Abstract
Very recently, we creatively put forward a new classification for ACLF patients, which lays the foundation for the establishment of prognostic model that can accurately predict the prognosis of ACLF patients. Herein, we found: galectin-3 levels were higher in type A ACLF patients compared to those of type B patients; galectin-3 expression was closely correlated with TBil, PTA/INR and MELD; galectin-3 is an independent predictive factor for rapid progression in ACLF, and exhibited superior predictive value for the prognosis of type A ACLF patients than MELD score; and the survival rate was remarkably higher in ACLF patients with lower galectin-3 expression. Collectively, galectin-3 can be considered as a non-invasive biomarker to predict the prognosis of ACLF patients with new typing. Our findings help advance the time window of prognosis prediction for type A and type B ACLF patients from 4 weeks to the baseline, thereby identifying ACLF patients who really need liver transplantation earlier and improving the survival of ACLF patients.
Similar content being viewed by others
Introduction
Acute-on-chronic liver failure (ACLF) refers to acute decompensation occurring in the setting of chronic liver diseases, such as chronic hepatitis, fibrosis or cirrhosis. The typical clinical manifestations commonly present in ACLF patients include jaundice, ascites, coagulation dysfunction, hepatorenal syndrome, and hepatic encephalopathy1,2. ACLF patients are often accompanied by multiple organ failure with rapid progression and high short-term mortality (50-70%)3. Liver transplantation is the only radical treatment for ACLF patient, however, it is faced with the challenge of severe shortage of liver source4. Therefore, how to screen patients for liver transplantation and determine the timing of transplantation is very important. For patients with ACLF, it is pivotal to determine which patients need liver transplantation early and which patients can wait for liver cell regeneration. The accurate judgment of the timing of liver transplantation is also very critical, because the implementation of liver transplantation too early or too late will lead to the waste of liver source or reduce the efficacy of liver transplantation. In view of this, early and accurate prediction of the prognosis of ACLF patients is of great significance for rational formulation of hierarchical diagnosis and treatment strategies (including whether to undergo liver transplantation) and improvement of the survival rate of patients.
What calls for special attention is that ACLF represents an extraordinarily dynamic syndrome, and the clinical course patterns of ACLF patients can be classified into the followings: resolution or improvement, steady or fluctuating, and worsening. Remarkably, it has been reported that the resolution occurs in 42.5% of ACLF patients5. In this regard, early and accurate identification of ACLF patients who are likely to recover can avoid unnecessary liver transplantation and lifelong immunosuppressive therapy.
Currently, a variety of scoring systems have been used to assess the severity of ACLF patients and judge the prognosis of ACLF patients6,7,8. Nevertheless, there are still divergence in the definition and diagnosis of ACLF in the domestic and overseas, which brings about the diversity and disagreement in the scoring systems for the prognosis of ACLF patients. Importantly, the disease evolution of ACLF is a dynamic process5, which means that the prognosis of patients should be judged based on the dynamic changes of key clinical and biochemical indicators and the clinical outcome of patients should be taken into account. Unfortunately, most scoring systems utilize 28d or 90d mortality as an end-point index for ACLF patients, and are developed based on the baseline clinical and laboratory indicators at diagnosis (single time-point), which cannot reflect the dynamic course of ACLF.
Very recently, we innovatively put forward a new dynamic classification for ACLF patients based on a multicenter, large-scale, retrospective study9. According to this new typing, ACLF patients was stratified based on the dynamic changes of bilirubin and coagulation function indicators. Especially, the novel classification took into account the relationship between the disease progression and the prognosis, and included the prognosis into the classification. ACLF patients can be classified into five types in the light of this novel criteria: type A (rapid progression), type B (rapid recovery), type C (slow progression), type D (slow recovery), and type E (slow persistence). This dynamic typing has been validated in a prospective cohort (unpublished data). Our new typing will aid in the development of more practical predictive scoring models, thereby laying the foundation for making appropriate treatment strategies. In this setting, screening out the indicators that can accurately predict the prognosis of ACLF patients with new typing at the early stage has important clinical significance for the treatment of ACLF patients, especially whether to undergo liver transplantation.
