V_{p}\) is a fixed value. The absolute adsorption capacity calculated by molecular simulation is only different from the excess adsorption capacity measured by physical experiment in numerical value, and its variation law is consistent under different temperature and pressure conditions. Therefore, it is completely feasible to analyze the adsorption behavior of coal gas molecules by using the absolute adsorption capacity calculated by molecular simulation.
Methane molecule adsorption by coal is simulated using the above optimized structure. Three-dimensional periodic boundary conditions are adopted for the simulation, and whether a methane molecule accepts or rejects adsorption is judged by the sampling calculation method in accordance with energy changes and the Metropolis operation rule. In the COMPASS force field, 1 × 107 Monte Carlo steps are used in each simulation, which makes the accuracy reach 4.184 × 10–4 kJ/mol when performing electrostatic interaction calculations, and the truncation radius of van der Waals interactions is 1.25 nm; the simulation is performed with Berendsen temperature control method, and the temperature is set to 303.15 K; the simulation is performed with the Andersen pressure control method, the time step of simulation calculation is 1.0 fs, and the total simulation calculation time is not less than 2 ns.
In the simulation, the 5-molecule lignite macro-molecular structure model and the chemical potential, volume and temperature of gas molecules established during the simulation calculation remain unchanged, and the adsorbate molecule model is regarded as a rigid small molecule group. Therefore, only the non-bonding interactions between coal molecules and gas molecules, including van der Waals interaction energy and electrostatic interaction energy, need to be considered regarding the entire system energy for simulation calculation. The two types of energy were treated by the Atom based method and the Ewald summation method, respectively.
The van der Waals interaction was represented by the Lennard–Jones potential function27:
where \(V\left( {r_{ij} } \right)\) is the non-bonding interaction energy between atom i and atom j; i and j are the atoms in the adsorbent and the adsorbate, respectively; Rij is the distance between the atoms; \(D_{ij}\) and \(\left( {R_{0} } \right)_{ij}\) are the Lennard–Jones potential parameters; \(q_{i} q_{j} /R_{ij}\) is the electrostatic interaction; qi and qj are atomic charges whose electrostatic interactions are:
Calculation of the equivalent heat of adsorption during gas adsorption based on the energy particle rise and fall in the μVT ensemble Qs28:
where \(U_{total}\) is total interaction energy in the system, kJ/mol; \(U_{{{\text{int}} ra}}\) is internal energy of CH4 gas molecule, kJ/mol.
Gas molecule adsorption by coal is an exothermic process, and the sum of the individual energies of adsorbate and adsorbent before adsorption is greater than the total energy of the system after adsorption. The adsorption energy can be calculated by:
where Eads is the interaction energy between coal molecules and gas molecules at adsorption equilibrium; EW is the energy of coal molecules before adsorption; EM is the energy of gas molecules before adsorption; EW/M is the total energy of the system at adsorption equilibrium.
Results and discussion
Variations in methane adsorption capacities of coal samples under different stress loads
With a coal rock triaxial adsorption and desorption experimental device, gas adsorption experiments were conducted on loaded coal samples by the above experimental method. The measured adsorption capacities of methane under different stress loads and the fitting variation curve are displayed in Fig. 6.
Under a constant temperature of 303.15 K, an adsorption equilibrium pressure of 2.0 MPa and a confining pressure of 3 MPa, the methane adsorption capacity of coal falls significantly with the rise of axial stress. Specifically, it falls from 13.96 mL/g to 11.28 mL/g, by 19.2%, as the axial stress rises from 0.0 to 30 MPa. Combined with the research of some scholars29, it is found that the increase of effective stress leads to the compression of the skeleton structure of coal, the pore volume becomes smaller, and the position available for gas adsorption decreases. Therefore, the adsorption capacity of coal decreases with the increase of effective stress.
Analysis on isotherm changes in methane adsorption by loaded coal
According to the above-mentioned simulation method, hydrostatic pressure was applied to the established 5-molecule lignite macro-molecular structure model after molecular optimization. Afterwards, the methane adsorption capacities of the coal molecular model were calculated under hydrostatic pressures of 0 MPa, 10 MPa, 20 MPa, and 30 MPa, respectively. The simulation calculation results and the Langmuir fitting curve are shown in Fig. 7.
