Abstract
This paper investigates the impact of treatment with chemical solutions of varying pH values on the micro-macroscopic damage in coal samples under load, employing a combination of Small Angle X-ray Scattering (SAXS) experiments and uniaxial compression tests. The experimental results show that soaking coal samples in NaOH, HCl, and distilled water for 7 days leads to reductions in uniaxial compressive strength by 39.19%, 47.26%, and 24.39%, respectively, compared to untreated coal. The elastic modulus exhibited similar reductions, further confirming the weakening effects of chemical solutions. SAXS experiments reveal that exposure to alkaline solutions promotes the expansion and connection of nanopores, while acidic solutions primarily dissolve mineral components, increasing brittleness. Distilled water causes milder effects on pore structure and mechanical properties. To model the synergic effects of chemical solution corrosion and stress, a chemical solution corrosion-stress coupling factor was introduced into the Weibull probability distribution-based constitutive model. The modified model accurately describes the strain–stress behavior and damage evolution under combined chemical and mechanical effects. These findings highlight the risks of chemical solution-induced damage to coal and provide theoretical support for disaster prevention and stability control in coal mining.
Similar content being viewed by others
Introduction
Coal, as a typical complex porous medium, plays a critical role in groundwater flow and gas migration processes through its pore structure. In recent years, significant progress has been made both domestically and internationally in elucidating the relationship between coal porosity and permeability as well as the impact of chemical solution corrosion on pore morphology1,2. For example, acidic solutions can significantly alter the pore structure of coal, while alkaline solutions accelerate the expansion of microfractures3. Under the influence of mining and tunneling activities, the instability of coal is closely associated with the erosion caused by water chemical solutions4,5,6,7. However, existing studies are predominantly focused on static loading conditions, lacking systematic investigations into the damage evolution of coal from the micro- to macro-scale under the coupled effects of chemical solution corrosion and stress. Therefore, conducting research on the damage and destruction mechanisms of coal under the synergic effects of chemical solutions and stress is of significant importance for the safe production of coal mines.
The action of chemical agents on the coal includes ion exchange, dissolution, hydrolysis. These actions can alter the mineral composition within coal, leading to changes in the internal pore structure and mechanical characteristics of coal. Previous studies have gained certain understanding into the impact of chemical agents on the mechanical characteristics and pore structure of coal. For instance, Jiang et al.8 investigated the effect of the water environment on the fracture toughness and strain energy index of coal, and proposed an intrinsic mechanism by which chemical solutions can alter the properties of rock masses. Xia et al.9 studied the effects of soaking time and concentration of alkaline solutions on the propensity of coal. Song et al. 10 suggested that coal will develop new open pores after soaking in an alkaline solution of a certain concentration. Li et al.11 investigated the effects of coal composition, original pores, and the selection of chemical agents on coal damage when using acidic permeability enhancers. Cai et al.12,13 studied the influence of water saturation on the mechanical properties and microstructural damage of rock masses, and established corresponding models. Lv et al.14 conducted uniaxial compression tests on coal samples treated with acid solutions and posited that the use of mixed acid solutions can effectively enhance the moldability of coal and reduce its strength. Wang et al.15 studied the effect of solution pH on the permeability enhancement of lignite and characterized factors influencing the reaction between functional groups in the coal and chemical solvents and the influence of the salt sensitivity effect. Nie et al.1 utilized methods such as Small-Angle X-ray Scattering (SAXS) and low-temperature CO2 adsorption to study the destruction of the nanoporous structure and the framework in gas-bearing coal. Wang et al. 16 studied the physico-mechanical properties of coal under the action of saline solutions and established a damage constitutive model. Han et al.17 conducted research that clarified the varying degrees of damage and deterioration in sandstone due to chemical solutions with different pH values. Zheng et al.18 introduced the concept of rock micro-element strength and, in conjunction with the Weibull distribution function, established a nonlinear evolution model to describe rock damage, which effectively reflects the relationship between elastic energy and strain. Zhang et al.19 investigated the damage evolution characteristics of sandstone under uniaxial compression after chemical corrosion using methods like CT scanning, and proposed a sandstone damage model that incorporates the effects of chemical corrosion and CT values. Zhang et al.20 studied the microscale mechanical behavior of soaked shale using atomic force microscopy (AFM) and environmental scanning electron microscopy (ESEM). Zhong et al.21 used Nuclear Magnetic Resonance (NMR) to study the influence of chemical solutions on the microscale damage and macroscopic mechanical property degradation of limestone samples under external forces, and calculated the microscale damage of the samples treated with chemical solutions during triaxial compression.