Galectin-3 (Gal-3) is a member of galectin family, which are small proteins that play a variety of functions by interacting with glycoproteins and glycolipids10. Galectin-3 has been confirmed to hold a pivotal place in the pathogenesis of multiple human diseases, such as cardiovascular diseases11,12, nervous system diseases13,14, metabolic diseases15, and liver diseases16. Most recently, we have demonstrated that galectin-3 critically mediates the hepatoprotection conferred by M2-like macrophages in ACLF through inhibiting pyroptosis but not necroptosis signaling17. Nevertheless, whether galectin-3 can be used as a prognostic predictor in ACLF patients with new typing remains to be confirmed.
To clarify this issue, we first measured the expression of galectin-3 in ACLF patients with different types; then, we analyzed the correlation between galectin-3 and the indexes reflecting disease severity of ACLF patients; next, we investigated the predictive power of galectin-3 for type A ACLF patients; finally, we compared the survival rates between ACLF patients with high or low expression of galectin-3. Our findings will help to predict the prognosis of ACLF patients with new typing at an earlier and more accurate manner.
Materials and methods
Ethics approval
Human studies were reviewed and approved by the Clinical Research Ethics Committee of Beijing YouAn Hospital, Capital Medical University (LL-2021-133-K) according to the Declaration of Helsinki. Informed consent was obtained from all participants.
Study subjects
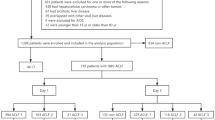
This prospective study consecutively recruited 87 patients with ACLF who were admitted to the Fourth Department of Beijing YouAn Hospital, Capital Medical University from September 2020 to June 2022. The diagnosis of ACLF was made according to APASL recommendation18. Subjects fulfilled the following criteria were enrolled: acute decompensation of liver function in the setting of chronic liver disease (namely, chronic hepatitis, compensated or decompensated cirrhosis), manifested by jaundice and coagulation dysfunction, and complicated within 4 weeks by ascites and/or encephalopathy. (1) rapid progression of jaundice, serum total bilirubin (TBil) ≥ 5 mg/dl or 85µmol/L; (2) coagulation dysfunction, prothrombin activity (PTA) ≤ 40%, or international normalized ratio (INR) ≥ 1.5. We excluded the following subjects: (1) liver cancer or other malignant tumors; (2) chronic obstructive pulmonary disease, severe pneumonia, and other severe respiratory diseases; (3) coronary heart disease, cardiomyopathy, and other serious cardiovascular diseases; (4) metabolic diseases with serious complications such as diabetes and hyperthyroidism; (5) severe hematological diseases; (6) chronic kidney disease with renal failure; and (7) pregnancy.
Data collection
The demographic and clinical information of enrolled subjects were collected at admission. Laboratory data were gathered prospectively at admission and during the followed-up period (3d, 1w, 2w, 3w, 4w, and before liver transplantation). Data assessed in this study were detailed in Table 1. Model for end-stage liver disease (MELD) score was calculated according to the following equation: MELD = 3.78×ln[TBil (mg/dl)] + 11.2×ln(INR) + 9.57×ln[Cr (mg/dl)] + 6.43.
Sample processing and laboratory test
Blood samples were centrifuged at 2,000 rpm for 15 min, and the plasma was harvested and stored at -80℃ until analysis. All laboratory parameters were measured using standard automated methods and commercially available kits according to the manufacturers’ protocols.
Enzyme-linked immunosorbent assay (ELISA)
The plasma levels of galectin-3 were measured using the commercial Human galectin-3 Quantikine ELISA Kit (R&D) according to the manufacturers’ instructions.
Statistics
Continuous variables were expressed as mean ± standard error of the mean (SEM) or median (Min, Max), and categorical variables were expressed as frequencies or percentages. Comparisons between the two variables were carried out using the student t-test or Mann-Whitney test for continuous variables and the Chi-Squared test or Fisher exact test for categorical variables. Comparisons among multiple variables were carried out using ANOVA followed by Tukey’s multiple comparison test. Linear correlation analysis (Pearson correlation analysis) was used to analyze the correlation between the two continuous variables. The diagnostic value of plasma biomarker was evaluated by the receiver operating characteristic (ROC) curve and area under the curve (AUC). The Kaplan-Meier survival curve was used to compare the outcomes of patients between the two groups. SPSS 22.0 and GraphPad Prism 9 software were used for statistical analysis and mapping of data. P < 0.05 was considered to be statistically significant.
