Abstract
Urinary tract injuries represent a significant clinical challenge, necessitating precise diagnosis and effective treatment strategies. Rat models are preferred for studying urinary tract injuries due to their size, visibility of external genitalia, and robust reproductive and growth capabilities. However, there is a lack of standardized methodologies for evaluating the endpoints of rat urinary tract injury models. This study aimed to investigate the methodology of model establishment, imaging evaluation techniques, and endpoint effectiveness. Twenty-four male Sprague-Dawley rats were randomly assigned to groups, including a blank control group and surgical groups. The surgical groups underwent urethral injury induced by recombinant transforming growth factor-β1 (TGF-β1) solution, followed by the random selection of one surgical group (n = 6) to receive treatment with mesenchymal stem cell (MSC) exosomes. High-frequency ultrasonography using a GE E10 device combined with a water bath method was employed to evaluate the urethral conditions of all rats. Ultrasonographic characteristics were scored for all surgical group rats, and an intergroup analysis was conducted between the surgical group and the control group rats. Statistical analysis was conducted using SPSS 22.0 software. Ultrasonographic assessment revealed significant differences in urethral echogenicity between the normal group and the surgical group, with noticeable changes in urethral morphology post-injury. Histopathological examination confirmed more severe urethral stenosis in the TGF group compared to the MSC group. High-frequency ultrasound could effectively differentiate between the two groups of rats. Additionally, there were significant differences in the scoring of ultrasonographic characteristics between the two groups. Urethral stricture presents a complex challenge in urology, often requiring invasive treatment modalities. The establishment of animal models plays a crucial role in understanding and addressing this condition. TGF-β1 induced fibrosis and MSC therapy represent promising avenues for urethral stricture management. High-frequency ultrasound combined with the immersion method offers a non-invasive and cost-effective approach for evaluating rat urethral stricture models. This study highlights the practicality of high-frequency ultrasound combined with the water bath method in evaluating rat urethral stricture models, as it can effectively differentiate between rats in the pure TGF-β1 induced group and those in the MSC treatment group based on urethral changes. The results underscore the importance of standardized methodologies for model assessment and the potential of non-invasive imaging techniques in preclinical research. Overall, this study contributes to advancing our understanding of urinary system injuries and holds implications for future therapeutic interventions in urology.
Similar content being viewed by others
Introduction
Animal models of urethral injury are of significant clinical value in guiding the evaluation of treatment efficacy for urethral injuries1. Male rats, due to their larger size, visible external genitalia, and strong reproductive and growth capabilities, are often the preferred animals for urethral injury models2. However, the random and concealed nature of animal excretory behaviors poses challenges in determining the endpoints of experimental animal models, potentially increasing the number of animals used in experiments, which contradicts the principles of animal welfare. Given the scarcity of methodological research on the establishment of endpoints in urethral injury animal models, this study focuses on exploring the methodology for model establishment, imaging evaluation methods at endpoints, and their effectiveness. This research was conducted as an additional study within a project on urethral stricture, without exceeding the original experimental design’s bodily trauma to the rats.
Materials and methods
General information
Twenty-four male Sprague-Dawley (SD) rats, clean grade, were purchased from the Experimental Animal Center of Fujian Medical University, the animal qualification certificate number: SCXK (Min) 2016-0002, with individual net weight ranging from 350 to 400 g. They were housed in a closed environment with artificially simulated alternating 12-hour light-dark cycles, and had ad libitum access to food and water.
Experimental reagents
(1). TGF-β1 cytokine, sourced from Sigma-Aldrich Corporation, USA; (2).Preparation of MSC-Exo solution: The MSC-Exo solution was extracted from human umbilical mesenchymal stem cells using differential centrifugation, aproximately the final concentration is 0.5 µg/µl .Umbilical mesenchymal stem cells (UMSC) were purchased from Salial Company (Guangzhou, China; http://www.saliai.com) and maintained in a medium containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific Life Sciences) at 37 °C and 5% CO2.
