Abstract
One of the popular subjects of millennia is the synthesis of nanostructures, their applications in numerous fields, and their interaction with various biological systems, making them appealing for drug delivery systems, and diagnostic and therapeutic agents. In this study, silver oxide nanoparticles were synthesized using E. sativa (ES) aqueous extract. The biosynthesis was followed via UV-vis spectroscopy by analysis, FTIR, XRD, TEM, and Zeta potential to further analyze the synthesized nanoparticles. Furthermore, the biosynthesized silver oxide nanoparticles (Ag2ONPs) were checked through various biological activities. The antioxidative potential was assessed by performing a DPPH radical scavenging assay, total reducing power assay, and total antioxidant capacity assay. Antimicrobial potential was observed against various bacterial and fungal strains. Likewise, Artemia salina (brine shrimps) was used to study cytotoxicity, while VERO and HEK-293 cell lines were applied to check the biocompatibility of synthesized NPs. Anticancer potential was evaluated against the Hep-2 cells by utilizing an MTT assay. A mean crystallite ~ 50 nm size is evidenced by TEM analysis. Notable antimicrobial activity was detected with various bacterial and fungal strains with maximum ZOI by B. subtilis was 18.5 ± 2.36 mm at 1000 µg/mL and A. niger reveals a minimum ZOP of 16 ± 1.7 mm at 1000 µg/mL respectively. A dose-dependent response was observed in biological evaluation against A. salina (LC50: 12.21 µg/mL), DPPH (IC50: 62.36 µg/mL), VERO cell line (IC50: 43.11 µg/mL), HEK-293 cell line (IC50: 26.56 µg/mL), and Hep-2 cell line (IC50: 9.97 µg/mL). The multifaceted attributes of ES-Ag2ONPs encompassing antimicrobial, antioxidant, cytotoxic, and anticancer properties render them a versatile platform in drug delivery and biomedical horizons. However, detailed investigation and clinical trials will undoubtedly provide translational applications in diverse fields.
Similar content being viewed by others
Introduction
One of the popular subjects of the millennia is the synthesis of nanostructures and their applications in numerous fields, such as biomedical sciences target-specific imaging, and drug delivery1,2,3. More specifically, the ability of nanostructures that interact with various biological systems, including cells, fungi, and bacteria, which makes them appealing for use as diagnostic agents, drug delivery systems, and therapeutic agents4. A range of biomedical applications is due to nanoparticles’ distinctive physical and chemical properties5. This comprises their extremely high surface area to volume ratio—consequently, greater reactivity than their greater counterparts. This property makes them ideal for their use in coating, catalysts, and other applications6. Moreover, the higher stability of nanoparticles than larger particles, makes their application more effective in drug delivery and storage7. The synthesis of nanoparticles with the appropriate characteristics for these applications is, anyhow, a complicated task. One of the more promising approaches for nanoparticle fabrication is “green synthesis”8; which comprises the utilization of eco-friendly, and renewable resources. This approach has been gaining more interest in recent decades due to its cost-effectiveness, environmentally friendly nature, and ability to produce nanostructures with unique characteristics without applying hazardous solvents and chemicals9. The development of nanoparticles using green practices is an attractive substitute for traditional techniques that require potentially hazardous and toxic chemicals. In the green synthesis approach, biological sources are involved as capping and reducing agents, resulting in nanoparticles generally being biocompatible and non-toxic, making them suitable entity for a long range of biomedical applications10. The usage of green nanoparticles in the domain of biomedical has several advantages over traditional treatments, as their high surface area to volume ratio allows greater loading of targeting agents11,12, along with their nontoxic and biocompatible nature making them safer for humans and animal systems13. Additionally, their wide range of optical properties make them useful for sensing and imaging applications14. Conclusively, green synthesized nanostructures can be used to target specific tissues or cells, providing a more profound diagnosis, as they can be used to generate high-resolution images of the body’s internal structures15. Furthermore, they can also be used to treat infectious diseases and to monitor the progress of treatments16.
The green synthesis of nanoparticles is a process that produces nanoscale material in a precise and environmentally friendly way17. In this method, renewable resources are used such as plants, vegetables, and fruits, which contain natural phytocompounds as their starting materials4. In this process, the metal ions reduction in organic media or in aqueous media is carried out in several ways, comprising chemical reduction, sonochemical reduction, and electrochemical reduction18. Particles produced by green methods are an effective and biocompatible alternative to the traditional production of nanostructures19. Additionally, introducing required functionalities specific to biomedical applications, surface modification is one of the most important modifications; this is because it alters the interactions at the surface of the nanoparticles to enhance their stability, biocompatibility toward interaction with biological systems. In many instances, functionalization strategies generally involve the attachment of certain specified molecules, polymers, or ligands on the surface of the nanoparticle, besides improving properties such as solubility, target specificity, and reduced cytotoxicity. These modifications are of particular significance in applications such as drug delivery, where functionalized nanoparticles may help in improving efficiencies in drug loading, targeted accuracy, and release mechanisms. Besides, the functionalization can effectively prevent aggregation, hence increasing the colloidal stability of nanoparticles in a biological medium20,21.
Among the major parameters, the physicochemical properties of nanomaterials most critically define their biological interactions, fate, and outcomes. These include shape, size, dissolution rate, agglomeration state, chemical composition, specific surface area, crystal structure, surface morphology, surface energy, surface coating, and surface charge. These properties will determine cellular uptake, biodistribution, and interaction with biomolecules, affecting the efficacy of desired therapeutic effects or possible adverse outcomes22.
Metals react with plant compounds to produce nanoparticles via a process called phytoreduction. Through this process, metal ions are allowed to reduce to their elemental form by the plant compounds, which behave like a reducing agent to produce a stable nanoparticle. The resulting nanoparticle can have numerous properties and compositions based on the nature of the metal used and phytocompounds23,24. In this context, biological methods have shown great promise for the ecologically friendly synthesis of a broad range of metal-based nanoparticles and are a more sustainable or eco-friendly approach than conventional ones. For instance, organically produced zirconium and zinc oxide nanoparticles showed superior antioxidant potential and enhanced activity, making them suitable for a variety of biomedical uses25,26. Tellurium and selenium nanoparticles that are environmentally safe can be used as antibacterial and cancer treatments27,28. These instances highlight the effectiveness and adaptability of green synthesis techniques in creating bioengineered nanoparticles for application in nanomedicine.
The prospects of bioengineered metallic NPs are enormous in nanomedicine, owing to their harmless synthesis with no hazardous by-product and easily tunable physicochemical properties in target applications such as drug delivery and imaging. However, challenges remain on limited FDA approval, difficulties in the processions of consistent characterization due to nanoscale variability, and barriers in scaling up production for industrial use. Overcoming these challenges by advanced research and optimization opens great perspectives for the application of bioengineered metallic NPs in future medical practices29. In this article, we present a study about the green synthesis of silver oxide nanoparticles through E. sativa aqueous extract, their characterization, and evaluation concerning their potential to combat the various microbes, pathogenic to humans and animals. The synthesized silver nanoparticles were fabricated with environment-friendly resources and their characteristics—such as size, composition, morphology, and surface charges were characterized via various analytical techniques. A sustainable and eco-friendly method is provided by employing E. sativa, which may improve biocompatibility and mitigate the need for hazardous chemicals that are frequently used in other methods of nanoparticle syntheses. Along with its wide range of biological activities, including cytotoxic, antioxidant, antibacterial, and anticancer properties, ES-Ag2ONPs are suitable for a range of applications. This study gives a novel and more comprehensive view of the cytotoxic effects of E. sativa mediated nanoparticles by assessing biocompatibility across different cell lines Hep-2, HEK-293, and VERO. Although the work shows interesting in vitro results, more in vivo research and clinical trials are required to completely confirm the biomedical uses of ES-Ag2ONPs.
Materials and methods
Processing of E. sativa
Fresh green plants of E. sativa were collected from District Bhakkar, Pakistan. The plants were identified by taxonomists (Mr. Amjad Khan, and Prof. Dr. Mushtaq Ahmad), and permission was obtained from the herbarium of the Department of Plant Sciences, Quaid-i-Azam University, Islamabad, Pakistan (Herbarium Accession No. 134106), where the specimens were then submitted. Plant species were washed with fresh water, and subsequently air dried under shade. Later the dried plants were ground, and around 30 g of plant powder was set to boil in 300 mL of ultrapure water at 72 °C for 3–4 h with continuous stirring to prepare the plant extract.