Results
Characteristics of the study subjects
Table 1 listed the information on the demographic and clinical characteristics as well as laboratory data of the study cohort. The majority (87.4%) of ACLF patients were male, HBV infection was the most common (57.5%) etiology of ACLF patients. Nearly half of all patients developed compensated cirrhosis. Eighty-seven patients were divided into five types according to our new typing, namely, type A (rapid progression), 22(25.3%); type B (rapid recovery), 36(41.4%); type C (slow progression), 11(12.6%); type D (slow recovery), 9(10.3%); and type E (slow persistence), 9(10.3%). Type A and type B patients accounted for two-thirds of all ACLF patients. In view of the rapid change in clinical course of type A and type B ACLF patients, it is urgently needed for these two types of ACLF patients to make treatment decisions quickly. Therefore, we enrolled only these two types of ACLF patients in subsequent tests and analyses.
Higher levels of galectin-3 are noticed in type A ACLF patients
We first detected and compared the plasma levels of galectin-3 between type A and type B ACLF patients. According to our data, the plasma concentrations of galectin-3 were significantly higher in type A patients than those in type B patients [18.59 (13.59 ~ 23.74) vs. 10.43 (6.96 ~ 14.55) ng/ml] (p = 0.0004). In addition, we analyzed the galectin-3 expression in subgroups classified by different etiology and cirrhosis status. And no significant difference was found in the levels of galectin-3 between ACLF patients with different etiology or cirrhosis status. Therefore, type A ACLF patients exhibit higher levels of galectin-3 (Fig. 1).
Galectin-3 expression is correlated with disease severity of ACLF patients
Next, we analyzed the correlation between galectin-3 and TBil, PTA/INR or MELD, these indicators have been utilized to assess the disease severity and classify ACLF patients. As shown in Fig. 2, galectin-3 levels manifested positive correlations with TBil (r = 0.444, p < 0.001), INR (r = 0.381, p = 0.003) and MELD score (r = 0.523, p < 0.001), but were negatively correlated with PTA (r=-0.31, p = 0.018), suggesting that galectin-3 expression may be closely related to the disease severity of ACLF patients.
Galectin-3 predicts the rapid progression in ACLF patients
We then conducted Cox univariate and multivariate analysis to screen out the predictive factors for rapid progression in ACLF patients. According to univariate analysis, TBil, PTA, INR, Na, WBC, PLT, galectin-3 and MELD were associated with rapid progression in ACLF patients (Table 2). Multivariate analysis showed that only galectin-3 was associated with rapid progression in ACLF patients [HR:1.05 (1.00-1.10), p = 0.0368] (Table 2). These findings suggest that galectin-3 is an independent factor for predicting the rapid progression in ACLF.
We also evaluated the probability of galectin-3 to predict the occurrence of type A (rapid progression) ACLF by ROC curve analysis. As shown in Table 3; Fig. 3, galectin-3 had the ability to predict the rapid progression in ACLF patients with the AUC value of 0.792. Subsequently, we calculated the Youden index, and identified the optimal cut-off of 16.0 ng/ml for predicting the occurrence of type A ACLF. Of note, galectin-3 exhibited a better prognostic efficacy for the occurrence of type A ACLF than MELD score (AUC 0.792 vs. 0.697) (Table 3). Hence, galectin-3 can be utilized to predict the occurrence of type A ACLF.
ACLF patients with higher expression of galectin-3 exhibit poorer survival
Finally, we evaluated the capability of galectin-3 to distinguish survival or death in ACLF patients (type A and type B). To this end, ACLF patients were divided into two groups based on the cut-off of galectin-3: high expression (≥ 16.0 ng/ml) and low expression (< 16.0 ng/ml), and Kaplan-Meier survival analysis was performed. As manifested in Fig. 4, the survival rate was evidently lower in ACLF patients with higher expression of galectin-3 (p < 0.001). Consequently, galectin-3 can be utilized to distinguish survival and death in type A and type B ACLF patients.