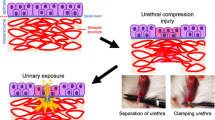
Rat model preparation (the grouping process is shown in Fig. 1)
Six rats were randomly selected as the control group, and the remaining rats (n = 18) form the surgery group. After anesthesia with intraperitoneal pentobarbital sodium (50 mg/kg), all rats (n = 24) are intubated. The control group (n = 6) receives 100ul of saline solution injected into the urethral wall, while the remaining rats (n = 18) receive TGF-β solution (1 mg TGF-β1 per 100 ml saline solution) 100ul injected into the urethral wall. Five minutes after injection, use a surgical blade to make transverse incisions on the mid-ventral side of the penis in the surgery group rats, perpendicular to the longitudinal axis of the penis, until the catheter is visible. Make four consecutive cuts at even intervals, ensuring smooth blade movement and avoiding intersection between the incisions. Then, use 5 − 0 absorbable sutures to stitch the skin of the penis, remove the catheter. Twenty-four hours later, the control group rats receive a second injection of 100ul saline solution into the urethral wall. One group of rats were randomly selected from the surgery group (n = 6) to simply receive 100ul of TGF-β1 solution (labeled as TGF group), and the remaining surgery group rats (n = 12) receive 100ul of TGF-β1 solution plus 30ug mesenchymal stem cell exosomes (MSC-Ex) (labeled as MSC group). One month later, all groups of rats underwent ultrasound examination blindly, and then they were immediately euthanized by intraperitoneal injection of 100 mg/kg of 2% pentobarbital sodium. The animals’ vital signs were reassessed after a 10-minute interval following the administration. Penile and urethral tissues are collected for pathological histological examination.
Histology
Penile tissues from the incision and injection sites were carefully harvested and fixed with 10% neutral buffered formalin (NBF) for further histological examination. Following standard operating procedures, these samples were then subjected to Masson’s trichrome staining and Hematoxylin and Eosin (HE) staining3. In conducting histopathological analysis, we used the Olympus (Model: BX51) microscope along with a Nikon (Model: Digital Sight 10) camera.
Instruments and methods
The GE E10 ultrasound diagnostic apparatus with a probe model 11 L-D and a frequency of 4.5–12.0 MHz was used. After intraperitoneal anesthesia with water-soluble pentobarbital sodium (50 mg/kg), the rat was placed in a supine position, and its hind legs were held by an assistant to expose the external genitalia. The rat was then immersed in a water basin up to the level of its waist ( Fig. 2). The high-frequency probe covered with a sterilized sheath (to prevent cross-contamination) was inserted into the water basin, and the ultrasound beam was directed parallel to and overlapping with the long axis of the rat’s penis to obtain a clear image of the entire urethral penile segment. The images were interpreted by a radiologist with 10 years of experience in ultrasound imaging, who was blinded to the grouping information of the rats. The images were evaluated based on criteria such as "Continuity of central line of urethral hyperechoic bands; Width of urethral hyperechoic bands; Echo intensity of urethral hyperechoic bands; Echo intensity distribution uniformity of urethral hyperechoic bands” and corresponding scores were assigned as detailed in Table 1.
Statistical methods
Statistical analysis was performed using SPSS 22.0 software. The respective measurement indices of urethral echogenicity were normally distributed metric data, expressed as mean ± standard deviation \((\overline{x} \pm s)\). The independent samples t-test was employed for comparisons between two groups, while one-way analysis of variance (ANOVA) followed by the LSD post hoc test was used for comparisons among three or more groups. Additionally, receiver operating characteristic (ROC) curves were constructed for rat scores, and the area under the curve (AUC) was calculated to determine the cutoff value. A significance level of P < 0.05 was considered statistically significant.
