Abstract
Sympathetic nerves regulate nearly all human organs. Their peripheral nerves are present in tumor tissue. Activation of the sympathetic nervous system promotes malignant transformation in several cancers. This study aimed to quantify sympathetic nerve density (SND) in gastric cancer and investigate the relationship between SND and nerve growth factor (NGF) in human clinical samples using immunohistochemistry. Patients with high SND in tumor tissue had significantly shorter survival. High NGF expression in tumor tissue was significantly associated with increased SND and poorer prognosis. In vitro studies demonstrated that nerve elongation of PC12 cells, a model for sympathetic neuron-like cells, was promoted by co-culture with gastric cancer cells expressing high NGF levels whereas nerve elongation was suppressed by NGF knockdown. Furthermore, noradrenaline, a neurotransmitter released from sympathetic nerve endings, induced malignant transformation by promoting epithelial–mesenchymal transition, increasing invasiveness and enhancing the ability of gastric cancer cells to migrate. These findings suggest that gastric cancer with high NGF expression might promote sympathetic innervation within tumor tissue, fostering malignant transformation through noradrenaline signaling. Thus, suppressing sympathetic nerve elongation or activation in gastric cancer might be a target for new therapeutic interventions.
Similar content being viewed by others
Introduction
Gastric cancer remains one of the most common gastrointestinal malignancies. It ranks as the fourth leading cause of cancer-related deaths globally1 and has the highest mortality rate and incidence in Japan2. While treatments like chemotherapy and immunotherapy are continually evolving for gastric cancer, there is a pressing need to innovate future therapeutic interventions.
The sympathetic nervous system regulates the function of almost all human organ systems via the local release of catecholamine neurotransmitters from sympathetic nerve endings and the systemic circulation of catecholamines from the adrenal glands3,4. Sympathetic nerves are also present in tumor tissue. High sympathetic nerve density (SND) within tumor tissue has been reported to be associated with poor prognosis in prostate cancer5, hepatocellular carcinoma6, pancreatic cancer7, and head and neck cancer8. Furthermore, noradrenaline, a neurotransmitter released from sympathetic nerve endings, is known to promote various tumor malignancies by binding to β-adrenergic receptors (ARs) in prostate cancer9 and pancreatic cancer7,10. Recently we reported that high β2-AR expression is a poor prognostic factor in gastric cancer11. However, it remains unclear whether high SND in gastric cancer tissue is a poor prognostic factor. Furthermore, it is unclear whether sympathetic innervation is increased within gastric cancer tumor tissue.
Nerve elongation requires neurotrophins such as nerve growth factor (NGF), which stimulates tyrosine kinase receptors expressed on nerve terminals to promote axonogenesis12,13. It has also been suggested that tumor cells release NGF, which elongates the axons of surrounding nerves in the tumor microenvironment and might induce innervation in breast cancer14and prostate cancer15. However, the impact of NGF expression on sympathetic nerve elongation within the tumor microenvironment remains unclear in gastric cancer. Therefore, we hypothesized that high NGF expression in gastric cancer cells increases SND and noradrenaline levels in tumor tissue, promote tumor malignancy.
In this study, the significance of SND and NGF expression in the tumor microenvironment of gastric cancer was evaluated with immunohistochemistry of human clinical samples. The correlation of SND and NGF expression with prognosis was investigated. We investigated the mechanism by which NGF and SND influence prognosis in vitro.
Results
SND and NGF expression in gastric cancer
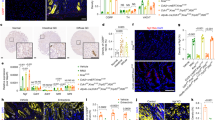
First, immunohistochemistry was performed to evaluate the association between SND and NGF expression using clinical samples from 131 patients with gastric cancer. Anti–tyrosine hydroxylase (TH) antibody was used to stain sympathetic nerve fibers. To verify that nerve fibers stained correctly, nerve fibers in normal tissue were subsequently stained using the anti–neurofilament-L (NF-L) antibody on consecutive sections and confirmed to be consistent with TH staining (Fig. 1a). Unlike sympathetic bundles in normal tissue, sympathetic nerves in tumor tissue were often stained in a scattered pattern (Fig. 1b). SND results in normal tissue and tumor tissue are shown in supplementary Fig. S1. Median SND in normal tissue was 2,180 (range: 0–12,814) µm²/field under 200× magnification. By contrast, median SND in tumor tissue was 264 (range: 0–4,268) µm²/field under 200× magnification. There was no correlation between SND in normal tissue and SND in tumor tissue (R = 0.054, P = 0.542) (Fig. 1c).