Discussion
In the present work, we evaluated the potential of galectin-3 for predicting the prognosis of ACLF patients classified by new typing. And we found: (1) galectin-3 levels are higher in type A ACLF patients compared to those of type B ACLF patients; (2) the expression of galectin-3 is closely correlated with TBil, PTA/INR and MELD score; (3) galectin-3 is an independent predictive factor for rapid progression in ACLF; (4) galectin-3 exhibits superior predictive value for the occurrence of type A ACLF patients than MELD score; and (5) the survival rate is remarkably higher in ACLF patients with lower galectin-3 expression. Collectively, galectin-3 can be considered as a non-invasive biomarker to predict the prognosis of ACLF patients classified by new typing. As far as our information goes, this is the first work to evaluate the potential of galectin-3 for predicting the prognosis of ACLF patients with new typing.
In the last decade, various international consortia have defined ACLF differently, depending on the nature of acute insult, the stage of the underlying chronic liver disease (CLD), and the resulting organ failures (OF)18,19,20,21,22. Nevertheless, there is no consensus on the best definition for ACLF23. In this setting, diverse scoring systems have been developed to assess the prognosis of ACLF patients, such as chronic liver failure-sequential organ failure assessment (CLIF-SOFA) 8, APASL ACLF Research Consortium (AARC) score24, and COSSH-ACLF score25. Unfortunately, there is no universally accepted scoring standard to assess the prognosis of ACLF patients. Especially, most of the scoring criteria utilized the 28-day or 90-day mortality of patients as evaluation indicator, and were made based on the baseline clinical indicators at the time of diagnosis of ACLF. Remarkably, ACLF has a dynamic course of disease, and the prognosis of patients can be divided into resolved/improved, steady or fluctuating, and worsening5. Thus, the ideal scoring system should be able to reflect the dynamic nature of the disease and the responsiveness to medical treatment26.
In recent years, the application of dynamic scoring model in the prognosis assessment of ACLF has attracted the attention of researchers24,27. However, a single-point outcome (death or liver transplantation) was still used as a prognostic variable. EASL-ACLF has assessed the changes of ACLF grading at the different time points (day 1, 3, 7, 28) 5, however, the diagnostic criteria of ACLF in the East and the West are different, so the reference significance is limited. APASL-ACLF believes the disease course of ACLF is reversible, and recovering patients exhibit improvements in coagulation and jaundice without hepatic encephalopathy18. Nevertheless, a dynamic pattern of disease evolution has not been reported. Therefore, there is an urgent need to establish a new clinical classification that can accurately reflect the dynamic outcome of ACLF patients at an early stage. Timely and dynamic assessment on ACLF patients is essential to avoid ineffective treatment and rational selection of liver transplantation.
Very recently, Chen Y and China Network for severe liver diseases (CNSLD) innovatively put forward a new clinical classification for ACLF patients9. The new classification is made based on clinical outcome (death or transplantation), time axis (4 weeks and 12 weeks), and dynamic indicators (PTA recovered to more than 40%, TBil declined to 50% of the peak). According to our new criteria, ACLF can be divided into five types: type A, rapid progression; type B, rapid recovery; type C, slow progression; type D, slow recovery; and type E, slow persistence. This classification is relatively simple and easy to calculate, so it is more practical. Our criteria are helpful to judge the prognosis of ACLF patients and make decision on liver transplantation. Furthermore, it facilitates the rational allocation of donor liver resources, helps to develop better hierarchical diagnosis and treatment strategy and follow-up plan, and lays the foundation for developing a new accurate prognostic model for ACLF patients 9. In view of this, it is of scientific value and clinical significance to screen out the biomarkers which can predict the prognosis of ACLF patients with this new typing early and accurately.
As mentioned in Introduction, galectin-3 has been documented to critically involve in the development of multiple human diseases28,29,30. Especially, galectin-3 has been reported to be a biomarker for predicting the prognosis of diverse diseases. For instance, high expressed galectin-3 is positively correlated with disease progression of pancreatic cancer, indicating that galectin-3 can be employed as a predictor of poor prognosis31. Conversely, low expression of galectin-3 predicts poor response to chemotherapy and poor survival in node-positive breast cancers32. Our newly published paper has reported that galectin-3 holds a pivotal place in the hepatoprotection conferred by M2-like macrophages in ACLF 17. Nevertheless, the predictive efficacy of galectin-3 on the prognosis of ACLF patients has not been clarified. In light of the fact that the novel typing proposed by our team is helpful to establish an accurate prognostic model for ACLF patients, it is worthwhile to evaluate the predictive power of galectin-3 for the prognosis of ACLF patients with new typing.