Synthesis of silver oxide nanoparticles (NPs)
Ag2ONPs were synthesized by mixing 3 g of silver nitrate (AgNO3) with 100 mL of E. sativa plant extract. According to a described method, with a few modifications30, the resultant mixture was placed on a hot plate for 3–4 h at 72 °C with continuous stirring, and the pH was adjusted to 10. The change of color shift of the solution directed the creation of Ag2ONPs, which was confirmed by UV-Vis spectroscopy under the range of 300 to 600 nm. The resultant mixture was then gone under centrifugation for 30 min at 10,000 rpm, supernatant was discarded, the obtained pallet was considered silver oxide nanoparticles, which were washed three times with dd H2O, and allowed to dry in an incubator. Figure 1 shows a general layout of the study.
Characterization of synthesized nanoparticles
Various microscopic and spectroscopic techniques were applied to understand the formation and properties of synthesized Ag2ONPs. UV-vis spectrophotometer was used to monitor the concentration of (ES-Ag2ONPs) in the solution, silver nitrate solution was applied as control. The absorbance spectrum was recorded 300–600 nm range. The zeta potential of synthesized Ag2ONPs was measured by the Zetasizer (ZS) Nano Series. To analyze the particles, the wavelength of -663 nm of the red laser was incident at 173° scattering angle, in a viscous medium (0.887 milli pascal), at 25 °C. The zeta potential value enables us to understand the interparticle forces of attraction. The ZP value influences the consistency of a colloidal system. The low density—small particles with high + / ̶ zeta potential repel each other. Moreover, Fourier Transform Infrared Spectroscopy (FTIR) spectroscopy (Alpha, Bruker, Ettlingen, Germany) in the range of 400–4000 cm−1 was used to examine the functional groups that were present in the produced nanoparticles and are thought to be responsible for their reduction and stabilization. X-ray diffraction is a useful technique to evaluate a broad range of structural patterns in a crystalline sample. X-ray Diffraction (XRD) provides insights into the phase, crystallinity, and texture of ES-Ag2ONPs via X-ray beam by observing the diffraction angle from 5° to 80° (2ϴ) Philips X’pert Pro, Panalytical. Using the Debye-Scherrer equation and the full width at half maximum (FWHM) of the face-centered cubic (111), the average size of ES-Ag2ONPs was determined. Moreover, TEM (transmission electron microscopy) was applied to get information about the crystal structure, shape and size of nanoparticles at the single-particle level with high resolution and selectivity. TEM is one of the most efficient methods to characterize the size of nanoparticles, as it provides precise information about the size, shape, and composition of a particle31,32. The synthesized nanoparticles are ready for their further biological evaluations.
Biological applications
Cytotoxicity evaluation of Es-Ag2ONPs against Artemia salina
Artemia salina (Brine shrimps) cyst larvae were used and retained under laboratory conditions to assess the cytotoxicity of biosynthesized ES-Ag2ONPs. Brine shrimp eggs (0.75 g) were held in 200 mL of saline solution (sea salt 20 g/L) inside a hatching tray. Larval hatching was carried out under an incandescent lamp light (40–60 watts), at 27 °C for 24 h, with continuous aeration. After hatching, free-floating active nauplii were picked and used for cytotoxicity analysis. Serially diluted concentrations of ES-Ag2ONPs ranging from 100 –10 µg/mL were used. For each concentration, 10 shrimp larvae were shifted into glass vials containing a 5 mL solution and left for 24 h at 27 °C. Finally, the number of dead and viable larvae was noted, and the LC50 value was determined by GraphPad Prism (USA 8.0.0) software. Dimethyl sulfoxide (DMSO) was used as a negative control33.
Antibacterial potential of ES-Ag2ONPs
The bactericidal potential of the ES-Ag2ONPs was carried out on some bacterial strains. The gram-positive strains including Staphylococcus aureus (ATCC 6538), Lactobacillus acidophilus (ATCC 4356), Enterococcus faecalis (ATCC 29212), and Bacillus subtilis (ATCC 19659) while gram-negative Pseudomonas aeruginosa (ATCC 90271), Klebsiella pneumoniae (ATCC 1705), and Escherichia coli (ATCC 33456) strains were tested. The disc diffusion technique was applied to confirm the antibacterial potential of synthesized nanoparticles. All bacterial strains were evenly spread on agar media plates. Afterward, sterile filter paper discs were embedded with 50 µL of various concentrations of synthesized ES-Ag2ONPs ranging from 1000 to 62.5 µg/mL. For positive control, an antibiotic (oxytetracycline) was applied. The treated plates were incubated at 37 °C, and after 24 h of incubation, the diameter of zones of inhibitions was measured in millimeters through Imag J software34.
Evaluation of antifungal activity
To test the antifungal potential of synthesized ES-Ag2ONPs, aflatoxin-causing fungal strains Aspergillus niger and Aspergillus flavus were used via Sabouraud dextrose liquid media (SDA). The fungal spores were first sub-cultured in SDA media and left for incubation at 37 °C for 48 h, before their fungicidal assay. Different concentrations of synthesized ES-Ag2ONPs (1000 –62.5 µg/mL) were mixed while pouring the autoclaved SDA media into Petri plates. Fungal spores were carefully picked and placed in the center of media plates containing ES-Ag2ONPs. The treated petri plates were incubated for 48–72 h at 37 °C to observe the potential of ES-Ag2ONPs. Mycelial growth was monitored, and their zone of proliferation (ZOP) was noted. For positive control, an antibiotic (ampicillin) was applied while for negative DMSO was used35.
Antioxidant efficiency of ES-Ag2ONPs
To assess the antioxidant capacity of the synthesized ES-Ag2ONPs, three antioxidant analyses were used.
2,2-diphenyl-1-picrylhydrazyl (DPPH)
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay was performed to evaluate the percentage of the scavenging potential of synthesized ES-Ag2ONPs with some minor modifications36. For this purpose, the freshly prepared 180 µL of DPPH (0.004% methanolic acid) was mixed with 20 µL of every concentration of synthesized NPs to achieve a final volume of 200 µL. The resulting mixtures were incubated for 30 min at room temperature in the dark, and the absorbance was measured at 517 nm. For positive control (standard), ascorbic acid (C6H8O6) was used, and methanol (CH3OH) was used as a blank. The percentage of free radical scavenging of DPPH was calculated by using Eq. 1:
Total antioxidant capacity (TAC) (phospho-molybdenum assay)
To check the total antioxidant capacity of synthesized ES-Ag2ONPs, a phospho-molybdenum assay was performed according to the already stated procedure37. In this method, 100 µL of each concentration of ES-Ag2ONPs ranging from 200 to 10 µg/mL mixed with TAC reagent, which was prepared by adding a 0.2 mL solution consisting of sodium phosphate, sulphuric acid (0.6 M NaHPO4, H2SO4, H2O 28mL), added by 4mM ammonium hepamolybdate ((NH4)6Mo7)24, 4H2O) maintained with an acidic pH. The resultant reaction mixture was incubated in the water bath for 90 min at 95 °C. Afterward, the reaction mixture was put at room temperature, and its absorbance was measured at 630 nm with a spectrophotometer. Gallic acid was used as standard in this assay.
Total reducing power
Moreover, a potassium-ferricyanide assay was performed to assess the total reduction power of the synthesized ES-Ag2ONPs38. In this assay, ascorbic acid was applied as a positive control while DMSO was used as a negative control, and the reduction power of samples is illustrated in terms of gram-positive control (ascorbic acid) equivalent per mg of test samples. The experiment involved mixing 100 µL of each concentration with 250 µL of 1% potassium ferricyanide solution and 200 µL of phosphate buffer (0.2 M), then incubating the mixture for 20 min at 50 °C in a water bath. Next, 200 µL TCA (trichloroacetic acid) was added and centrifuged for 10 min at 3000 rpm. Following centrifugation, 150 µL supernatant was picked and mixed well with 50 µL (0.1%) ferric chloride, and absorbance of the resultant was recorded at 630 nm.