Results
Ultrasonographic echogenicity of urethra in different groups
All rats survived healthy until the completion of ultrasonographic image acquisition. In the normal group, there were 6 rats whose urethral long axis exhibited a straight and strong echogenic stripe, with the strongest echo at the central line and weakened but still noticeable echoes at the edges, which were stronger than the surrounding corpus cavernosum echoes (Fig. 3). The overall stripe appeared as a “three-line pattern,” and on the short axis, it presented as a circular central echo with the strongest echo forming a target-shaped high echo area (Fig. 4). The mean width \((\overline{x} \pm s)\) of the long axis stripe (based on the outer edge) was 0.89 ± 0.11 mm. All surgical group rats showed a narrowing of the strong echogenic stripe width in the middle segment of the penile urethra (Fig. 5), with a mean length of narrowing of 0.22 ± 0.06 mm, ranging from 0.14 to 0.30 mm. The mean width \((\overline{x} \pm s)\) of the strong echogenic stripe in the narrowed segment of the urethra (recorded at the narrowest point) was 0.40 ± 0.10 mm, ranging from 0.20 to 0.50 mm; among them: (1) The mean width \((\overline{x} \pm s)\) of the strong echogenic stripe in the urethra for the MSC group was 0.47 ± 0.05 mm, with a 95% confidence interval ranging from 0.41 mm to 0.52 mm; (2) The mean width \((\overline{x} \pm s)\) of the strong echogenic stripe in the urethra for the TGF group was 0.30 ± 0.06 mm, with a 95% confidence interval ranging from 0.23 mm to 0.37 mm.
The urethral stripe echogenicity decreased in all surgical groups (Fig. 6), with some stripes becoming blurred and discontinuous (Fig. 7), detailed changes are shown in Table 1. All measurement data were normally distributed and expressed as continuous variables. Comparisons between two groups were performed using the independent samples t-test, while comparisons among three or more groups were conducted using one-way analysis of variance (ANOVA) followed by the LSD post hoc test. Statistical analysis revealed significant differences in the urethral hyperechoic band width between the normal group and both the MSC and TGF groups, as well as between the MSC and TGF groups (P < 0.01).
Scoring of changes in rat urethral echogenicity
Different scores were assigned to changes in the continuity of the central line, width, echo intensity, and echo intensity distribution uniformity of the urethral hyperechoic bands. Changes in the continuity of the central line include basic continuity and interruption. Changes in the width of the urethral hyperechoic bands include stability and stenosis. Changes in the echo intensity of the urethral hyperechoic bands include basic consistency, weakening of the central line echo with blurred edge lines, and weakening of the central line echo with disappearance of the edge line. The echo intensity distribution uniformity of the urethral hyperechoic bands includes basic uniformity and nonuniformity. Specific scoring criteria are detailed in Table 1, with a maximum possible score of 9 points. The cumulative score range for all rats is summarized in Table 2. All the control group rats’ urethral ultrasounds did not show any of the changes described above, so they were not included in the statistical tables for display.
Surgical group rat urethral tissue pathology and ultrasound scoring comparison
All rats in the surgical group exhibited more pronounced urethral stenosis compared to the control group, with the TGF group showing more severe urethral stenosis than the MSC group. Histopathological examination revealed abundant dense collagen fibers at the urethral incision site in the TGF group, with disordered arrangement of newly formed collagen fibers. In contrast, the arrangement of collagen fibers at the incision site in the MSC group was also disordered but with fewer overall quantities, and there were significantly more blood sinuses within the surrounding corpus cavernosum tissue (Figs. 8, 9 and 10).
(A) shows urethral histopathology in normal rats; (B) shows a large number of fibroblasts are proliferative in the surgical group; (C) showed a large number of blood sinuses appeared in the surgical group (HE staining, the images were taken at a magnification of 10x, with the scale bar representing 100 μm). (This pathological image was obtained one month after surgery.)
Pathological images of urethra of rats in the surgical group (HE Staining, The image was taken at a magnification of 10x, with the scale bar representing 100 μm); TGF group (A), MSC group (B), The MSC group, compared to the TGF group, exhibited similarly disorganized collagen fibers but in a lesser overall quantity. (This pathological image was obtained one month after surgery.)
Pathological images of rat urethras in various groups (Masson’s trichrome staining, The image was taken at a magnification of 40x, with the scale bar representing 25 μm); Control group (A), TGF group (B), MSC group (C). The surgical group shows similarly disorganized collagen fibers under HE staining, but the overall quantity is less in the MSC group compared to the TGF group. (This pathological image was obtained one month after surgery.)