The clinical and laboratory information from 87 ACLF patients was assessed. As shown in Table 1, Type A and type B patients account for two-thirds of all ACLF patients. Especially, both type A and type B patients are characterized by a rapid change in disease course (rapid progression or rapid recovery), which requires clinicians to make the treatment decision quickly and accurately. Thus, these two types of ACLF patients were subjected to detection and analysis in this work.
According to our data, the plasma levels of galectin-3 were higher in type A ACLF patients compared to those of type B patients. To our knowledge, this is the first time to assess the expression of galectin-3 in plasma from ACLF patients with new typing. Our finding is similar to the report by Zheng et al. who demonstrated that serum galectin-3 levels are significantly higher in patients with liver failure compared to those in CHB patients and healthy controls33. On the other hand, a recent study showed that galectin-3 mRNA levels were lower in PBMCs from ACLF patients compared to those in CHB patients and healthy controls, namely, lower galectin-3 expression suggests ACLF with poor survival34. This finding is consistent with our latest report, namely, galectin-3 expression is lower in the liver of acutely injured mice compared to that of ACLF mice 17. Considering that we have demonstrated the mortality is higher in acutely injured mice compared to that of ACLF mice, the above-mentioned result indicates that lower galectin-3 levels in the liver correspond to poor survival. Thus, the expression of galectin-3 in PBMCs/liver tissues and serum/plasma may be reversed in ACLF patients. We speculated it may be attributed to the different distribution of galectin-3 between the liver and serum/plasma in diverse damage degree. Galectin-3 expression is elevated in response to hepatic injury. When the morphology of liver cells (e.g. M2-like macrophages) is intact, the synthesized galectin-3 cannot be released into the blood, thus leading to low serum/plasma levels of galectin-335. When massive liver cell necrosis occurs, a large amount of galectin-3 is released into blood, therefore the levels of galectin-3 in serum will be elevated.
We then analyzed the correlation between the levels of galectin-3 and indicators including TBil, PTA/INR and MELD score which reflect the disease severity of ACLF and are utilized to classify ACLF patients according to new criteria. The correlation analysis manifested that the expression of galectin-3 is positively correlated with TBil levels, INR value and MELD score. Conversely, galectin-3 levels exhibit a negative correlation with PTA value. Our finding is partially consistent with the report by Yoeli et al. who found a positive correlation between galectin-3 expression and fibrosis score, TBil, ALT, AST, and APRI score, as well as a negative correlation between galectin-3 levels and albumin in patients with biliary atresia36.
Next, we evaluated the predictive power of galectin-3 for type A or type B ACLF patients. After univariate analysis, we selected galectin-3, MELD, PTA, INR and TBil to conduct multivariate analysis in view of their clinical relevance. And only galectin-3 is significantly associated with rapid progression in ACLF, showing that galectin-3 is an independent predictive factor for the prognosis of ACLF patients. In addition, ROC curve analysis manifested that galectin-3 possesses better predictive power than the MELD score in distinguishing between type A and type B ACLF patients. Therefore, galectin-3 can be considered as a non-invasive biomarker to predict the prognosis of ACLF patients.
We then classified ACLF patients into high-expression and low-expression groups according to the cut-off of galectin-3 and conducted the 4-week survival analysis. According to our data, patients with lower expression of galectin-3 (lower than the cut-off of galectin-3) exhibit better survival than those with higher expression of galectin-3 (equal to or higher than the cut-off of galectin-3). Therefore, ACLF patients with higher plasma expression of galectin-3 may have a poorer prognosis. This finding is similar to a previous report that showed that the dead subjects with liver failure exhibit remarkably higher levels of galectin-3 than the surviving patients33. On the contrary, another work by Zhao et al. reported that galectin-3 mRNA content is higher in survivors than in non-survivors. And a positive correlation was noticed between galectin-3 mRNA level and the survival time in ACLF patients34. In our latest work, we documented that galectin-3 levels in the liver of ACLF mice are higher than those in ALF mice (the latter has higher mortality)17. This result is consistent with Zhao’s report considering the reverse correlation between the expression of galectin-3 in PBMCs/liver tissues and serum/plasma as we discussed previously.