Evaluation of sympathetic nerve density (SND) and nerve growth factor (NGF) expression. (a) Figures show sympathetic nerves in non-tumor tissue stained with anti–neurofilament-L (NF-L) and anti–tyrosine hydroxylase (TH) antibodies. Arrows point to stained sympathetic nerves. The two slides were made from consecutive sections. Scale bars are 100 μm. (b) Sympathetic nerves of tumor tissue stained with anti-TH antibodies. Arrows point to stained sympathetic nerves. Scale bars are 100 μm. (c) Scatter plots showing the correlation between SND in non-tumor versus tumor tissue. The correlation was analyzed using Spearman’s rank correlation coefficient. (d) Positive staining in the cytoplasm of cancer cells. NGF immunostaining expression was scored as 0, 1+, 2+, or 3 + according to staining intensity. Scale bars are 100 μm. (e) Boxplot shows a comparison of SND by NGF expression level. Circled markers indicate patients with values more than 1.5 times the interquartile range value. The results were compared using the Mann–Whitney U test.
NGF staining was evident in gastric cancer cells. It was localized predominantly in the cytoplasm. Staining intensities of 0, 1+, 2+, and 3 + were observed in 58, 20, 27, and 26 patients, respectively (Fig. 1d). We defined 0, 1 + as the low NGF group and 2+, 3 + as the high NGF group. The high NGF group had significantly higher SND than the low NGF group (median [range], 495 [0–3,876] vs. 216 [0–4,268] µm²/ field under 200× magnification; P = 0.003) (Fig. 1e).
Correlation between SND and NGF expression with prognosis
The low and high groups of SND were classified based on the median value. Relationships between patient background characteristics and SND or NGF expression are shown in Table 1. The high SND group had a significantly higher proportion of patients with undifferentiated adenocarcinoma based on histological examination and significantly more advanced pathological T status, N status, and stage than the low SND group. On the other hand, no significant differences were observed in clinicopathological factors between the two groups in terms of NGF expression.
Kaplan–Meier analysis of overall survival (OS) by SND is shown in Fig. 2a. The high SND group had significantly worse prognosis than the low SND group. Likewise, in the Kaplan–Meier analysis of OS based on NGF expression, the high NGF group had significantly worse prognosis than the low NGF group (Fig. 2b). In the public Human Protein Atlas database, high expression of NGF at the mRNA level was also found to be a poor prognostic factor (supplementary Fig. S2).
Nerve elongation in co-culture experiments
We confirmed NGF expression by western blot analysis in the MKN45 and KATOІІІ gastric cancer cell lines (Fig. 3a, supplementary Fig. S3a). The KATOІІІ cell line had significantly lower NGF expression than the MKN45 cell line (Fig. 3b). We also confirmed NGF expression in other gastric cancer cell lines (NUGC3, MKN74, AGS), but only KATOІІІ had low NGF expression (supplementary Fig. S4).
Neurite elongation by nerve growth factor (NGF) secretion in gastric cancer cell lines. (a) Protein expression levels of NGF in MKN45 and KATOІІІ cells analyzed with western blotting. The blot was cut horizontally and immunoblotting was performed for each section. (b) Quantification of NGF band intensity was normalized to that of β-actin in each cell line. Data are means ± standard error (n = 3 for each group). (c) The positive control was PC12 with 50 ng/mL of NGF added. The negative control was monocultured PC12 cells. The other panels show PC12 cells co-cultured with MKN45 or KATOІІІ cells. All were photographed after 72 h. Representative images of PC12 cells with neurite elongation by co-culture condition are shown. Scale bars are 100 μm. (d) The figure shows the comparison of the proportion of cells with nerve elongation in the control group with those in PC12 cells co-cultured with MKN45 or KATOІІІ cells. Data are means ± standard error (n = 3 for each group). (e) Results of quantitative real-time PCR of NGF in MKN45 cells transfected with NGF smallinterfering RNA (Si 1, Si 2) and negative control. Data are means ± standard error (n = 3, each group). (f) PC12 cells were co-cultured with MKN45, Si 1, or Si 2, and photographed 72 h later. Representative images of PC12 cells with neurite elongation by co-culture condition are shown. Scale bars are 100 μm. (g) Comparison of the proportion of cells with neurite elongation in each co-culture condition. Data are means ± standard error (n = 3 for each group).