Statistical analysis revealed that the ultrasound scores in the TGF group were significantly higher than those in the MSC group. In the TGF group, all 6 rats had ultrasound scores of 8 or above, with a mean score of (8.67 ± 0.52), while in the MSC group, only 2 out of 12 rats scored 8, and the remaining 10 rats scored below 8, with a mean score of (6.83 ± 0.94). The score difference between the two groups was statistically significant (P < 0.01). Further analysis using receiver operating characteristic (ROC) curve (Fig. 11) showed an area under the curve (AUC) of 0.972, indicating that at a cutoff value of 7.5, the sensitivity and specificity for distinguishing between the TGF and MSC groups were 100% and 83.3%, respectively. These results suggest that the TGF group had significantly higher ultrasound scores for urethra assessment compared to the MSC group, and the ROC curve analysis supports the effectiveness of this scoring system in differentiating between the two groups of rats.
Discussion
Urethral stricture, a prevalent condition in the realm of urology, presents a formidable challenge to practitioners within this medical field4. Its pathological progression is characterized by an overabundance of fibrosis in the urethral mucosa and the scarring of the surrounding spongiosum tissue5. In the contemporary clinical management of urethral stricture, invasive methodologies reign supreme6, including but not limited to, urethral dilation, urethrotomy, and a myriad of urethroplasty techniques. Among these procedures, urethroplasty emerges7as the vanguard surgical intervention in clinical practice, attributed to its refined surgical methodologies and commendable longitudinal outcomes. However, it still carries risks of complications such as a high recurrence rate 8.9and erectile dysfunction10.
Hence, many scholars are devoted to exploring more ideal treatment modalities or adjunctive therapeutic approaches. The establishment of an animal model for urethral stricture holds significant importance for both the fundamental understanding and clinical research of this condition. In this study, we induced urethral stricture in rats by injuring their urethra and investigated the methodology of model establishment, along with the efficacy of imaging evaluation methods for assessing the endpoint of model establishment.
Currently, there is no unified standard for establishing a rat urethral stricture model, particularly one that mimics the formation of urethral stricture resulting from abnormal wound healing. In actual practice, regardless of the establishment method, the formation of urethral stricture is primarily a pathological healing process characterized by fibrosis at the cellular and tissue levels, which is associated with inflammatory reactions, abnormal or excessive activation of fibroblasts11.
Studies have found that both human and rat urethras contain a large number of TGF-β1 receptors12. After the injection of TGF-β1 into urethral tissues, the expression of type I and III collagens significantly increases. Therefore, most experiments induce urethral fibrosis using TGF-β1 to construct rat urethral stricture models2.
Mesenchymal stem cells (MSCs) are a type of multipotent stem cells derived from the mesoderm with the potential for multidirectional differentiation13. In addition to their differentiation ability, MSCs can promote tissue repair by regulating cellular responses in structure and immune cells, thereby creating a regenerative and anti-inflammatory environment. It is well known that inflammatory mediators can attract MSCs and alter their secretions, thereby promoting immune responses14and improving the therapeutic progress of inflammatory diseases, such as urethral stricture.
Many studies have shown that during cell therapy, MSCs primarily enhance wound healing efficiency through paracrine mechanisms15,16, and in the repair of urethral stricture, they may improve repair rates through the secretion of extracellular vesicles17. Extracellular vesicles derived from MSCs typically possess many properties associated with MSCs, including tissue healing, attenuation of inflammatory reactions, and reduction of fibrosis18,19,20. Meanwhile, extracellular vesicles derived from MSCs may inhibit the differentiation of myofibroblasts during the wound healing process21.
On the other hand, TGF-β1, as an inflammatory factor, also has the effect of inducing MSCs to inhibit inflammation22. The histopathological results of this study showed that compared to the TGF group alone, the severity of urethral stricture in the MSC group rats was milder, indicating that MSCs play an important role in the repair of urethral stricture.