In sum, galectin-3 can be considered as a non-invasive marker to predict the prognosis of ACLF patients with new typing, and patients with high expression of galectin-3 are more likely to develop type A ACLF and exhibit poor survival and prognosis. Our findings help advance the time window of prognosis prediction for type A and type B ACLF patients from 4 weeks to the baseline, thereby identifying ACLF patients who really need liver transplantation earlier, guiding clinicians to develop more accurate individualized diagnosis and treatment strategies, optimizing the utilization of medical resources, and improving the survival rate of ACLF patients.
Limitations of the study
There are some limitations in our study. Our data lack a validation set, and we plan to further validate the predictive power of plasma galectin-3 for the prognosis of ACLF patients with new typing in the future. A second limitation of the study is the relatively small sample size.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Kulkarni, A. V. & Sarin, S. K. Acute-on-chronic liver failure - steps towards harmonization of the definition! J. Hepatol. (2024).
Bajaj, J. S. et al. Acute-on-chronic liver failure clinical guidelines. Am. J. Gastroenterol. 117, 225–252 (2022).
Jalan, R., Moreau, R. & Arroyo, V. Acute-on-chronic liver failure. Reply. N. Engl. J. Med. 383, 893–894 (2020).
Ogura, Y., Kabacam, G., Singhal, A. & Moon, D. B. The role of living donor liver transplantation for acute liver failure. Int. J. Surg. 82S, 145–148 (2020).
Gustot, T. et al. Clinical course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology 62, 243–252 (2015).
Ma, K. et al. Entecavir treatment prevents disease progression in hepatitis B virus-related acute-on-chronic liver failure: establishment of a novel logistical regression model. Hepatol. Int. 6, 735–743 (2012).
Kim, W. R. et al. MELD 3.0: The Model for End-Stage Liver Disease Updated for the Modern Era. Gastroenterology 161, 1887–1895 e1884 (2021).
Jalan, R. et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J. Hepatol. 61, 1038–1047 (2014).
Xu, M. M. et al. Clinical course and outcome patterns of Acute-on-chronic liver failure: a Multicenter Retrospective Cohort Study. J. Clin. Transl. Hepatol. 9, 626–634 (2021).
Garcia-Revilla, J. et al. Galectin-3, a rising star in modulating microglia activation under conditions of neurodegeneration. Cell. Death Dis. 13, 628 (2022).
Suthahar, N. et al. Galectin-3 activation and inhibition in Heart failure and Cardiovascular Disease: an update. Theranostics 8, 593–609 (2018).
Chen, Y. et al. Galectin 3 enhances platelet aggregation and thrombosis via Dectin-1 activation: a translational study. Eur. Heart J. 43, 3556–3574 (2022).
Garcia-Revilla, J. et al. Galectin-3 shapes toxic alpha-synuclein strains in Parkinson’s disease. Acta Neuropathol. 146, 51–75 (2023).
Soares, L. C. et al. Novel Galectin-3 Roles in Neurogenesis, Inflammation and Neurological Diseases. Cells 10 (2021).
Li, Y., Li, T., Zhou, Z. & Xiao, Y. Emerging roles of Galectin-3 in diabetes and diabetes complications: a snapshot. Rev. Endocr. Metab. Disord. 23, 569–577 (2022).
Mackinnon, A. C., Tonev, D., Jacoby, B., Pinzani, M. & Slack, R. J. Galectin-3: therapeutic targeting in liver disease. Expert Opin. Ther. Targets. 27, 779–791 (2023).
Bai, L. et al. Galectin-3 critically mediates the hepatoprotection conferred by M2-like macrophages in ACLF by inhibiting pyroptosis but not necroptosis signalling. Cell. Death Dis. 13, 775 (2022).
Sarin, S. K. et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol. Int. 13, 353–390 (2019).
European Association for the Study of the Liver. Electronic address eee and European Association for the study of the L. EASL Clinical Practice guidelines on acute-on-chronic liver failure. J. Hepatol. 79, 461–491 (2023).
Bajaj, J. S. et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology 60, 250–256 (2014).