To evaluate whether gastric cancer cells cause nerve elongation, we co-cultured PC12 cells with MKN45 or KATOІІІ cells. PC12 cells cultured without NGF had only slight neurite elongation. By contrast, PC12 cells co-cultured with MKN45 cells had comparable neurite elongation as PC12 cells treated with NGF (Fig. 3c). On the other hand, PC12 cells co-cultured with KATOІІІ cells, which expressed low levels of NGF, had a significantly lower percentage of cells with neurite elongation compared with MKN45 (Fig. 3d). The NGF concentration in each co-cultured medium was measured. The co-culture with MKN45 showed a concentration of 40.2 pg/mL, while the levels in the co-culture with KATOIII and the control were below the detection limit.
Next, we used small-interfering RNA (siRNA)-mediated RNA interference, which suppresses NGF expression, in MKN45 cells to evaluate the role of NGF in gastric cancer. NGF expression was reduced at the protein level by siRNA-mediated knockdown of NGF (Fig. 3e). Representative figures of co-cultures of MKN45 cell lines (control) and NGF knockdown cell lines are shown in Fig. 3f. NGF knockdown of MKN45 cells significantly suppressed neurite elongation in PC12 cells (Fig. 3g).
Evaluation of tumor malignancy induced by noradrenaline
We examined the expression of ARs in gastric cancer cell lines. In all cell lines (MKN45, KATOІІІ, and NUGC3), β2-ARs were strongly expressed while other ARs (α1, α2, β1, and β3) were poorly expressed (Fig. 4a, supplemental Fig. S3b). To determine the effect of sympathetic activation on gastric cancer cells, we conducted experiments using noradrenaline, a sympathetic neurotransmitter, as an AR agonist. First, we found that noradrenaline administration did not affect proliferation of MKN45 cell lines (Fig. 4b). Nevertheless, the MKN45 and KATOІІІ cell lines had significant decreases in E-cadherin mRNA levels and significant increases in N-cadherin mRNA levels with 10 µM noradrenaline treatment compared to controls, demonstrating that noradrenaline induced epithelial–mesenchymal transition (Fig. 4c). Subsequently, alterations in migratory capacity resulting from noradrenaline administration were assessed using the Wound Healing Assay (Fig. 4d). MKN45 cells had significantly smaller wound area and increased migratory ability when treated with 10 µM noradrenaline compared to control (mean, 60% vs. 73%; P = 0.008). Similarly, NUGC3 cells also had smaller wound area with noradrenaline 10 µM administration (mean, 24% vs. 38%; P = 0.007) (Fig. 4e). In addition, an invasion assay was performed to assess tumor invasion potential (Fig. 4f). In both MKN45 and NUGC3 cells, samples treated with 10 µM noradrenaline had a significant increase in the number of invasive cells compared to control (P = 0.003 and P = 0.003, respectively) (Fig. 4g).
Tumor malignancy induced by noradrenaline. (a) Protein expression levels of adrenaline receptors in MKN45, KATOІІІ, and NUGC3 cells analyzed with western blotting. The blot was cut horizontally and immunoblotting was performed for each section. (b) Proliferation assay using the MKN45 cell line. Data are means ± standard error (n = 6 for each group). ns, not significant. (c) Quantitative real-time PCR for E-cadherin and N-cadherin in MKN45 and KATOІІІ cells 24 h after the addition of 10 μM noradrenaline and compared with non-treated control. Data are means ± standard error (n = 3 for each group). (d) Wound Healing Assay was performed on MKN45 and NUGC3 cells with 10 μM of noradrenaline added to determine cell migration ability. MKN45 cells were evaluated at 0 and 72 h and NUGC3 cells were evaluated at 0 and 24 h after the addition of noradrenaline. Scale bars are 500 μm. (e) The scratch area at hour 0 was set as 100% and the percentage decrease in total area was measured. Data are means ± standard error (n = 3 for each group). (f) Invasion assay was performed on MKN45 and NUGC3 cells with 10 μM of noradrenaline added to determine cell invasion ability. MKN45 cells were evaluated at 0 and 96 h and NUGC3 cells were evaluated at 0 and 48 h after the addition of noradrenaline. Scale bars are 100 μm. (g) The percentage increase in invasive cells was measured, with control invasive cells defined as 100%. Data are means ± standard error (n = 3 for each group).