At the same temperature, the isothermal adsorption curve of loaded coal gradually decreases as the stress load gradually increases, indicating that the methane adsorption quantity declines as the stress load increases under the same adsorption equilibrium pressure, which is consistent with the adsorption amount change rule obtained from physical experiments. Combined with the research and analysis of other relevant scholars30,31, it can be found that stress affects the methane adsorption of coal by changing the characteristics of the pore-fracture system. as the stress increased, the coal molecular structure model mass shrank under pressure, the pore-fissure system closed, and reduces the adsorption potential energy of gas molecules on the coal surface on the other hand. Eventually, it weakens the ability of the coal surface to adsorb gas molecules through vacancies, resulting in a decrease in the adsorption capacity.
Analysis on equivalent adsorption heat variation of methane molecules by loaded coal
The equivalent adsorption heat in coal adsorption simulation under the same temperature was extracted, and the variation curves of equivalent adsorption heat of methane molecules were plotted (Fig. 8).
A rising stress load induces a decline in the equivalent adsorption heat of methane molecules in the adsorption process. Combined with the findings of other scholars32,33, it was found that the increase in stress loading leads to the gradual compression of the skeleton cracks and the coal molecular layer, which reduces the adsorption potential energy of gas molecules when adsorbed on the coal surface. Consequently, the adsorption between the coal molecule surface and gas molecules weakens, and the adsorption quantity (adsorption capacity) of coal under stress loads is reduced accordingly.
Analysis on energy variation of the system during methane molecule adsorption
The adsorption of gas molecules is an exothermic process in which heat is released during the adsorption of methane molecules by lignite molecules, causing a gradual decrease in the system energy. As the number of adsorbed gas molecules grows, the system energy reaches its minimum. In this case, if more gas molecules are supplemented, repelling will occur on the coal surface, and the system energy will increase instead. The energy variation curves of the established lignite molecular model when a single lignite molecule adsorbs methane molecules are presented in Fig. 9.
A single molecular cell of the loaded lignite molecular model starts to repel methane molecules after it adsorbs more than 7 methane molecules. In both the adsorption stage and the repelling stage, the van der Waals force plays the main role while the electrostatic and intra-molecular forces are rather weak, suggesting that methane molecule adsorption by coal is mainly controlled by the van der Waals force.
Methane molecule adsorption by coal under different stress loads is also a physical adsorption process, during which heat is released to decrease the system energy. The only difference between this process and methane molecule adsorption by free-state coal only lies in its smaller number of methane molecules adsorbed on a single molecular cell. When a stress load is applied, the total energy in the system increases. However, the depth of adsorption potential wells on the coal surface drops, thereby affecting the adsorption ability of coal to methane molecules.
Probability density distribution of gas molecules adsorbed by loaded coal
Based on the stable structure of the macro-molecular crystal cell structure of lignite coal optimized by molecular dynamics, an adsorption equilibrium of the stable structure was achieved under a pore pressure of 2.0 MPa and external loading stresses of 0 MPa, 10 MPa, 20 MPa, and 30 MPa through the Sorption module. The adsorption structure of loaded coal and the adsorption occupancy of methane molecules at adsorption equilibrium are exhibited in Fig. 10.
In Fig. 10, methane molecules in the saturated adsorption structure of loaded coal are distributed along both sides of the coal molecular chains, and adsorbate methane molecules are mostly stacked together, displaying a significant stacking effect. The methane molecules adsorbed by the coal macro-molecular structure often cross in pairs, exhibiting cross conformation similar to that of ethane. As can be observed from the 5-molecule lignite structure model, when the stress load rises, the number of methane molecules adsorbed within the crystal cell of coal macro-molecular structure gradually decreases. This also demonstrates that the ability of coal to adsorb methane molecules weakens in the presence of stress loading.
From the probability density distribution of methane molecules adsorbed by the 5-molecule lignite model under different stress loads, it can be concluded that the application of an external stress load significantly reduces the number of methane molecules adsorbed by coal, and that the ability of coal molecules to adsorb gas molecules gradually weakens with the rise of stress load.
Microscopic mechanism of gas adsorption by loaded coal
Combining the physical experiments of gas adsorption by loaded coal samples and numerical simulations of gas molecules adsorbed by coal, we analyze the effect of stress load on the adsorption capacity of coal from the perspective of adsorption energy and adsorption potential well, and elucidate the microscopic mechanism of gas molecules adsorbed by loaded coal.