This paper integrates synchrotron SAXS experiments with uniaxial compression tests and introduces the concept of a chemical solution corrosion-stress coupling factor to reflect the nonlinear coupling effects of solution corrosion and stress. Based on this, a micro- to macro-scale mechanical constitutive model for damage under the combined effects of chemical solution corrosion and stress was established. The study systematically analyzes the synergistic mechanisms of chemical solution corrosion on the microstructural damage of coal pores and the evolution of its macroscopic mechanical properties. The findings provide new theoretical insights and practical guidance for disaster warning and stability control in coal mines.
Experimental methods and preparation
Samples preparation
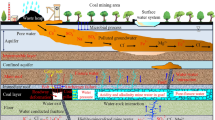
The coal samples were taken from Daliuta Coal Mine in Shenmu County, Shaanxi Province, China. The proximate analysis results, density, and porosity of the experimental coal samples are presented in Tables 1 and 2. The bulk coal obtained from the working face were sealed with plastic cling film and transported back to the laboratory to prevent oxidation during transit. The retrieved raw coal was cut into standard specimens of Φ 50 × 100 mm and separately immersed in solutions with different characteristics: the 0.01 mol/L NaOH solution with a pH of 12, the HCl solution with a pH of 2, and distilled water with a pH of 7, for a period of 7 days (Fig. 1)8,13. During the soaking process, the pH value of the solutions was regularly monitored and solvents were timely replenished to maintain a stable pH level.
After the coal samples were soaked, they were placed in a vacuum drying oven for drying. The temperature of the vacuum drying oven was set to 105 °C. The samples were removed for weighing every 6 h until the mass of the coal samples remained stable, after which the drying process was stopped. For each group of soaked coal samples, five specimens were prepared. Among these, three specimens were selected for mechanical loading experiments, with each experiment repeated three times to ensure reliability. The remaining two specimens were cut into smaller pieces and ground with sandpaper of various grit sizes into thin, flat slices with a thickness of 1 mm and a diameter of 10 mm, ensuring both surfaces were smooth for effective X-ray transmission during the SAXS experiments. The experimental samples were categorized into three groups: samples A (coal samples soaked in HCl solution), samples B (coal samples soaked in distilled water), and samples C (coal samples soaked in NaOH solution).
Mechanical loading experiment
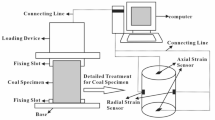
The mechanical behavior of coal samples were measured with the triaxial loading experimental apparatus, which comprises a loading and unloading control system, strain and stress–strain testing system, acoustic emission system, and other auxiliary equipment. The loading and unloading control system is the Tianshen testing machine test system, consisting of a press and an automatic control program TensonTest. During the experiment, the TensonTest program controls the change of loading and unloading modes and the real-time recording of data such as stress, strain, and displacement, ensuring the observation mode can be switched at any time for efficient data collection. The strain testing system primarily consists of the Micro-Measurements-7000 strain tester and StrainSmart data acquisition software, using BX120-5AA type strain gauges for precise collection of lateral and axial strains. The AMSY-6 full-information acoustic emission signal analysis system is used to collect acoustic emission signals during the experiment. The main components include acoustic emission probes, preamplifiers, signal acquisition devices, and data processing software, as shown in the figure. This system is equipped with eight acoustic emission channels. To prevent external noise interference with the acoustic emission signals collected during coal sample experiments, the signal acquisition threshold is set to 40 decibels based on the laboratory’s environmental conditions. A total of six acoustic emission probes are used, and they are tightly attached to the surface of the coal specimen by applying petroleum jelly. Three probes are arranged at each end of the coal sample, and their relative positions are shown in Fig. 2b.Other auxiliary materials include shock-absorbing pads to adjust the level of the press working platform. The experimental testing system is shown in Fig. 2a.
The stress loading system applies an axial load at a rate of 100 N/s until the coal sample fractures. At least three experiments were conducted for each type of coal sample to ensure the reliability of the results.