Discussion
This study investigated the association between sympathetic nerves and gastric cancer. The results based on clinical samples revealed that patients with higher SND had more advanced tumor stages and poorer prognosis. This was consistent with previous reports in several other cancers5,6,7,8. In addition, there was no correlation between SND in normal tissue and SND in tumor tissue. This result suggests that the density of sympathetic innervation in gastric cancer tumor tissue is not influenced by the density of sympathetic innervation in normal tissue. NGF is a type of neurotrophin required for sympathetic nerve growth and maintenance12,13. However, there is growing evidence from the recent study that neural invasion within tumors is enhanced by NGF secreted from the tumor cells in pancreatic cancer7. In breast and prostate cancer, it has also been reported that the release of NGF by cancer cells stimulates neuroaxonal formation within the tumor and is associated with higher tumor grade15,16. In addition, similar to results from the public database, the high NGF group had significantly poorer prognosis in gastric cancer. Since the mechanism by which high NGF expression in gastric cancer is associated with poor prognosis remains unclear, we hypothesize that increased SND as a result of NGF secreted by gastric cancer cells might be involved in poor prognosis.
To further evaluate this hypothesis, we performed co-culture experiments using PC12 cells as sympathetic neuron–like cells and gastric cancer cells. High NGF expression by gastric cancer cell lines significantly elongated neurites of PC12 cells, similar to previous studies in breast cancer16 and esophageal squamous cell carcinoma17. In addition, NGF knockdown in gastric cancer cells significantly suppressed nerve elongation. These results indicate that NGF expression promotes neurite outgrowth and that nerve elongation is dependent on NGF secretion from gastric cancer cells. The development of axons of nerves in tumor tissue has also been highlighted as a new cancer feature in pancreatic and prostate cancer7,15. Thus, NGF expression might increase SND in the tumor microenvironment. However, it is unclear which signaling pathway the secreted NGF utilizes to elongate nerve fibers. NGF is known to bind its receptor TrkA and activate signals such as MAPKs and PI3K18. So further studies are needed to clarify the effects of tumor-derived NGF on neurons.
Noradrenaline-induced malignant transformation has been reported in several cancers. In ovarian and pancreatic cancer, noradrenaline has been shown to act on β-ARs, enhancing MMP-2 and MMP9 expression and increasing potential invasiveness19,20. It has also been reported that noradrenaline acts on β-ARs in colon and esophageal cancer, activating STAT3 signaling and increasing migration capacity10,21. In gastric cancer, noradrenaline has also been reported to promote malignant transformation. It has been shown to promote epithelial-mesenchymal transition by activating the β2-AR–HIF-1a–Snail signaling pathway22. It also causes hyperactivation of the β2-AR–STAT3/ERK–YKL-40 signaling pathway, resulting in increased capacity for cancer cells to invade and migrate23. Conversely, no reports have indicated the function of noradrenaline on the proliferation of gastrointestinal cancer cells. In our study using gastric cancer cell lines, noradrenaline promoted epithelial–mesenchymal transition, enhanced invasive capacity, and promoted migration, but no effect on proliferation was observed, which was consistent with previous studies. We also confirmed the presence of ARs in gastric cancer cells. β2-ARs were strongly expressed in each gastric cancer cell line while β1-ARs and β3-ARs were less strongly expressed. In our previous reports, blockade of β-adrenergic receptors inhibited tumor growth and the ability to migrate in gastric cancer cells11. Since the biological effects of α-adrenergic agonists have been reported to be complex and inconsistent24,25, β2-ARs might be strongly involved in noradrenergic-induced malignant transformation.
In a report measuring catecholamine levels in ovarian cancer, Lutgendorf et al. found no adrenaline in tumor tissue, but noradrenaline levels were higher in more advanced ovarian cancers. Nevertheless, there were no differences between plasma noradrenaline levels and patients with ovarian cancer26. Renz et al. also found that catecholamines promote β2-AR–mediated pancreatic carcinogenesis and NGF secretion, which promoted innervation in pancreatic cancer, leading to increased noradrenaline levels and tumor growth7. Therefore, local sympathetic innervation might supply much of the catecholamines in tumors. In our study, the high NGF group showed higher SND, and the high SND group was associated with significantly poorer prognosis in human clinical samples. NGF expression was also observed in several gastric cancer cell lines, with significant neurite elongation in co-culture experiments. Furthermore, noradrenaline treatment, a sympathetic neurotransmitter, was shown to promote malignant transformation of gastric cancer cell lines. This indicates that gastric cancer cells might increase SND in the tumor microenvironment and acquire malignant transformation by secreting NGF.