Adsorption energy and adsorption potential wells
The dual-porosity structure of coal endows it with strong adsorption ability. Gas adsorption by coal is a physical process arising from the adsorption potential energy between the coal surface and methane gas molecules. The potential energy adsorption vacancy curve obtained using the Lennard–Jones potential function is shown in Fig. 11 where α is the adsorption equilibrium distance and ε is the depth of potential wells.
Scholars have analyzed the thermodynamic properties and adsorption characteristics of the coal adsorption process34,35,36,37. In previous analysis, after an external stress σij is applied to coal, the adsorption potential distribution of coal molecules on the loaded coal matrix surface changes significantly. When a single gas molecule changes from a free state to an adsorption state, the change in its potential energy can be expressed as: \(\Delta e^{\sigma } = e_{s}^{{P_{0} }} + \Delta e^{{\left( {P - P_{0} } \right)}} + \Delta e^{{\left( {\sigma - P} \right)}} - e_{g}\). Such a result demonstrates that a rising adsorption potential energy is indicative of a decrease in the depth of adsorption potential wells. When a single gas molecule changes from a free state to an adsorption state, less heat is released by the gas–solid system composed of coal surface and gas molecules. This explains why the application of an external load stress leads to a decrease in the adsorption capacity of coal.
Microscopic mechanism of gas molecule adsorption by loaded coal
Through macroscopic adsorption experiments on coal samples in the presence of external load stresses, it is found that the quantity of methane adsorption by coal decreases with increasing effective stress. However, in actual coal seam mining, the stress load changes continuously. Therefore, stress load is one of the important influencing factors for gas occurrence in coal seams. On the one hand, the stress state of coal exerts an important influence on the characteristics of gas adsorption by coal. On the other hand, as a macroscopic factor, it also changes the energy of the gas–solid system formed on the coal surface, thereby affecting the micro-structure of gas adsorption by coal; resultantly, it triggers a transfer and exchange of substance and energy and determines the adsorption performance of coal. In short, the process of gas adsorption by coal is jointly affected by common macroscopic factors that have been extensively studied previously, such as temperature, pore pressure, particle size, and stress load. Moreover, gas adsorption by coal releases heat while gas desorption from the coal matrix absorbs heat. During the two different processes of energy exchange and transfer, if the stress load increases, small fractures and large and medium pores in coal will be compressed, and the adsorption capacity of coal will be weakened. This will break the original equilibrium and changes gas from an adsorbed state to a free state, greatly raising pore pressure. As a result, non-steady gas flow will occur in the stress concentration area, which leads to macroscopic changes in aspects such as coal temperature and pore pressure.
The adsorption of gas molecules by coal is an integration of macroscopic physical phenomena and micro-structural changes. The macroscopic factors directly change the micro-structure, and such changes in turn act on macroscopic factors, thereby directly affecting the adsorption capacity of coal. The theoretical model analysis, macroscopic physical phenomena observation, and micro-structural simulation calculation all reveal that gas adsorption by coal (as a porous medium) is a combination of macro factors and micro-structure.
Conclusions
-
(1)
By analyzing the distribution characteristics of adsorption potential wells on the surface of the loaded coal matrix, we elucidated five main microscopic influences on the gas adsorption capacity of loaded coal and found that the stress load σij on the coal matrix plays an important role in gas adsorption by coal when adsorbent, adsorbate, adsorption pore pressure, and ambient temperature are fixed.
-
(2)
This study adopts the coal rock triaxial adsorption and desorption experimental device for gas adsorption experiments on loaded coal and the results show that the quantity of methane adsorption decreases with the increase in the stress load. A 5-molecule lignite molecular model based on Wiser model was constructed, and the adsorption capacity and isothermal adsorption line of the loaded coal were calculated under the conditions of force field selection and hydrostatic pressure. It was found that the excess adsorption capacity gradually decreased with the increase of stress load and the same adsorption equilibrium pressure.
-
(3)
By calculating the equal heat of adsorption, it is found that the equal heat of adsorption decreases with the increase of stress load at the same temperature, and the adsorption capacity of coal sample decreases under the action of stress load. In addition, the adsorption phenomenon of coal and methane under load is physical adsorption, and it is an exothermic process. There is no chemical bond between the molecules, and there is no problem of energy level structure. The adsorption of CH4 gas molecules is mainly controlled by Van Der Waals force.