Anticancer potential of synthesized ES-Ag2ONPs
Cell culture
Hepatocellular carcinoma cells (Hep-2) (National Institute of Health, Pakistan https://www.nih.org.pk/ ) were added to a cell culture flask (25 cm2) and allowed to grow in Dulbecco’s Modified Eagle’s Medium (15% DMEM) supplemented with heat-inactivated 10% fetal bovine serum (FBS), added by an antibiotic solution of penicillin (1% w/v) and streptomycin 5 mg/mL, and incubated in a humified atmosphere having 5% carbon dioxide (CO2), at 37 °C for 48 h. After the incubation was done, the attached cells were trypsinized about 3–4 times to separate the cells. Afterward, the detached cells were counted and shifted to 96-well Enzyme-Linked Immunosorbent Assay (ELISA) plates with each well containing 104 cells. For 24 h, the plates were incubated at 37 °C with 5% CO2 to enable the cells to reach a monolayer confluence of approximately 80–90%.
Cell treatment with Ag2ONPs
Hep-2 cells were treated with various concentrations of ES-Ag2ONPs (100 µg/mL, 50 µg/mL, 25 µg/mL, 12 µg/mL, and 10 µg/mL) in 96-well plates. For this purpose, different doses of Ag2ONPs were prepared in the same media which was used to grow cell cultures. Again, the plates were incubated in the above-mentioned conditions for 24 h.
(3-(4,5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium Bromide) (MTT) assay
Following the incubation, flicked off media and ES-Ag2ONPs solution as described before, by inverting the plates carefully. MTT solution (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was prepared by adding 4 mg/mL MTT reagent in PBS solution. A volume of 200 µL was added in each culture well and left for 4–5 h of incubation. Post-incubation, the plates were reinverted to remove unconverted MTT in the cells, leaving behind the formazan crystals at the bottom of 96-well plates. After that, 100 µL of DMSO was added to each well to dissolve the formazan crystals by agitating on a plate shaker for 10–15 min. The optical density of the product was measured at 570 nm by a spectrophotometer. The effect of Ag2ONPs was determined by applying Eq. 2.
Biocompatibility potential of ES-Ag2ONPs
The MTT assay was processed to confirm the biocompatible nature of synthesized ES-Ag2ONPs. Two cell lines VERO and HEK-293 were used in this assay. The various concentrations of tested samples were 10, 12, 25, 50, and 100 µg/mL, respectively. Initially, 1 × 104 cells were seeded in each well of the 96-well plate, which already contains 200 µL of DMEM media. The plate was then incubated for 24 h at 37 °C with 5% CO2. Cells were treated with different doses of ES-Ag2ONPs and kept under the same above-mentioned conditions for 24 h. After that, 20 µL of MTT (4 mg/mL) was added to each well and left for 3–4 hours of incubation. The culture media was discarded and 200 µL of DMSO was added and agitated for 10 min. The reading was measured at 570 nm through a spectrophotometer39. The % viability was calculated by using Eq. 3:
Results and discussion
Synthesis of E. sativa silver oxide nanoparticles
Tough nanoparticles can be synthesized via multiple routes; however, the green approach of nanoparticle synthesis is excellent over chemical and physical methods of synthesis. It’s a simple, cost-effective, and environmentally friendly approach with no use of harmful or toxic substances. In chemical and physical methods, expensive and toxic chemicals are applied to synthesize nanoparticles, which also require high temperatures and pressure. These toxic elements occasionally persist to nanoparticle surfaces, and ultimately, their use in biomedical purposes cannot be granted safe. The green synthesis approach is substituting other approaches due to its cost-effectiveness and non-use of hazardous chemicals. Plant extracts are used as reducing and stabilizing agents in the environmentally friendly fabrication of nanoparticles. These biological materials contain phytochemicals such flavonoids, alkaloids, terpenoids, and phenolic compounds that aid in the reduction of metal ions and the stabilization of the ensuing nanoparticles40. Previously, silver nanoparticles have been employed with several medicinal plants41,42. In the current study, the E. sativa plant was used to synthesize silver oxide nanoparticles. E. sativa was selected due to its treasures of multiple chemical compounds flavonoids, glucosinolates, phenolics, tannins, saponins, and essential oils in various parts43. These biomolecules play an essential role in capping, reducing, and stabilizing silver nanoparticles44. Biosynthesis of Ag2ONPs using the E. sativa extract involves reduction of Ag⁺ to Ag⁰, followed by further oxidation to produce Ag₂O nanoparticles. The plant extract can act as a reducing agent and a stabilizing agent due to its bioactive compounds. The major compounds responsible for this extract process include flavonoids, phenolic acids, terpenoids, and alkaloids45. Electron donation by these chemicals brings about the reduction of silver ions. For example, the phenolic groups (-OH) in flavonoids and phenolic acids are easily oxidized in order to donate electrons to Ag⁺ ions, thus leading to the formation of nanoparticles. Furthermore, the proteins and polysaccharides in the extract can also contribute to nanoparticle stabilization by preventing further particle aggregation through surface capping46. The formation of ES-Ag2ONPs was first confirmed by a change in the color shift from light brown to black color. This color shift resulted from optical properties especially due to resonance in the surface plasmon when E. sativa extract was reacted with silver nitrate47. The obtained blackish material which assumed as ES-Ag2ONPs, underwent further physical characterizations.
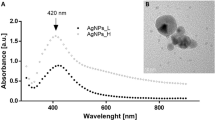
Via the use of an optical UV–Vis spectrophotometer operating at wavelengths between 300 and 600 nm, the reduction of silver ions in the reaction solution was tracked. A spectroscopic measurement of the synthesized ES-Ag2ONPs exposed a symmetric absorption spectrum with a peak maximum at 400–402 nm (Fig. 2). This was a characteristic peak for the confirmation and initial characterization of silver oxide nanoparticles. After two weeks of synthesis, spectroscopic analysis was also carried out to verify the stability of ES-Ag2ONPs. The synthesized NPs showed no discernible variation in the spectroscopic results, indicating their stability. This implies that the E. sativa extract may be stabilizing Ag2ONPs by functioning as a capping or binding agent.
Furthermore, FT-IR explorations were conducted to investigate the capping ability of the functional groups present on the surface of E. sativa extract-mediated Ag2ONPs48. The presence of Ag2ONPs is evident in the FTIR spectrum, characterized by prominent broad bands. Additionally, the stretching vibrations observed indicate the reduction of Ag + to Ag (Fig. 3). Table 1 provides an overview of the various functional groups present in the Ag2ONPs, along with relevant bonding information. Notably, the Ag2ONPs exhibited capping with the O-H stretch, N-H bend, C-N, C-Cl, and C-Br groups, which were identified by reduced band intensities at frequencies around 3253, 1638, 1052, 620, 579, 553, 544, 526, and 519 cm− 1. The bio-reduction process responsible for the formation of Ag2ONPs might have contributed to the disappearance of several functional groups, including the carbonyl group.
Transmission electron microscopy (TEM) was employed to gain valuable insights into the morphology and size characteristics of ES-Ag2ONPs. The TEM micrograph vividly illustrates the uniform distribution of the synthesized ES-Ag2ONPs, exhibiting predominantly spherical shapes with an average diameter of approximately 50 nm (Fig. 4). It can also be observed that synthesized nanoparticles are well-distributed and are not clumped together. The well-dispersed nature of nanoparticles is important for their stability, as the particles clumped together are more likely to form their aggregate which leads to the loss of their properties. This observation provides a comprehensive understanding of the physical attributes and dimensions of the Ag2ONPs under investigation.
Moreover, the zeta potential serves as a crucial indicator of the surface charge potential, playing a significant role in assessing the stability of nanoparticles within aqueous suspensions. In this study, the Ag2ONPs exhibit a value of -17.7 mV, signifying the presence of negative charges on the synthesized nanoparticles (Fig. 5). This negative charge on the Ag2ONPs may be attributed to the coverage of E. sativa extract. Understanding the zeta potential is vital in comprehending the stability of nanoparticles in aqueous suspensions, as the positive or negative charges on their surface promote stability and hinder nanoparticle aggregation by repelling like charges. Existing literature suggests that nanoparticles possessing a zeta potential higher than + 30 mV or lower than − 30 mV are deemed remarkably stable in the dispersion medium. The capping of bioorganic components from plant extracts, which stabilizes the nanoparticles and inhibits agglomeration, is responsible for the potential values of silver oxide nanoparticles. This negative zeta potential indicates that the nanoparticles have enough electrostatic repulsion to keep them from aggregating, which is important for their use in environmental and medicinal sciences among other domains49. These results highlight the long-term preservation of Ag2ONP’s structure and unequivocally show the successful formation of Ag2ONPs for cellular uptake.