Currently, various treatments, especially surgery and multidisciplinary treatments, are progressing for locally advanced gastric cancer27,28. The potential of anti-neurogenic cancer therapies is garnering attention as a future avenue. For example, similar to our previous reports11, non-selective β-blockers have been reported to reduce mortality in breast cancer29, prostate cancer30, and ovarian cancer31. They are expected to be new drugs for cancer treatment. Studies using mouse models of prostate cancer5, gastric cancer32, and basal cell carcinoma33 have also shown that surgical and chemical removal of nerves might lead to tumor suppression. However, some studies have reported that vagotomy conversely promotes tumor growth34,35. Because the sympathetic–parasympathetic network of each organ is complex, inhibition of one system might cause negative feedback on the other; further validation is needed. In addition, tanezumab, an anti-NGF antibody, has been shown to have analgesic activity in chronic rheumatoid arthritis and metastatic cancer; it is in clinical trials36,37. One study showed that anti-NGF drugs suppress neuroinvasion and metastasis in a mouse model of pancreatic cancer38. These anti-neurogenic therapies might inhibit neural invasion in cancer and could be a new type of therapeutic intervention for cancer.
This study has several limitations. First, this study did not clarify that sympathetic nerve elongation raises levels of noradrenaline. However, previous reports have shown that sympathetic nerve elongation elevates levels of noradrenaline8. Second, the present study did not evaluate vagal or sensory nerves. In pancreatic and basal cell carcinoma, ablation of sensory neurons has been reported to inhibit cancer growth. Therefore, the potential involvement of sensory and parasympathetic nerves in gastric cancer cannot be ruled out39. Third, PC12 cells can not completely replicate actual nerve axons and synapses. We believe that future studies will elucidate the role of sympathetic nerves in the tumor microenvironment in vivo experiments, as well as in vivo-derived neuronal cell cultures.
In conclusion, our results suggest that gastric cancer cells secrete NGF, which increases sympathetic innervation in the tumor microenvironment and results in the release of noradrenaline from sympathetic nerve endings and promotion of tumor malignancy. Further research is needed to elucidate this crosstalk, which may lead to new therapeutic interventions such as anti-neurogenic therapy.
Methods
Cell lines
Human gastric cancer cell lines MKN45 (JCRB0254), NUGC3 (JCRB0822) and MKN74 (JCRB0255) were obtained from the Japanese Collection of Research Bioresources (Osaka, Japan). The KATOІІІ (HTB103) and AGS (CRL1739) cell line was obtained from the American Type Culture Collection (Rockville, MD, USA). MKN45 and NUGC3 are used as poorly differentiated adenocarcinomas and KATOIII as a signet ring cell carcinoma. The PC12 cell line (JCRB0733), a neuronal cell model, was obtained from the Japanese Collection of Research Bioresources. All cell lines were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium (Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO, USA), 100 U/mL penicillin, and 100 µg/mL streptomycin (Life Technologies, Carlsbad, CA, USA) at 37 °C, in a humidified atmosphere of 5% CO2.
Cell proliferation assay
Cell proliferation assays were performed using the MKN45 cell line. Cells were seeded in 96-well plates at a density of 1 × 103 cells per well and incubated for 24 h. Next, cells were incubated with various concentrations of noradrenaline. Cell proliferation was evaluated using 2-(2-methoxy-4-nitro-phenyl)−3-(4-nitrophenyl)−5-(2,4-disulfophenyl)−2 H-tetrazolium, monosodium salt (WST-8) assays (Cell Counting Kit-8; Dojindo Laboratories, Kumamoto, Japan). The absorption of WST-8 was measured at a wavelength of 450 nm using a microplate reader (iMark; Bio-Rad Laboratories, Hercules, CA, USA). The growth rate was expressed as the percentage of absorbance for treated cells versus that of control cells. Experiments were performed with six replicate wells for each sample. Data are presented as averages.
Small-interfering RNA (siRNA) transfection
SiRNAs (including negative control siRNA and NGF siRNA) were procured from Thermo Fisher Scientific (Waltham, MA, USA). MKN45 gastric cancer cells were seeded in a 6-well plate using antibiotic-free RPMI 1640 medium supplemented with 10% FBS. On the following day, cells were transfected with siRNAs using Lipofectamine 3000 Reagent and Opti-MEM Reduced Serum Medium (Thermo Fisher Scientific).