-
(4)
The adsorption mechanism of loaded coal on gas molecules was analyzed by physical adsorption experiments and molecular dynamics simulations. Changes in the stress state of coal samples affect the microstructure of coal, which in turn affects the adsorption of gases. The transfer and exchange of matter and energy on the coal samples are formed, which ultimately determines the adsorption properties of coal. Adsorption of gas molecules by coal is a combination of macroscopic physical phenomena and microstructural changes.
Data availability
All data included in this study are available upon request by contact with the corresponding author.
References
Skoczylas, N., Kudasik, M., Pajdak, A. & Braga, L. I. C. T. Study of CO2/CH4 exchange sorption in coal under confining pressure conditions. Int. J. Greenh. Gas Control. 124, 103845 (2023).
Hol, S., Peach, C. J. & Spiers, C. J. Applied stress reduces the CO2 sorption capacity of coal. Int. J. Coal Geol. 85, 128–142 (2011).
Hol, S., Peach, C. J. & Spiers, C. J. Effect of 3-D stress state on adsorption of CO2 by coal. Int. J. Coal Geol. 93, 1–15 (2012).
Liu, J., Spiers, C. J., Peach, C. J. & Vidal-Gilbert, S. Effect of lithostatic stress on methane sorption by coal: Theory vs. experiment and implications for predicting in-situ coalbed methane content. Int. J. Coal Geol. 167, 48–64 (2016).
Tian, H., Tang, J., Zhang, S. & Zhang, X. Adsorption-desorption characteristics of coal-bearing shale gas under three-dimensional stress state studied by low field nuclear magnetic resonance spectrum experiments. Sci. Rep. 14, 5566 (2024).
Li, S. et al. First principles-based study of the influence of pressure on the gas adsorption performance of coal. Mater. Today Commun. 34, 105269 (2023).
Ren, J. et al. Structure feature and evolution mechanism of pores in different metamorphism and deformation coals. Fuel. 283, 119292 (2021).
Zhang, Q. et al. Insight into the effect of biaxial compression strain on adsorption structure of bituminous coal matrix as well as gas diffusion and permeability properties by macromolecule simulation. Fuel. 338, 127223 (2023).
Ren, J. et al. Fractal-time-dependent fick diffusion model of coal particles based on desorption-diffusion experiments. Energy Fuels. 36, 6198–6215 (2022).
Wang, H., Xiang, J. & Deng, X. Molecular simulation of the pore structure and adsorption properties of coal under compression stress. Energy Fuels. 37, 1887–1895 (2023).
Yan, J., Jia, B., Liu, B. & Zhang, J. Simulation study on molecular adsorption of coal in Chicheng coal mine. Molecules. 28, 3302 (2023).
Zhao, D. & Liu, X. Monte Carlo and molecular dynamics simulations of CH4 molecules adsorption behavior in bituminous coal. Int. J. Low-Carbon Technol. 17, 879–887 (2022).
Sakurovs, R., Day, S., Weir, S. & Duffy, G. Application of a modified Dubinin–Radushkevich equation to adsorption of gases by coals under supercritical conditions. Energy Fuels. 21, 992–997 (2007).
Hill, T. L. An Introduction to Statistical Thermodynamics (Courier Corporation, 1986).
Xie, H., Gao, F. & Ju, Y. Research and development of rock mechanics in deep ground engineering. Chin. J. Rock Mech. Eng. 34, 2161–2178 (2015).
Xie, H. Research review of the state key research development program of China: Deep rock mechanics and mining theory. J. Chin. Coal Soc. 44, 1283–1305 (2019).
Marzec, A. Towards an understanding of the coal structure: A review. Fuel Process. Technol. 77, 25–32 (2002).
Wang, H., Feng, Y., Zhang, X., Lin, W. & Zhao, Y. Study of coal hydropyrolysis and desulfurization by ReaxFF molecular dynamics simulation. Fuel. 145, 241–248 (2015).
Zhang, J., Wang, J., Li, Z., Zhu, J. & Lu, B. Molecular dynamics simulation and gas generation tracking of pyrolysis of bituminous coal. ACS Omega. 7, 11190–11199 (2022).
Li, S. H., Wu, S. W., Wang, B., Zhang, J. Y. & Wang, L. Investigation of desorption and diffusion of gas within low-rank bituminous coal under moisture-stress constraints. Fuel. 373, 132320 (2024).