XRD was applied to uncover the crystalline structure and average crystallite size of the synthesized silver oxide nanoparticles. Different diffraction peaks at 2θ values of 31.12245°, 43.50128°, 45.28061°, 55.55485°, 64.50893°, 75.24235°, and 90.07015° can be seen in XRD pattern (Fig. 6). The effective synthesis of ES-Ag2ONPs is confirmed by these peaks, which correspond to the (111), (200), (220), (311), (400), and (420) planes of the face-centered crystalline nature of Ag2ONPs48. The peak’s sharpness and intensity suggest that the synthesized nanoparticles have a high degree of crystallinity. The Debye-Scherrer equation (Eq. 4) was applied to get the average crystallite size:
ES-Ag2ONPs were found to have a mean crystallite size of 59.52 nm, which is in line with the nanoscale dimension and close to the size observed by TEM analysis. The nanoparticles comparatively tiny sizes are beneficial because it advances the surface area-to-volume ratio, which may improve the biological and catalytic qualities of the particles. Eswari et al. (2018) reported that the XRD patterns of silver nanoparticles generated using biosurfactants showed peaks suggestive of both silver and silver oxide phases, with prominent peaks at 31.69° indicating the presence of silver oxide. This result is consistent with the peaks found in our current study, supporting the crystalline nature of synthesized nanoparticles50.
Biological applications
Antioxidant potential of synthesized Ag2ONPs
In this study, a phosphomolybdate assay was utilized to check the total antioxidant capacity of the synthesized ES-Ag2ONPs. One important approach for examining the association between antioxidants and oxidative stress-induced diseases is total antioxidant capacity47. This assay is good for the evaluation of the ability of a sample to scavenge reactive oxygen species (ROS) in a system. During this assay, if some antioxidants (reducing agents) are present with the system, then the phosphomolybdate (V) will be indicated by the generation of a green color, which confirms the reduction of molybdenum (VI) to molybdenum (V) and that is spectrophotometrically can be assessed51. Total antioxidant capacity is a profound assay to inspect the association of antioxidants and pathologies stimulated by oxidative stress. The total antioxidant capacity of the synthesized Ag2ONPs was expressed as gallic acid equivalent to mg/g of the sample depicted in Fig. 6. The maximum TAC value was found to be 50.31 ± 3.2 µg/mL at the highest concentration of 200 µg/mL. A cell under stress may generate reactive oxygen species (ROS) because of oxidative chain reactions. Production of free radicals by unpaired electrons are extremely unstable entities, as a result, they can easily interact with other molecules, removing their electrons and making them unstable by destroying their normal metabolic processes. To manage these free radicals and help the cell recover normal metabolic function, antioxidants play a critical role. Antioxidants work by donating electrons to free radicals, allowing them to stabilize, thus preventing them from reacting with other cellular components. Free radical DPPH scavenging is the most important assay to evaluate the antioxidant capacity. For the DPPH assay, at the highest concentration of 200, the %inhibition was found to be 36.98 ± 2.99 µg/mL and the IC50 value of ES-Ag2ONPs was found to be 62.36 µg/mL. To find out the percentage scavenging activity of synthesized ES-Ag2ONPs by calculating their IC50, GraphPad Prism Software was applied (Fig. 7).
The principle of reducing power assay works as a reducer i.e., antioxidants present in a sample cause the reduction of ferricyanide complex/Fe3+ to ferrous form. Consequently, Perl’s Prussian Blue color production can be employed to observe the Fe2+ contents52. The reducing potential of our synthesized ES-Ag2ONPs is determined by total reducing power calculation as shown in (Fig. 7) with a maximum value of 59.61 ± 1.75 µg/mL at the maximum concentration of 200 µg/mL.
Antibacterial efficiency of Ag2ONPs
Multidrug-resistant bacteria (MDR) are a main risk to human health. They can affect treatment results, which can eventually have adverse effects on treatment strategies53. Some of the extremely challenging MDR organisms comprise P. aeruginosa, E. coli, S. aureus, K. pneumoniae, and M. tuberculosis54,55. These bacteria are becoming progressively more general, due to unnecessary consumption of antibiotics. These bacteria can produce a broad scale of infectious diseases, including meningitis, sepsis, pneumonia, etc. These infections are often challenging to handle and can cause death56,57. Comparably, antimicrobial resistance poses a serious risk to human health since it contributes to a rise in morbidity and death from the most prevalent bacterial diseases58. Antimicrobial usage protocols will need to be modified in order to handle the resistance issue. Thus, there is a considerable deal of interest in non-traditional antibacterial drugs to overcome resistance59.
As a potential solution to the issue of bacterial multidrug resistance, nanoparticles are currently seen as a competitive alternative to antibiotics60. Specifically, in the field of science, silver nanoparticles (AgNPs) have garnered a lot of interest61. Because of its low cytotoxicity, silver has long been employed as an antiseptic and antibacterial against both Gram-positive and Gram-negative bacteria62,63. In the past, it was also used as an antiseptic against other diseases64,65. Ag2ONPs have gained particular attention recently for their potential to produce a novel class of antimicrobials, providing an entirely new means of fighting a variety of bacterial infections66,67. In this study, the agar disc diffusion method was applied against different bacterial strains to study the effectiveness of ES-Ag2ONPs (Fig. 8). For this purpose, various concentrations of synthesized NPs ranging from 1000 to 62.5 µg/mL were used against bacterial strains. The results revealed that most of the studied strains were sensitive to ES-Ag2ONPs, with the most susceptible strain being B. subtilis. The ZOI for B. subtilis was 18.5 ± 2.36 mm at 1000 µg/mL and 9.6 ± 0.62 mm at 62.5 µg/mL. E. coli showed ZOI of 18.4 ± 1.41 mm ZOI at 1000 µg/mL and 11.3 ± 0.36 mm at 62.5 µg/mL. In the case of S. aureus, the ZOI were 16.25 ± 1.13 mm and 8.5 ± 0.94 mm at the concentrations of 1000 and 62.5 µg/mL respectively. Furthermore, P. aeruginosa ZOIs were noted 15.83 ± 1.44 mm at 1000 µg/mL and 6.74 ± 0.54 mm at 62.5 µg/mL. The susceptibility for L. acidophilus was found 15.55 ± 0.50 mm at the concentration of 1000 µg/mL and 9.53 ± 0.72 mm at 62.5 µg/mL of concentration. Similarly, the ZOIs for K. pneumoniae and E. faecalis observed at 1000 µg/mL of concentration were 14.20 ± 0.64 mm and 12.6 ± 1.30 mm and at 62.5 µg/mL, the zone of inhibitions was 9.70 ± 1.01 mm and 7.49 ± 0.18 mm respectively, while oxytetracycline was observed more effective than any single test sample in this study. Overall, a mixed response was observed by both gram-positive and negative strains as B. subtilis is a gram-positive strain showed maximum ZOI 18.5 ± 2.36 mm while minimum ZOI 12.6 ± 1.30 mm was also exhibited by a gram-positive strain E. faecalis. The maximum ZOI for gram negative strains was 18.4 ± 1.44 mm by E. coli which is very close to the maximum ZOI of gram-positive. Figure 9 (A) shows the graphical presentation of antibacterial results. Nanoparticles, during the interaction with bacterial cells, may induce structural disruptions, penetrate into the cell interior, interfere with the cell organelles as well as other cellular processes, thus inducing disturbances and oxidative stress, ultimately leading to damage of cellular components such as DNA, proteins, lipids, etc., and/or death of the cell68. The MIC values of E. sativa mediated silver oxide nanoparticles, ranged between 500 and 62.5 µg/mL, which shows the inhibitory potential of synthesized nanoparticles at relatively low concentrations. The MBC values ranged from 1000 to 125 µg/mL, found two-fold higher than MIC indicating that a relatively higher concentrations are required to completely kill a bacterium. These findings suggest that Ag2ONPs have bacteriostatic potential at low concentrations while bactericidal potential require higher concentrations (Table 2).