Wu, T. et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut 67, 2181–2191 (2018).
Aggarwal, A. et al. Definitions, etiologies, and outcomes of Acute on Chronic Liver failure: a systematic review and Meta-analysis. Clin. Gastroenterol. Hepatol. (2024).
Li, F. & Thuluvath, P. J. EASL-CLIF criteria outperform NACSELD criteria for diagnosis and prognostication in ACLF. J. Hepatol. 75, 1096–1103 (2021).
Choudhury, A. et al. Liver failure determines the outcome in patients of acute-on-chronic liver failure (ACLF): comparison of APASL ACLF research consortium (AARC) and CLIF-SOFA models. Hepatol. Int. 11, 461–471 (2017).
Li, J. et al. Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure. J. Hepatol. 75, 1104–1115 (2021).
Hernaez, R., Sola, E., Moreau, R. & Gines, P. Acute-on-chronic liver failure: an update. Gut 66, 541–553 (2017).
Ha, J. M. et al. Static and dynamic prognostic factors for hepatitis-B-related acute-on-chronic liver failure. Clin. Mol. Hepatol. 21, 232–241 (2015).
Dong, R. et al. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (review). Int. J. Mol. Med. 41, 599–614 (2018).
Bouffette, S., Botez, I. & De Ceuninck, F. Targeting galectin-3 in inflammatory and fibrotic diseases. Trends Pharmacol. Sci. 44, 519–531 (2023).
Cao, Z. Q., Yu, X. & Leng, P. Research progress on the role of gal-3 in cardio/cerebrovascular diseases. Biomed. Pharmacother. 133, 111066 (2021).
Yao, Y. et al. HH1-1, a novel Galectin-3 inhibitor, exerts anti-pancreatic cancer activity by blocking Galectin-3/EGFR/AKT/FOXO3 signaling pathway. Carbohydr. Polym. 204, 111–123 (2019).
Ilmer, M. et al. Low expression of galectin-3 is associated with poor survival in node-positive breast cancers and mesenchymal phenotype in breast cancer stem cells. Breast Cancer Res. 18, 97 (2016).
Zheng, Y. et al. [Relationship between serum galectin-3 levels and mortality of subacute on chronic liver failure]. Zhonghua Gan Zang Bing Za Zhi 22, 295–298 (2014).
Zhao, J. et al. Hypermethylation of the galectin-3 promoter is associated with poor prognosis of acute-on-chronic hepatitis B liver failure. Dig. Liver Dis. 49, 664–671 (2017).
Zhang, X. et al. The different expression of caspase-1 in HBV-related liver disease and acts as a biomarker for acute-on-chronic liver failure. BMC Gastroenterol. 19, 148 (2019).
Yoeli, D. et al. 431 Galectin-3 as a biomarker and potential therapeutic target in biliary atresia. J. Clin. Transl. Sci. 6, 84–84 (2022).
Acknowledgements
This study was supported by Beijing Municipal Natural Science Foundation (7222094, 7232081); National Key Research and Development Program of China (2022YFC2304400); Beijing Hospitals Authority’s Ascent Plan (DFL20221501); Construction Project of High-level Technology Talents in Public Health (Discipline leader − 01–12); Beijing You’an Hospital Construction of Talent Pool Program (YARCKC2024001);Scientific Research Project of Beijing Youan Hospital, CCMU, 2024 (BJYAYY-YN2024-10);and Open project of Beijing Municipal Key Laboratory of Liver Failure and Artificial Liver Treatment Research (BJYAHKF2023004).
Author information
Authors and Affiliations
Contributions
Z.D. and Y.C. designed and supervised the experiments. L.B., W.L., and Q.Y. performed the ELISA test. X.L. performed the statistical analysis. W.L., Q.Y. and X.L. harvested the plasma samples of the patients and collected the basic information of the patients. L.B. wrote the manuscript. Z.D. and Y.C. polished the manuscript, and provided the funding support. All of authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bai, L., Lu, W., Yang, Q. et al. Plasma galectin-3 can be considered as a non-invasive marker to predict the prognosis of ACLF patients with new typing. Sci Rep 15, 3916 (2025). https://doi.org/10.1038/s41598-025-87557-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87557-9