Western blot analysis
For western blot analysis, cells were seeded in 6-well plates at a density of 1 × 105 cells per well and incubated for 72 h. Proteins were extracted from cell lines using protease and phosphatase inhibitors in RIPA buffer (Thermo Fisher Scientific). Proteins were separated using Mini-PROTEAN TGX Precast Gel (Bio-Rad Laboratories), transferred to an Immun-Blot PVDF Membrane (Bio-Rad Laboratories), and incubated with primary antibodies at 4 °C overnight. After incubation with secondary antibodies, signals were detected with the ECL Prime Western Blotting Detection reagent (GE Healthcare Bioscience, Piscataway, NJ, USA). Bands were quantified by densitometry using Image Lab software Version 6.0.0 (Bio-Rad Laboratories). The following antibodies were used at 1:1,000 dilution: anti-NGF (ab52918), anti–α1-AR (ab313577), anti–α2-AR (ab85570), anti–β2-AR (ab182136), and anti–β3-AR (ab300483) from Abcam (Cambridge, UK) and anti–β1-AR (GTX23546) from Gene Tex (Irvine, CA, USA). The experiment was performed at least in triplicate.
RNA extraction and quantitative real-time PCR
Total RNA was extracted from cell lines using TRIzol RNA Isolation Reagent (Thermo Fisher Scientific). Quantitative real-time PCR analyses were performed with Thunderbird SYBR qPCR Mix (TOYOBO, Osaka, Japan). PCR products were continuously measured using the Roche LightCycler 1.5 PCR System (Roche Holding AG, Basel, Switzerland); values were normalized to the expression levels of β-actin.
Neurite elongation assay with co-culture
The capacity of gastric cancer cells to extend nerves was assessed using PC12 cells, which are recognized for their ability to differentiate and transform into cells with characteristics similar to sympathetic nerve cells when exposed to NGF. PC12 cells have been used extensively for studying neurite outgrowth40. In co-culture experiments, 1 × 105 PC12 cells were seeded into the lower chamber of a 12-well Transwell plate (Corning, NY, USA) pre-coated with rat tail collagen I (Invitrogen, Carlsbad, CA, USA). Each gastric cancer cell line was co-cultured in Transwell inserts (diameter, 12.0 mm; pores, 0.4 mm; Corning). After 3 days of co-culture, the percentage of cells with neurite elongation among all PC12 cells within the field of view was measured using BZ-X Analyzer (Keyence Corporation, Osaka, Japan) at 100× magnification. PC12 cells with neurites at least twice the size of the cell body were considered to be elongated. Each co-culture experiment was repeated at least thrice. Cell lines were maintained in RPMI 1640 medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37 °C in a humidified atmosphere of 5% CO2. NGF levels of supernatant were measured using an ELISA kit specific for human (R&D Systems, Minneapolis, MN).
Wound healing assay
A wound healing assay was conducted using MKN45 and NUGC3 cells. Cells were seeded in 6-well plates and incubated until they reached confluence. Linear scratch wounds were made in the cell monolayer using a pipette tip. We added 10 µM noradrenaline to each well and incubated the cells in RPMI 1640 medium supplemented with 0.1% FBS. At 48 and 72 h after scratching, we assessed percent reduction in the scratched area using the BZ-X Analyzer (Keyence Corporation). A total of five samples were used for each experiment.
Invasion assay
Invasiveness was measured using 24-well plates with inserts, 8-µm membrane pores and Matrigel coating (#354480; Corning). A total of 5 × 10⁴ cells (MKN45 or NUGC3) was seeded in the upper chamber and 0.75 mL of medium was added to the lower chamber. The cells were incubated at 37 °C in a CO2 incubator for 48 and 96 h. Subsequently, non-invasive cells in the upper chamber were removed using cotton swabs. Invasive cells on the underside of the membrane were fixed and stained using the Diff-Quick stain kit (16920; Sysmex, Kobe, Japan). The number of invasive cells was counted in a field under 200× magnification. Each experiment consisted of three samples.