Antifungal response of Ag2ONPs
Despite the developments of upgraded medical technology and huge investments in combating microbes, infectious diseases originated by pathogenic fungi are still a consistent threat to human as well as animal health. Antifungal drug remedies are no exception, however, the resistance to various antifungal agents in use now has merged. Although the resistance shown to antifungal drugs is as considerably problematic as the resistance faced by antibacterial agents, one extended period of concern is that the availability of various antifungal agents remains tremendously inadequate69,70. Therefore, there is a certain and immediate need for antibiotics that have unique mechanisms of action against fungi, because being eukaryotic organisms, fungi have similar metabolic and structural features to those of eukaryotic host organisms71. With an increase in the development of metal nanoparticles, Ag2ONPs attain more attention responding as a strong antimicrobial agent in the field of nanotechnology72. Since ages, silver and its composites have been acknowledged for exhibiting robust fungicidal potentials and a wide range of antimicrobial capacities against fungi, bacteria, and viruses. Silver metal was found to be more toxic to microorganisms and it was revealed as less toxic to mammalian cells when compared with other different metals73,74. It has been realized that Ag2ONPs formulated with different synthetic manners have shown effective antimicrobial potential, thus the synthesis of nanosized uniform silver particles with precise requirements in terms of shape, size, chemical, and physical properties is of immense interest to formulate new avenues in the field of pharmaceutics. In the present work, the susceptibility of the selected strains A. niger and A. flavus was calculated by determining the mycelial growth of fungal strains on SDA media plates at different concentrations of Ag2ONPs (1000 –62.5 µg/mL). By pursuing the activity, A. flavus was found with a Zone of Proliferation (ZOP) of 17 ± 2.64 mm at 1000 µg/mL and the lowest concentration of ES-Ag2ONPs 62.5 µg/mL was observed with a ZOP value of 21.6 ± 2.82 mm. Similarly, A. niger reveals a ZOP of 16 ± 1.7 mm at the highest concentration of 1000 µg/mL while its highest ZOP was recorded at 22 ± 2.6 mm at the lowest applied concentration of ES-Ag2ONPs at 62.5 µg/mL. A. niger was more inhibited at highest applied concentration (1000 µg/mL) of synthesized nanoparticles showing ZOP of 16 ± 1.7 mm as compared to A. flavus at highest concentration showed ZOP of 17 ± 2.64 mm. The interaction of nanoparticles with the fungal cell membrane may result in more permeable and disrupted membranes, eventual cell lysis, and possible death75. According to earlier reported studies, Ag2ONPs inhibit fungal growth by demolishing fungal spores and hype, in addition to the generation of ROS76. Previously reported works also demonstrate the significant dose-dependent response of Ag2ONPs against various fungal strains, which are compatible with current findings77,78. Silver nanoparticles synthesized from Cassia roxburghii leaf extract were found with exhibited higher antifungal activity compared to the conventional antifungal drug amphotericin B against human and plant pathogens79. Similarly, silver NPs mediated from Psidium guajava leaf extracts displayed significant antifungal activity against resistant strains of A. niger and Candida glabrata80. Notably, our finding revealed that robust antifungal activity is associated with an increase in the concentrations of E. sativa-mediated silver oxide nanoparticles. Figure 9(B) presents the graphical expression of the antifungal activity of synthesized nanoparticles.
Brine shrimp cytotoxic response
To determine the cytotoxicity of green synthesized ES-Ag2ONPs to freshly hatched A. salina, a brine shrimp lethality assay (BSLA) was conducted. The BSLA is a standard test used to assess the toxicity of any compound. In this assay, ES-Ag2ONPs were tested at concentrations of 10 to 200 µg/mL. At the highest applied concentration of 200 µg/mL, the mortality rate was observed at 100% and for the lowest concentration of 10 µg/mL, the death rate was recorded at 20%. The toxicity of ES-Ag2ONPs was concentration-dependent, with the LC50 value which represents the concentration at which 50% mortality occurs, 12.21 µg/mL (Fig. 10). The higher the value of Ag2ONPs, the more toxic they were to the brine shrimps. Previous work collectively suggests that silver nanoparticles have cytotoxic effects on brine shrimps (A. salina). According to a study it was found that increasing concentrations of Ag2ONPs led to increased mortality rates, aggregation in the gut region, apoptotic cells, and DNA damage in Artemia nauplii81. In a similar study a concentration-dependent immobilization of Artemia nauplii was checked when exposed to silver nanoparticles, with sensitivity varying between different instar stages and being influenced by environmental conditions82. Likewise, silver NPs synthesized using Stereospermum kunthianum Cham exhibited strong cytotoxicity against brine shrimps83.
These results confirm that the ES-Ag2ONPs can be applied as cytotoxic agents. However, none of the ES-Ag2ONPs concentrations tested were more cytotoxic than vincristine sulfate, which was used as a positive control in this assay.
Anticancer potential of synthesized Ag2ONPs
It is already stated that the distinctive features and size properties of nanoparticles may play some key role in their potential biomedical applications, especially in cancer therapy. In this contribution, the present study explored the cytotoxic effects of Ag2ONPs on Hep-2 cell lines. According to MTT outcomes, cytotoxicity was observed in dose-dependent manners, which was like the earlier studies of Ag2ONPs on MTT assay84,85. The percentage cytotoxicity of Hep-2 cells treated with various concentrations of Ag2ONPs (10,12,25,50, and 100 µg/mL) is shown in the Fig. 10. As concentrations of Ag2ONPs increase, cytotoxicity is seen to increase; it reaches around 40% at maximum concentration of 100 ug/mL, having IC50 for cytotoxic effects is 9.97 ug/mL. At all concentrations, doxorubicin exhibits more cytotoxicity than Ag2ONPs. The cytotoxic effects of silver oxide NPs can be linked to multiple processes, including induction of apoptosis, impairment of mitochondrial functions, and induction of reactive oxygen species (ROS). The anticancer effects of synthesized nanoparticles may be strengthened by the phytocompounds present in E. sativa. Silver nanoparticles robustly decrease the ATP content of the cells which eventually results in mitochondrial damage86 and an increase in the generation of reactive oxygen species (ROS) in a dose-dependent response87. Silver nanoparticles synthesized via the green process were found to be strongly anticancer against various cell lines B16F10, 3LL, and EAC88. Ag2ONPs synthesized from E. sativa, Pandanus odorifer, Panax ginseng, and Lagerstroemia indica demonstrated strong anticancer potential. Specifically, silver nanoparticles were found to inhibit the movement of cancer, which is a crucial phenomenon in the progression and metastasis of cancer. The IC50 value for the inhibition of A549 was observed at 25.15 µg/mL, and 46.22 µg/mL89,90,91. The following results thus stated that the stress triggered by Ag2ONPs is affecting the viability of cells and inhibiting their proliferation. However, to the best of knowledge, this is the first study on the cytotoxic effects of E. sativa-mediated Ag2ONPs against human cancer cell line Hep-2. Figure 11 shows the demonstration of this assay. To more understand the effectiveness of these nanoparticles, more research should concentrate on clarifying the exact mode of action and streamlining the synthesis protocols.
Biocompatibility of ES-Ag2ONPs
The biocompatibility of the green synthesized ES-Ag2ONPs was estimated via two cell lines, HEK-293 and VERO (Fig. 10). The cells were treated with different concentrations of NPs for 24 h. The viability of cells was assessed using the MTT assay. The IC50 value for HEK-293 was 26.56 µg/mL; for VERO, it was calculated at 43.11 µg/mL. At 10 µg/mL the viability for HEK-293 and VERO was 70% and 80% respectively. There is a gradual decrease in viability observed by increasing the dose concentrations. VERO cells have shown a moderate level of cytotoxicity while HEK-293 is showing a higher level of sensitivity towards Ag2ONPs. Doxorubicin, a strong chemotherapeutic agent, showed a strong decline in cell viability at all concentrations. Ag2ONPs displayed a degree of cytotoxic at higher concentrations towards both cell lines, more specifically with HEK-293, while the lower concentrations of Ag2ONPs could be considered biocompatible specifically when compared with the more toxic nature of doxorubicin. Though, a summary of studies on various human cell lines indicates the biocompatibility of Ag2ONPs and their potential role in biomedical applications92. Silver nanoparticles can induce targeted cytotoxicity due to the production of ROS. Because of their changed redox state, cancer cells are more vulnerable to ROS. This intracellular ROS initiates programmed cell death due to oxidative stress. In addition, nanoparticles may induce pathways of cell apoptosis, including mitochondrial pathways. This may be achieved by the manufacture of ROS, the loss of mitochondrial membrane potential, and DNA fragmentation93. These results indicate that the green synthesized ES-Ag2ONPs could be considered biocompatible at lower doses and further work is required to evaluate their potential in biomedical applications like drug delivery and tissue engineering to optimize their uses in biomedical sciences.