The excretion behavior of rats is secretive and highly random, making it impossible to assess the endpoint of model establishment or stratify the severity of strictures based on excretion behavior changes. Moreover, relying on pathological tissue assessment to determine the endpoint of urethral stricture model establishment would require an increase in the number of experimental rats, which contradicts the optimization principle of the “3R” principle of animal welfare23.
Ultrasound, as a technique with good repeatability, convenience of use, and clear and reliable imaging, naturally possesses advantages as a method for determining model endpoints. The high-frequency ultrasound probe used in this study is widely used in clinical practice, eliminating the need to purchase expensive small animal ultrasound equipment and thus saving experimental costs.
The immersion method used in this study effectively removes air from the rat’s fur and increases the distance between the high-frequency ultrasound probe and the scanning site, avoiding near-field interference of the ultrasound beam and further enhancing image clarity. Additionally, the immersion method avoids the process of shaving the rats, reducing physical and emotional harm to the experimental animals, improving their survival rate, and reducing the number of animals used in experiments, thereby adhering to the “3R” principle of animal welfare.
Under high-frequency ultrasound, the normal rat urethra appears as a “three-line pattern” with a central strong echo and slightly weaker edges on the longitudinal axis. The central line represents a thin continuous band formed by the compression of the urethral lumen, while the slightly weaker bands at the edges represent the smooth muscle tissue of the urethral wall. On the short axis, the rat urethra appears as a target-shaped high echo area.
Due to surgical injury affecting the urethral wall, the urethral strong echo bands in the surgical group rats showed continuous changes. After postoperative scar repair, the proliferation of collagen fibers replaced the original smooth muscle tissue, leading to urethral lumen and surrounding tissue contraction, resulting in uneven distribution of echo intensity and changes in band width on ultrasound images. There was a statistically significant difference in urethral echo band width between the control group and the surgical group measured by ultrasound. The repair degree of urethral injury in the MSC group rats was higher than that in the TGF group, as reflected in the histopathological examination in this study.
High-frequency ultrasound, due to its high spatial resolution, can correlate changes in echo intensity and form with changes in tissue structure, thus distinguishing between urethral changes in the MSC group and the TGF group. There was a significant statistical difference in the width of the urethral strong echo bands between the two groups of rats, and the corresponding evaluation scores also revealed differences in urethral echo band form changes between the two groups.
The results of this study confirm that high-frequency ultrasound combined with the immersion method has a good imaging evaluation effect in establishing rat urethral stricture models and has good judgment effects on the endpoint of model establishment and the degree of stricture among different groups. However, the sample size in this study was small, which may have biased the determination of scoring thresholds between groups.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due the funding agency’s internal requirements for data management but are available from the corresponding author on reasonable request. Any requests for data must specify the required information in detail and state the purpose for which the data will be used.
References
Xu, N. et al. Fasudil inhibits proliferation and collagen synthesis and induces apoptosis of human fibroblasts derived from urethral scar via the Rho/ROCK signaling pathway [J]. Am. J. Transl Res. 9 (3), 1317–1325 (2017).
Liang, Y. C. et al. TNF-alpha-induced exosomal miR-146a mediates mesenchymal stem cell-dependent suppression of urethral stricture [J]. J. Cell. Physiol. 234 (12), 23243–23255 (2019).
Castiglione, F. et al. Intratunical injection of human adipose tissue-derived stem cells prevents fibrosis and is associated with improved erectile function in a rat model of Peyronie’s disease. Eur. Urol. 63, 551–560 (2013).
Rosenbaum, C. M. et al. Urethral stricture after radiation therapy [J]. Urologe A. 56 (3), 306–312 (2017).
Mundy, A. R. & Andrich, D. E. Urethral strictures [J]. BJU Int. 107 (1), 6–26 (2011).
Karl, H. et al. A systematic review and Meta-analysis of adjuncts to minimally invasive treatment of Urethral. Stricture Men [J] Eur. Urol. 80, 0 (2021).