Immunohistochemistry
We prepared 4.0-µm–thick sections of resected specimens obtained from formalin-fixed paraffin-embedded blocks. The sections were deparaffinized using xylene and subsequently rehydrated with multistep descending concentrations of ethanol. After autoclaving the sections in citrate buffer at 115 °C for 20 min, they were immersed in 0.3% hydrogen peroxide to block endogenous peroxidase. Next, they were incubated in horse serum for 20 min to prevent nonspecific staining. The slides were incubated overnight at 4 °C with a primary antibody (1:1,000), followed by incubation with the avidin–biotin-peroxidase complex (VECTASTAIN Elite ABC HRP Kit; Vector Laboratories, Burlingame, CA, USA) for 20 min and with 3,3ʹ-diaminobenzidine tetrahydrochloride (DAB Tablet; FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) for 3 min to visualize the reactions. The technique for staining sympathetic nerves used anti-TH antibody. TH is an enzyme required for noradrenaline production. Antibodies are widely used as sympathetic nerve markers. The following primary antibodies were used: anti-TH (NB300-109, Novus Biological, Centennial, CO, USA); anti-NGF (ab52918, Abcam); and anti–NF-L (2835 S, Cell Signaling, Danvers, MA, USA). All sympathetic nerves in normal tissue and tumor tissue were photographed in a field under 200× magnification. The stained area was measured using BZ-X Analyzer (Keyence Corporation). The SND was then calculated as the average of the five hotspot areas within a 200× field of view. This measurement method was based on previous reports5,8. NGF staining intensities were quantified as previously described41. Staining intensity of tumor tissue was classified as 0, 1+, 2+, or 3+. It was evaluated overall along with the percentage of stained area.
Human clinical samples
We enrolled 131 consecutive patients diagnosed with gastric cancer with stage greater than pT2 and pStage ІІ–ІІІ who underwent R0 resection between January 2013 and December 2016. The 15th edition of the Japanese classification of gastric carcinoma was used to determine pathological stage. Primary gastric cancer was analyzed using the resected specimens derived from formalin-fixed paraffin-embedded blocks after written informed consent was obtained from each patient. The institutional review board of Osaka University Hospital approved the study (approval number: 23255). This study was performed in accordance with the Declaration of Helsinki.
Statistical analysis
Data are expressed as means ± standard error. Statistical differences were evaluated using Student’s t-test or the Mann–Whitney U test. Two-sided P values were calculated; P < 0.05 was considered statistically significant. Survival rates were estimated using the Kaplan–Meier method and compared with the log-rank test. Cox proportional hazards models were used for both univariate and multivariate analyses to identify independent predictors of OS. All statistical analyses were performed with SPSS software, version 24.0 (IBM Corp, Armonk, NY, USA).
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Higashi, T. & Kurokawa, Y. Incidence, mortality, survival, and treatment statistics of cancers in digestive organs—Japanese cancer statistics 2024. Ann. Gastroenterol. Surg. 8, 958–965 (2024).
Karemaker, J. M. An introduction into autonomic nervous function. Physiol. Meas. 38, R89–R118 (2017).
Wehrwein, E. A., Orer, H. S. & Barman, S. M. Overview of the anatomy, physiology, and Pharmacology of the autonomic nervous system. Compr. Physiol. 6, 1239–1278 (2016).
Magnon, C. et al. Autonomic nerve development contributes to prostate cancer progression. Science 341, 1236361 (2013).
Zhang, L. et al. Sympathetic and parasympathetic innervation in hepatocellular carcinoma. Neoplasma 64, 840–846 (2017).
Renz, B. W. et al. β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 33, 75–90 (2018).
Amit, M. et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 578, 449–454 (2020).
Zahalka, A. H. et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 358, 321–326 (2017).
Guo, K. et al. Interaction of the sympathetic nerve with pancreatic cancer cells promotes perineural invasion through the activation of STAT3 signaling. Mol. Cancer Ther. 12, 264–273 (2013).
Koh, M. et al. Propranolol suppresses gastric cancer cell growth by regulating proliferation and apoptosis. Gastric Cancer 24, 1037–1049 (2021).
Kaplan, D. R., Martin-Zanca, D. & Parada, L. F. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature 350, 158–160 (1991).
Sharma, N. et al. Long-distance control of synapse assembly by target-derived NGF. Neuron 67, 422–434 (2010).
Bloom, A. P. et al. Breast cancer-induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J. Pain 12, 698–711 (2011).
Pundavela, J. et al. ProNGF correlates with Gleason score and is a potential driver of nerve infiltration in prostate cancer. Am. J. Pathol. 184, 3156–3162 (2014).
Pundavela, J. et al. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol. Oncol. 9, 1626–1635 (2015).
Griffin, N. et al. Clinicopathological significance of nerves in esophageal cancer. Am. J. Pathol. 190, 1921–1930 (2020).
Marlin, M. C. & Li, G. Biogenesis and function of the NGF/TrkA signaling endosome. Int. Rev. Cell. Mol. Biol. 314, 239–257 (2015).
Sood, A. K. et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin. Cancer Res. 12, 369–375 (2006).
Guo, K. et al. Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncol. Rep. 22, 825–830 (2009).
Masur, K., Niggemann, B., Zanker, K. S. & Entschladen, F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 61, 2866–2869 (2001).