Conclusion
This work has successfully demonstrated the synthesis of stable ES-Ag2ONPs characterized through various analytical techniques. One of the standout features of these nanoparticles is their promising antimicrobial properties as evidenced by a substantial clear zone of inhibitions, which marks them as a solution for the global issue of antibiotic resistance. This study also demonstrated the ES-Ag2ONPs as a potential cytotoxic agent against A. salina, highlighting their role as an environment-friendly pest-control agent. Their antioxidative potential harnesses them in various biomedical and pharmaceutical fields, notably, their biocompatible nature at lower doses and anticancer effectiveness observed via various cell lines suggest their importance in the biomedical field and a promising advancement in cancer therapy. The multifaceted attributes of these synthesized ES-Ag2ONPs positioned them as a versatile platform for diagnostic tools, drug delivery, and therapeutic agents. The use of green nanoparticles opens new avenues in the field of nanotechnology and biomedicine by introducing innovative solutions with the potential to address global health challenges. Additionally, the addition of Es-Ag2ONPs to diagnostic instruments has the potential to transform current approaches to disease detection. Their unique optical qualities make it possible to create sensitive biosensors that can identify biomolecules at low concentrations, which is essential for the early diagnosis of disease. However, further investigations and clinical studies will shed more light on their diverse implications and address the challenges in their practical applications.
Data availability
All the raw data in this research can be obtained from the corresponding authors upon reasonable request.
References
Gotov, O., Battogtokh, G. & Ko, Y. T. Docetaxel-loaded hyaluronic acid–cathepsin b-cleavable-peptide–gold nanoparticles for the treatment of cancer. Mol. Pharm. 15 (10), 4668–4676 (2018).
Swy, E. R. et al. Dual-modality, fluorescent, PLGA encapsulated bismuth nanoparticles for molecular and cellular fluorescence imaging and computed tomography. Nanoscale 6 (21), 13104–13112 (2014).
Wu, M. et al. Hierarchical polyelemental nanoparticles as bifunctional catalysts for oxygen evolution and reduction reactions. Adv. Energy Mater. 10 (25), 2001119 (2020).
Bouafia, A. & Laouini, S. E. Plant-mediated synthesis of iron oxide nanoparticles and evaluation of the antimicrobial activity: A review. Mini-Rev. Org. Chem. 18 (6), 725–734 (2021).
Ullah, Z. et al. Assessment of Gus expression induced by anti-sense os PPO gene promoter and antioxidant enzymatic assays in response to drought and heavy metal stress in transgenic Arabidopsis thaliana. Sustainability 15 (17), 12783 (2023).
Ullah, Z. et al. Biogenic synthesis, characterization, and in vitro biological investigation of silver oxide nanoparticles (AgONPs) using Rhynchosia capitata. Sci. Rep. 14 (1), 10484 (2024).
Naseri, T. & Pour-Khavari, F. Bimetallic core-shell with graphene coating nanoparticles: Enhanced optical properties and slow light propagation. Plasmonics 15 (4), 907–914 (2020).
Sushobhan, B. R. & Kar, S. P. Thermal modeling of melting of nano based phase change material for improvement of thermal energy storage. Energy Procedia. 109, 385–392 (2017).
Velsankar, K., Sudhahar, S. & Maheshwaran, G. Effect of biosynthesis of ZnO nanoparticles via Cucurbita seed extract on Culex Tritaeniorhynchus mosquito larvae with its biological applications. J. Photochem. Photobiol., B. 200, 111650 (2019).
Apsana, G., George, P. P., Devanna, N. & Yuvasravana, R. Biomimetic synthesis and antibacterial properties of strontium oxide nanoparticles using Ocimum sanctum leaf extract. Asian J. Pharm. Clin. Res. 11, 384–389 (2018).
Sharmila, C., Kumar, R., Shekar, C. & R., &, B Psidium guajava: A novel plant in the synthesis of silver nanoparticles for biomedical applications. Asian J. Pharm. Clin. Res. 11 (1), 341–345 (2018).
Ridolfo, R. et al. Exploring the impact of morphology on the properties of biodegradable nanoparticles and their diffusion in complex biological medium. Biomacromolecules 22 (1), 126–133 (2020).
Barua, S. et al. Silver nanoparticles as antibacterial and anticancer materials against human breast, cervical and oral cancer cells. J. Nanosci. Nanotechnol. 17 (2), 968–976 (2017).
Li, M. et al. Red blood cell membrane-coated upconversion nanoparticles for pretargeted multimodality imaging of triple-negative breast cancer. Biomaterials Sci. 8 (7), 1802–1814 (2020).
Zhang, D. et al. Gd–Al co-doped mesoporous silica nanoparticles loaded with Ru (bpy) 3 2 + as a dual-modality probe for fluorescence and magnetic resonance imaging. Analyst 139 (18), 4613–4619 (2014).
Hasan, I. et al. Eco-friendly green synthesis of dextrin based poly (methyl methacrylate) grafted silver nanocomposites and their antibacterial and antibiofilm efficacy against multi-drug resistance pathogens. J. Clean. Prod. 230, 1148–1155 (2019).
Naraginti, S. & Li, Y. Preliminary investigation of catalytic, antioxidant, anticancer and bactericidal activity of green synthesized silver and gold nanoparticles using Actinidia deliciosa. J. Photochem. Photobiol., B. 170, 225–234 (2017).
Rónavári, A. et al. Biological activity of green-synthesized silver nanoparticles depends on the applied natural extracts: A comprehensive study. Int. J. Nanomed. 12, 871 (2017).
Velsankar, K. et al. Bio-derived synthesis of MgO nanoparticles and their anticancer and hemolytic bioactivities. Biocatal. Agric. Biotechnol. 53, 102870 (2023).
Majeed, S. et al. Bioengineering of green-synthesized TAT peptide-functionalized silver nanoparticles for apoptotic cell-death mediated therapy of breast adenocarcinoma. Talanta 253, 124026 (2023).
Barabadi, H. et al. Functionalized bioengineered metal-based nanomaterials for cancer therapy. in Functionalized Nanomaterials for Cancer Research 219–260. (Academic, 2024).
Soares, S., Sousa, J., Pais, A. & Vitorino, C. Nanomedicine: Principles, properties, and regulatory issues. Front. Chem. 6, 360 (2018).
Francis, S., Joseph, S., Koshy, E. P. & Mathew, B. Synthesis and characterization of multifunctional gold and silver nanoparticles using leaf extract of Naregamia alata and their applications in the catalysis and control of mastitis. New J. Chem. 41 (23), 14288–14298 (2017).
Bibi, S., Ullah, R., Burni, T., Ullah, Z. & Kazi, M. Impact of resorcinol and biochar application on the growth attributes, metabolite contents, and antioxidant systems of tomato (Lycopersicon esculentum Mill). ACS Omega. 8 (48), 45750–45762 (2023).
Rather, G. A. et al. Biosynthesis of zinc oxide nanoparticles using Bergenia ciliate aqueous extract and evaluation of their photocatalytic and antioxidant potential. Inorg. Chem. Commun. 134, 109020 (2021).
Ghomi, A. R. G. et al. Fungus-mediated extracellular biosynthesis and characterization of zirconium nanoparticles using standard penicillium species and their preliminary bactericidal potential: A novel biological approach to nanoparticle synthesis. Iran. J. Pharm. Research: IJPR. 18 (4), 2101 (2019).
Vahidi, H., Kobarfard, F., Alizadeh, A., Saravanan, M. & Barabadi, H. Green nanotechnology-based tellurium nanoparticles: exploration of their antioxidant, antibacterial, antifungal and cytotoxic potentials against cancerous and normal cells compared to potassium tellurite. Inorg. Chem. Commun. 124, 108385 (2021).
Vahidi, H. et al. Mycosynthesis and characterization of selenium nanoparticles using standard penicillium chrysogenum PTCC 5031 and their antibacterial activity: A novel approach in microbial nanotechnology. Nanomed. J. 7 (4), 315–323 (2020).