Beatriz, G. et al. Surgical Treatment for recurrent Bulbar Urethral stricture: a Randomised Open-label superiority trial of Open Urethroplasty Versus Endoscopic Urethrotomy (the OPEN Trial).[J].Eur Urol, 78: 0. (2020).
Castiglione, F. et al. Adipose-derived stem cells counteract urethral stricture formation in rats [J]. Eur. Urol. 70 (6), 1032–1041 (2016).
Fossati, N. et al. The Surgical Learning curve for one-stage Anterior Urethroplasty: a prospective single-surgeon study [J]. Eur. Urol. 69 (4), 686–690 (2016).
Filippo, P. et al. Regenerative and engineered options for urethroplasty[. J] Nat. Rev. Urol. 16, 0 (2019).
Wynn, T. A. & Ramalingam, T. R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease [J]. Nat. Med. 18 (7), 1028–1040 (2012).
Sangkum, P. et al. Transforming growth Factor-beta1 Induced Urethral Fibrosis in a rat model [J]. J. Urol. 194 (3), 820–827 (2015).
Alfarano, C. et al. Intraparenchymal injection of bone marrow mesenchymal stem cells reduces kidney fibrosis after ischemia-reperfusion in cyclosporine-immunosuppressed rats [J]. Cell. Transpl. 21 (9), 2009–2019 (2012).
Broekman, W. et al. TNF-alpha and IL-1beta-activated human mesenchymal stromal cells increase airway epithelial wound healing in vitro via activation of the epidermal growth factor receptor [J]. Respir Res., 173. (2016).
Wu, Y. et al. Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice [J]. Int. Wound J. 11 (6), 701–710 (2014).
Dittmer, J. & Leyh, B. Paracrine effects of stem cells in wound healing and cancer progression (review) [J]. Int. J. Oncol. 44 (6), 1789–1798 (2014).
Luo, J. et al. Bone marrow mesenchymal stem cells reduce ureteral stricture formation in a rat model via the paracrine effect of extracellular vesicles [J]. J. Cell. Mol. Med. 22 (9), 4449–4459 (2018).
Li, T. et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis [J]. Stem Cells Dev. 22 (6), 845–854 (2013).
Cervio, E. et al. Exosomes for Intramyocardial Intercellular Communication [J]. Stem Cells Int., 2015482171. (2015).
Sole, C. et al. miR-29c in urinary exosomes as predictor of early renal fibrosis in lupus nephritis [J]. Nephrol. Dial Transpl. 30 (9), 1488–1496 (2015).
Fang, S. et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal MicroRNAs suppress myofibroblast differentiation by inhibiting the transforming growth Factor-beta/SMAD2 pathway during Wound Healing [J]. Stem Cells Transl Med. 5 (10), 1425–1439 (2016).
Putra, A. et al. The role of TNF-alpha induced MSCs on suppressive inflammation by increasing TGF-beta and IL-10 [J]. Open. Access. Maced J. Med. Sci. 6 (10), 1779–1783 (2018).
Russell, W. M. The progress of humane experimental technique [J]. Altern. Lab. Anim. 27 (6), 913–924 (1999).
Funding
This research was supported by the following fund: Fujian Provincial Health Technology Project (Grant number: 2020GGB032); Provincial subsidy fund for health and wellness from Fujian Provincial Department of Finance (Grant number: BPB-2022YXJ).
Author information
Authors and Affiliations
Contributions
Y.C was responsible for the animal surgery part; X.Y Responsible for experimental design and data analysis, writing; X.L is responsible for picture editing; R.X and J.Z were responsible for image acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our experiment has been reviewed and approved by the Experimental Animal Ethics Committee of Fujian Medical University. The specific methods of implementation during the experimental process strictly adhere to the guidelines for the use of animals. A scanned copy of the ethics review approval document has been uploaded to the submission website.
Competing interests
The authors declare no competing interests.
Ethical standards
We confirm that this study is reported in accordance with the ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ye, X., Lin, X., Chen, Y. et al. The role of ultrasound combined with water bath in the establishment of animal models of rat urethral stricture. Sci Rep 15, 3035 (2025). https://doi.org/10.1038/s41598-025-87511-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87511-9