Shan, T. et al. Novel regulatory program for norepinephrine-induced epithelial-mesenchymal transition in gastric adenocarcinoma cell lines. Cancer Sci. 105, 847–856 (2014).
Qi, Y. H. et al. Sympathetic nerve infiltration promotes stomach adenocarcinoma progression via norepinephrine/β2-adrenoceptor/YKL-40 signaling pathway. Heliyon 8, e12468 (2022).
Szpunar, M. J., Burke, K. A., Dawes, R. P., Brown, E. B. & Madden, K. S. The antidepressant desipramine and α2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure. Cancer Prev. Res. 6, 1262–1272 (2013).
Lamkin, D. M. et al. α2-Adrenergic blockade mimics the enhancing effect of chronic stress on breast cancer progression. Psychoneuroendocrinology 51, 262–270 (2015).
Lutgendorf, S. K. et al. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav. Immun. 25, 250–255 (2011).
Yanagimoto, Y., Kurokawa, Y. & Doki, Y. Essential updates 2021/2022: Perioperative and surgical treatments for gastric and esophagogastric junction cancer. Ann. Gastroenterol. Surg. 7, 698–708 (2023).
Kurokawa, Y. et al. Phase 2 trial of neoadjuvant docetaxel, oxaliplatin, and S-1 for clinical stage III gastric or esophagogastric junction adenocarcinoma. Ann. Gastroenterol. Surg. 7, 247–254 (2023).
Barron, T. I., Connolly, R. M., Sharp, L., Bennett, K. & Visvanathan, K. Beta blockers and breast cancer mortality: A population- based study. J. Clin. Oncol. 29, 2635–2644 (2011).
Grytli, H. H., Fagerland, M. W., Fosså, S. D. & Taskén, K. A. Association between use of β-blockers and prostate cancer-specific survival: A cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur. Urol. 65, 635–641 (2014).
Watkins, J. L. et al. Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer 121, 3444–3451 (2015).
Zhao, C. M. et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 6, 250ra115 (2014).
Peterson, S. C. et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell. Stem Cell. 16, 400–412 (2015).
Renz, B. W. et al. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 8, 1458–1473 (2018).
Erin, N., Barkan, G. A. & Clawson, G. A. Vagus nerve regulates breast cancer metastasis to the adrenal gland. Anticancer Res. 33, 3675–3682 (2013).
Jimenez-Andrade, J. M., Ghilardi, J. R., Castañeda-Corral, G., Kuskowski, M. A. & Mantyh, P. W. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain 152, 2564–2574 (2011).
Sopata, M. et al. Efficacy and safety of tanezumab in the treatment of pain from bone metastases. Pain 156, 1703–1713 (2015).
Saloman, J. L. et al. Systemic depletion of nerve growth factor inhibits disease progression in a genetically engineered model of pancreatic ductal adenocarcinoma. Pancreas 47, 856–863 (2018).
Saloman, J. L., Albers, K. M., Rhim, A. D. & Davis, B. M. Can stopping nerves, stop cancer? Trends Neurosci. 39, 880–889 (2016).
Suter, D. M. & Miller, K. E. The emerging role of forces in axonal elongation. Prog Neurobiol. 94, 91–101 (2011).
Ma, J., Jiang, Y., Jiang, Y., Sun, Y. & Zhao, X. Expression of nerve growth factor and tyrosine kinase receptor A and correlation with perineural invasion in pancreatic cancer. J. Gastroenterol. Hepatol. 23, 1852–1859 (2008).
Author information
Authors and Affiliations
Contributions
Conception and design of the work: T.I., Y.Ku., T.H., T.S., and T.T. Acquisition of data: T.I., S.N., R.N., and Y.Ka. Analysis and interpretation of data: T.I., Y.Ku., T.H., T.S., and T.T. Writing, review, or revision of the manuscript: T.I., Y.Ku., T.H., S.N., R.N., Y.Ka., T.S., K.Y., T.T., K.M., K.Y., K.T., T.M., K.N., H.E., and Y.D. Study supervision: H.E. and Y.D.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Itami, T., Kurokawa, Y., Hagi, T. et al. Sympathetic innervation induced by nerve growth factor promotes malignant transformation in gastric cancer. Sci Rep 15, 3824 (2025). https://doi.org/10.1038/s41598-025-87492-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87492-9
This article is cited by
-
Tumor microenvironment dynamics in gastric cancer pathogenesis and therapeutic resistance
Molecular Cancer (2026)