Nayak, D. et al. Opportunities and challenges for bioengineered metallic nanoparticles as future nanomedicine. Bioengineered Nanomateri. Wound Healing Infect. Control, 517–540. (2023).
Abbasi, B. A. et al. Plant-mediated synthesis of nickel oxide nanoparticles (NiO) via Geranium wallichianum: Characterization and different biological applications. Mater. Res. Express. 6 (8), 0850a7 (2019).
Ullah, R., Ullah, Z., Iqbal, J., Chalgham, W. & Ahmad, A. Aspartic acid-based nano-copper induces resilience in Zea mays to applied lead stress via conserving photosynthetic pigments and triggering the antioxidant biosystem. Sustainability 15 (16), 12186 (2023).
Chirayil, C. J., Abraham, J., Mishra, R. K., George, S. C. & Thomas, S. Instrumental techniques for the characterization of nanoparticles. In Thermal and Rheological Measurement Techniques for Nanomaterials Characterization 1–36 (Elsevier, 2017).
Uddin, S. et al. Green synthesis of nickel oxide nanoparticles using leaf extract of Berberis Balochistanica: Characterization, and diverse biological applications. Microsc. Res. Tech. 84 (9), 2004–2016 (2021).
Iqbal, J. et al. Biogenic synthesis of green and cost effective iron nanoparticles and evaluation of their potential biomedical properties. J. Mol. Struct. 1199, 126979 (2020).
Abbasi, B. A. et al. Green formulation and chemical characterizations of Rhamnella Gilgitica aqueous leaves extract conjugated NiONPs and their multiple therapeutic properties. J. Mol. Struct. 1218, 128490 (2020).
Iqbal, J. et al. Biogenic synthesis of green and cost effective cobalt oxide nanoparticles using Geranium wallichianum leaves extract and evaluation of in vitro antioxidant, antimicrobial, cytotoxic and enzyme inhibition properties. Mater. Res. Express. 6 (11), 115407 (2019).
Iqbal, J. et al. Phytogenic synthesis of nickel oxide nanoparticles (NiO) using fresh leaves extract of Rhamnus Triquetra (wall.) and investigation of its multiple in vitro biological potentials. Biomedicines 8 (5), 117 (2020).
Iqbal, J. et al. Green synthesis of zinc oxide nanoparticles using Elaeagnus angustifolia L. leaf extracts and their multiple in vitro biological applications. Sci. Rep. 11 (1), 20988 (2021).
Gudkov, S. V., Serov, D. A., Astashev, M. E., Semenova, A. A. & Lisitsyn, A. B. Ag2O nanoparticles as a candidate for antimicrobial compounds of the new generation. Pharmaceuticals 15 (8), 968 (2022).
Singh, J. et al. Green’synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 16, 1–24 (2018).
Velsankar, K., Aravinth, K., Yong, W., Mohandoss, S. & Paiva-Santos, A. C. NiO nanoparticles, an algorithm of their biosynthesis, toxicity, and biomedical activities. J. Mol. Struct. 1291, 136012 (2023).
Kannan, R. R. R., Arumugam, R., Ramya, D., Manivannan, K. & Anantharaman, P. Green synthesis of silver nanoparticles using marine macroalga Chaetomorpha linum. Appl. Nanosci. 3, 229–233 (2013).
Ullah, Z. et al. Biogenic synthesis of multifunctional silver oxide nanoparticles (Ag2ONPs) using parieteria alsinaefolia Delile aqueous extract and assessment of their diverse biological applications. Microorganisms 11 (4), 1069 (2023).
Paker, N. P. et al. Elucidating molecular characterization of chlorpyrifos and profenofos degrading distinct bacterial strains for enhancing seed germination potential of Gossypium arboreum L. Environ. Sci. Pollut. Res., 1–18. (2023).
Velsankar, K., Sudhahar, S., Parvathy, G. & Kaliammal, R. Effect of cytotoxicity and aAntibacterial activity of biosynthesis of ZnO hexagonal shaped nanoparticles by Echinochloa frumentacea grains extract as a reducing agent. Mater. Chem. Phys. 239, 121976 (2020).
Zuhrotun, A., Oktaviani, D. J. & Hasanah, A. N. Biosynthesis of gold and silver nanoparticles using phytochemical compounds. Molecules 28 (7), 3240 (2023).
Yang, C. S. et al. Antioxidants: Differing meanings in food science and health science. J. Agric. Food Chem. 66 (12), 3063–3068 (2018).
Abbasi, B. A. et al. Environmentally friendly green approach for the fabrication of silver oxide nanoparticles: Characterization and diverse biomedical applications. Microsc. Res. Tech. 83 (11), 1308–1320 (2020a).
Liaqat, N., Jahan, N., Anwar, T. & Qureshi, H. Green synthesized silver nanoparticles: Optimization, characterization, antimicrobial activity, and cytotoxicity study by hemolysis assay. Front. Chem. 10, 952006 (2022).
Eswari, J. S., Dhagat, S. & Mishra, P. Biosurfactant assisted silver nanoparticle synthesis: A critical analysis of its drug design aspects. Adv. Nat. Sci. NanoSci. NanoTechnol. 9 (4), 045007 (2018).
Benslama, A. & Harrar, A. Free radicals scavenging activity and reducing power of two Algerian Sahara medicinal plants extracts. Int. J. Herb. Med. 4 (6), 158–161 (2016).
Van Duin, D. & Paterson, D. L. Multidrug-resistant bacteria in the community: Trends and lessons learned. Infect. Disease Clin. 30 (2), 377–390 (2016).
Dahiya, P. & Purkayastha, S. Phytochemical screening and antimicrobial activity of some medicinal plants against multi-drug resistant bacteria from clinical isolates. Indian J. Pharm. Sci. 74 (5), 443 (2012).
Dey, D., Ray, R. & Hazra, B. Antimicrobial activity of pomegranate fruit constituents against drug-resistant Mycobacterium tuberculosis and β-lactamase producing Klebsiella pneumoniae. Pharm. Biol. 53 (10), 1474–1480 (2015).
Zumla, A. et al. Host-directed therapies for infectious diseases: Current status, recent progress, and future prospects. Lancet. Infect. Dis. 16 (4), e47–e63 (2016).
Houri, H. et al. Distribution of capsular types and drug resistance patterns of invasive pediatric Streptococcus pneumoniae isolates in Teheran, Iran. Int. J. Infect. Dis. 57, 21–26 (2017).
Alavi, M. & Rai, M. Recent advances in antibacterial applications of metal nanoparticles (MNPs) and metal nanocomposites (MNCs) against multidrug-resistant (MDR) bacteria. Expert Rev. anti-infective Therapy. 17 (6), 419–428 (2019).
Franci, G. et al. Silver nanoparticles as potential antibacterial agents. Molecules 20 (5), 8856–8874 (2015).
Dos Santos, C. A. et al. Silver nanoparticles: Therapeutical uses, toxicity, and safety issues. J. Pharm. Sci. 103 (7), 1931–1944 (2014).
Rai, M. K., Deshmukh, S. D., Ingle, A. P. & Gade, A. K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 112 (5), 841–852 (2012).
Khan, T., Yasmin, A. & Townley, H. E. An evaluation of the activity of biologically synthesized silver nanoparticles against bacteria, fungi and mammalian cell lines. Colloids Surf. B. 194, 111156 (2020).
Velsankar, K., Parvathy, G., Sankaranarayanan, K., Mohandoss, S. & Sudhahar, S. Green synthesis of silver oxide nanoparticles using Panicum miliaceum grains extract for biological applications. Adv. Powder Technol. 33 (7), 103645 (2022).
Akintelu, S. A. & Folorunso, A. S. Characterization and antimicrobial investigation of synthesized silver nanoparticles from Annona muricata leaf extracts. J. Nanotechnol Nanomed. Nanobiotechnol. 6, 1–5 (2019).
Adur, A. J., Nandini, N., Mayachar, K. S., Ramya, R. & Srinatha, N. Bio-synthesis and antimicrobial activity of silver nanoparticles using anaerobically digested parthenium slurry. J. Photochem. Photobiol., B. 183, 30–34 (2018).
Rodríguez-Félix, F. et al. Sustainable-green synthesis of silver nanoparticles using safflower (Carthamus tinctorius L.) waste extract and its antibacterial activity. Heliyon 7 (4), e06923 (2021).