Sun, H. COMPASS: An ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds. J. Phys. Chem. B. 102, 7338–7364 (1998).
Zhang, J. S., Ni, X. M., Su, E. R. & Liu, X. L. Simulation of the influences of different minerals contained in acidified coal on CH4 adsorption. J. Mol. Struct. 1294, 136328 (2023).
Zhang, Q., Zhu, H. Q. & Kang, R. X. Influence of uniaxial strain loading on the adsorption-diffusion properties of binary components of CH4/CO2 in micropores of bituminous coal by macromolecular simulation. Powder Technol. 427, 118715 (2023).
Wang, W. C. et al. Study on adsorption-diffusion-seepage behavior of oxygen in coal gangue under the coupling of temperature and pressure. Mater. Today Commun. 36, 106817 (2023).
Liu, Y. X. et al. Micropore distribution and methane adsorption process and mechanism in bituminous coals: A molecular dynamics simulation study. J. Environ. Chem. Eng. 12, 112319 (2024).
Salgueiro, G., de Moraes, M., Pessoa, F., Cavalcante, R. & Young, A. New volume translation functions for biodiesel density prediction with the Peng–Robinson Equation of state in terms of its raw materials. Fuel. 293, 120254 (2021).
Peng, D. & Robinson, D. B. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 15, 59–64 (1976).
Li, S. et al. A comprehensive review of deep coalbed methane and recent developments in China. Int. J. Coal Geol. 279, 104369 (2023).
Meng, Q. R., Wang, L., Yang, D. & Xing, J. W. Methane and helium adsorption of coal and its related deformation under different temperature and pressures. Energy Sources A 44, 3929–3944 (2022).
Fang, S. H. et al. Microscopic investigation of the effect of uniaxial stress on the structure of pore-fissure system and methane adsorption in lean coal. Energy. 288, 129837 (2024).
Tian, H. N., Tang, J. P., Zhang, S. P. & Zhang, X. Adsorption-desorption characteristics of coal-bearing shale gas under three-dimensional stress state studied by low field nuclear magnetic resonance spectrum experiments. Sci. Rep. 14, 532 (2024).
Jia, J., Song, H., Jia, P. & Li, B. Adsorption mechanism of CO2/CH4/N2 by the synergistic effect of N/S-doped and functional groups in coal at different temperatures and pressures. Arab. J. Chem. 16, 105333 (2023).
Lin, H. et al. CH4 adsorption and diffusion characteristics in stress-loaded coal based on molecular simulation. Fuel. 333, 126478 (2023).
Du, X. et al. CO2 and CH4 adsorption on different rank coals: A thermodynamics study of surface potential, Gibbs free energy change and entropy loss. Fuel. 283, 118886 (2021).
Zhang, X. et al. CO2 and N2 adsorption/desorption effects and thermodynamic characteristics in confined coal. J. Pet. Sci. Eng. 207, 109166 (2021).
Liu, H. et al. Evolution of thermodynamic properties of tectonic coal with mass ratios determined by isothermal adsorption test. Nat. Resour. Res. 32, 1795–1807 (2023).
Yuan, J., Zhang, H., Guo, Y. & Cai, N. Thermodynamic properties of high-rank tectonically deformed coals during isothermal adsorption. Arab. J. Geosci. 10, 1–11 (2017).
Acknowledgements
This work was supported by the Program for National Natural Science Foundation of China (52274191), Project funded by China Postdoctoral Science Foundation (2021M700132).
Author information
Authors and Affiliations
Contributions
Zhihui Wen: Conceptualization, Funding acquisition, Methodology, Investigation, Writing—original draft, Writing—review & editing. Shuqian Guo: Methodology, Formal analysis, Investigation, Writing—original draft. Yanxia Zhao: Conceptualization, Funding acquisition, Formal analysis, Investigation, Writing—review & editing. Yanping Wang: Investigation, Writing—original draft. Ran Jia: Investigation, Writing—original draft. Jiangang Ren: Investigation, Writing—original draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wen, Z., Guo, S., Zhao, Y. et al. Mechanism of microscopic behind the influence of stress loading on gas adsorption by coal. Sci Rep 15, 3253 (2025). https://doi.org/10.1038/s41598-025-87477-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87477-8