Buszewski, B., Rafiſska, K., Pomastowski, P., Walczak, J. & Rogowska, A. Novel aspects of silver nanoparticles functionalization. Colloids Surf., a. 506, 170–178 (2016).
Jampilek, J. Novel avenues for identification of new antifungal drugs and current challenges. Expert Opin. Drug Discov. 17 (9), 949–968 (2022).
Ahmed, B., Hashmi, A., Khan, M. S. & Musarrat, J. ROS mediated destruction of cell membrane, growth and biofilms of human bacterial pathogens by stable metallic AgNPs functionalized from bell pepper extract and quercetin. Adv. Powder Technol. 29 (7), 1601–1616 (2018).
Lockhart, S. R., Etienne, K. A., Vallabhaneni, S., Farooqi, J., Chowdhary, A., Govender, N. P., Litvintseva, A. P. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infecti. Dis., 64 (2), 134–140.
Nasrollahi, A., Pourshamsian, K. H. & Mansourkiaee, P. Antifungal activity of silver nanoparticles on some of fungi antibacterial activity. Int. J. Appl. Sci. Biotechnol. 5 (4), 523–531 (2011).
Khan, N. T. & Mushtaq, M. Determination of antifungal activity of silver nanoparticles produced from Aspergillus Niger. Biology Med. 9 (1), 1 (2017).
El-Bassuony, A. A. Effect of Al addition on structural, magnetic, and antimicrobial properties of Ag nanoparticles for biomedical applications. JOM 72 (3), 1154–1162 (2020).
Safarpoor, M. et al. Ultrasound-assisted extraction of antimicrobial compounds from Thymus daenensis and Silybum marianum: Antimicrobial activity with and without the presence of natural silver nanoparticles. Ultrason. Sonochem. 42, 76–83 (2018).
Xia, Z. K. et al. The antifungal effect of silver nanoparticles on Trichosporon Asahii. J. Microbiol. Immunol. Infect. 49 (2), 182–188 (2016).
Nisar, P., Ali, N., Rahman, L., Ali, M. & Shinwari, Z. K. Antimicrobial activities of biologically synthesized metal nanoparticles: An insight into the mechanism of action. J. Biol. Inorg. Chem. 24, 929–941 (2019).
Artunduaga Bonilla, J. J. et al. Silver chitosan nanocomposites as a potential treatment for superficial candidiasis. Med. Mycol. 59 (10), 993–1005 (2021).
Narasimha, G. et al. Fungicidal activity of silver nanoparticles synthesized by Agaricus Bisporus (white button mushrooms). J. Nanosci. Nanotechnol. 7 (3), 114–115 (2013).
Balashanmugam, P., Balakumaran, M. D., Murugan, R., Dhanapal, K. & Kalaichelvan, P. T. Phytogenic synthesis of silver nanoparticles, optimization and evaluation of in vitro antifungal activity against human and plant pathogens. Microbiol. Res. 192, 52–64 (2016).
Shehensha, S. & Vijaya, J. M. In vitro antifungal activity of Psidium Guajava based silver nanoparticles. J. Pure Appl. Microbiol. 14 (3), 2075–2083 (2020).
Arulvasu, C., Jennifer, S. M., Prabhu, D. & Chandhirasekar, D. Toxicity effect of silver nanoparticles in brine shrimp Artemia. Sci. World J., 2014. (2014).
Lish, R. A. D., Johari, S. A., Sarkheil, M. & Yu, I. J. On how environmental and experimental conditions affect the results of aquatic nanotoxicology on brine shrimp (Artemia salina): A case of silver nanoparticles toxicity. Environ. Pollut. 255, 113358 (2019).
Kabiru, H. D., Ahmad, K. B., Bello, N. M. & Chiegero, C. E. Biogenic silver nanoparticles from Stereospermum Kunthianum cham stem bark extract: Aynthesis, characterization, in vitro antimicrobial study and cytotoxicity effects against brine shrimp artemia. Int. J. Sci. Global Sustain. 8 (4), 9–9 (2022).
Dziedzic, A., Kubina, R., Bułdak, R. J., Skonieczna, M. & Cholewa, K. Silver nanoparticles exhibit the dose-dependent anti-proliferative effect against human squamous carcinoma cells attenuated in the presence of berberine. Molecules 21 (3), 365 (2016).
Mohanta, Y. K., Mishra, A. K., Nayak, D., Patra, B., Bratovcic, A., Avula, S. K., Saravanan, M. (2022). Exploring dose-dependent cytotoxicity profile of gracilaria edulis-mediated green synthesized silver nanoparticles against MDA-MB-231 breast carcinoma. Oxidative Med. Cell. Longev. (2022).
Nayak, D., Ashe, S., Rauta, P. R., Kumari, M. & Nayak, B. Bark extract mediated green synthesis of silver nanoparticles: Evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Engineering: C. 58, 44–52 (2016).
Ijaz, S., Iqbal, J., Abbasi, B. A., Ullah, Z., Yaseen, T., Kanwal, S., Cho, W. C. Rosmarinic acid and its derivatives: Current insights on anticancer potential and other biomedical applications. Biomed. Pharmacother. 162, 114687 (2023).
Teodoro, J. S. et al. Assessment of the toxicity of silver nanoparticles in vitro: A mitochondrial perspective. Toxicol. In Vitro. 25 (3), 664–670 (2011).
Awadelkareem, A. M., Al-Shammari, E., Elkhalifa, A. O., Adnan, M., Siddiqui, A. J., Patel, M., Ashraf, S. A. Biosynthesized silver nanoparticles from E. sativa Miller leaf extract exhibits antibacterial, antioxidant, anti-quorum-sensing, antibiofilm,and anti-metastatic activities. Antibiotics, 11 (7), 853 (2022).
Hussain, A., Alajmi, M. F., Khan, M. A., Pervez, S. A., Ahmed, F., Amir, S., Rehman, M. T. Biosynthesized silver nanoparticle (AgONP) from Pandanus odorifer leaf extract exhibits anti-metastasis and anti-biofilm potentials. Front. Microbiol. 10 8 (2019)
Castro-Aceituno, V. et al. Anticancer activity of silver nanoparticles from Panax ginseng fresh leaves in human cancer cells. Biomed. Pharmacother. 84, 158–165 (2016).
Behera, A. & Awasthi, S. Anticarcinogenic potentials of silver oxide nanoparticles synthesized from Lagerstroemia Indica. Int. J. Nanosci. 20 (06), 2150060 (2021).
Debnath, D., Lee, Y. & Geckeler, K. E. Biocompatible polymers as a tool for the synthesis of silver nanoparticles: Size tuning and in vitro cytotoxicity studies. Polym. Int. 66 (4), 512–520 (2017).
Ullah, I. et al. Green-synthesized silver nanoparticles induced apoptotic cell death in MCF‐7 breast cancer cells by generating reactive oxygen species and activating caspase 3 and 9 enzyme activities. Oxidative Med. Cell. Longev. 2020 (1), 1215395 (2020).
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP2025R301), King Saud University, Riyadh, Saudi Arabia. The study was financially supported by the University of Maryland System Wilson E. Elkins Professorship, Constellation, an Exelon Company, E2- Energy to Educate grant program (163893), and Dept. of Education, SAFRA Title III Grant.
Author information
Authors and Affiliations
Contributions
Author Contributions: F.G, Z.U, J.I, T.M, and B.A.A designed and conceived the study idea. F.G. completed the experiments. Z.U, F.G, B.A.A, S.A, S.K, and J.U., analyzed the data and performed visualizations and statistical data analysis. F.G. and Z.U. wrote the original draft. F.G, Z.U, J.I, T.M, B.A.A and M.K. reviewed and edited the manuscript. J.U. and M.K. reviewed the manuscript and provided funds. T.M. provided the resources and supervision. All authors made valuable revisions edited the manuscript and approved the last version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
This study does not include human or animal subjects.
Statement on guidelines
All experimental studies and experimental materials involved in this research are in full compliance with relevant institutional, national and international guidelines and legislation posing a conflict or bias.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gul, F., Ullah, Z., Iqbal, J. et al. Ecofriendly synthesis characterization and biological activities of Eruca sativa mediated silver oxide nanoparticles. Sci Rep 15, 13466 (2025). https://doi.org/10.1038/s41598-025-87670-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87670-9