SAXS experiment
The SAXS experiments in this paper were conducted with the 1W2A SAXS station of the Beijing Synchrotron Radiation Facility (BSRF). The 1W2A SAXS station is capable of effectively determining the nanoscale (1 ~ 100 nm) geometric structures of experimental samples. From the analysis of the two-dimensional images obtained from the SAXS experiments, it is possible to calculate the pore size, pore size distribution, specific surface area, fractal dimension, molecular weight, porosity, and other nanopore structural information of the experimental samples22. In SAXS, when there are samples with nanoscale (1 ~ 100 nm) inhomogeneities in electron density, such as pores and particles, scattering occurs at small angles due to the difference in electron cloud density between these features. The maximum scattering angle is 25°, and the range of the incident light beam is between 2° and 5°23. Therefore, it can be used as an important tool for studying porous materials.
For this experiment, the distance between the sample and the detector was 1810 mm, and the incident X-ray wavelength for the synchrotron SAXS was 1.54 Å. The horizontal acceptance angle range of the 1W2A beamline was − 3.5 to 0 mrad, and the vertical acceptance angle range was (0.36 ± 0.18) mrad. A detailed description of the working principle of the experimental station can be found in reference23.
Before the experiment, all light transmission channels were checked for airtightness and vacuumed. The experimental samples were fixed onto the carrying platform using 3M tape, followed by light correction to ensure that the X-ray could effectively irradiate the samples to be tested. The SAXS experimental system is shown in Fig. 3.
Results and analysis
Changes in the microstructure of coal
Scattering vector-scattering intensity relationship and deviation calibration.
The SAXS system produces two-dimensional scattering images for characterizing the porosity features in coal (Fig. 4). From these images, it can be seen that there are slight differences in the scattering rings of different samples, indicating that there is a disparity in the scattering intensity between different coal samples. With these images, one-dimensional relative scattering intensity data and effective scattering vector q can be further obtained with the Fit2d software24 and S software22. Figure 5 shows the one-dimensional relative scattering intensity of the coal samples. The highest scattering intensity was in the central part of the detector, decreasing as the distance from the center increases. A schematic diagram of a typical SAXS experiment is shown in Fig. 2. The quantitative relationship between the scattering vector q and the scattering angle θ is as follows25:
where q is the scattering vector (nm-1), λ is the incident wavelength with a value of 1.54 Å, θ is the scattering angle.
The effective scattering vector q value range was determined to be 0.00805 nm-1 < q < 1.6452 nm-1. The quantitative relationship between the scattering vector q value and the corresponding pore size d conforms to the Bragg equation (as shown in Eq. 14)26, calculating the main observed pore size range for this experiment as 4 nm < d < 78 nm.
The fractal geometry principles were used to simplify the complex and disordered scattering pores in coal into spherical structures. The relative scattering intensity I(q) can be calculated using the following integral formula22,27,28,29. The relative and absolute scattering data of the experimental coal samples are shown in Figs. 5 and 6.
where I(q) is the relative scattering intensity (a.u.), C is the comprehensive constant, r represents the radius of the simplified individual pore (nm), DV(r) is the distribution function with respect to pore diameter r, \(\varsigma (q,r)\) is the scattering form factor, the calculation method for \(\varsigma (q,r)\) is as follows27:
There are several different methods to calculate the pore size distribution of coal through Eq. (2). This paper uses the Carlo method30, which does not require an assumption of the type of distribution function.
According to Porod’s Law31, for an ideal two-phase system, the two phases have uniform but different electron density distributions with a clear interface. The porous theory mainly describes the relative relationship between scattering intensity and scattering angle. When there is no clear interface between the two phases, the scattering intensity of the test sample will exhibit a positive/negative deviation32. However, coal, as a non-ideal material, has inhomogeneous electron density between its internal matrix and pores. Fluctuations in electron density during the experiment cause additional scattering, leading to a positive slope (porous positive deviation phenomenon) in the porous curve at high q-value angles. Therefore, it is necessary to calibrate for these deviations. The specific scattering intensity deviation correction formula is as follows.
where K is the Porod constant.
According to Fig. 6, which shows the relationship between the effective scattering vector and the absolute scattering intensity of the three coal samples, it can be seen: in the low q-values range, the scattering intensity relationship of the three types of coal samples is I(q)(A) > I(q)(B) > I(q)(C). In the high q-values range, I(q)(C) > I(q)(B) > I(q)(A).
The results indicate that acidic and alkaline solution treatments have a significantly different impact on the scattering intensity of coal. At larger pore scales, the acidic solution results in a greater difference in electron density compared to the other two treatments. However, at smaller pore scales, the alkaline solution leads to a greater difference in electron density. This difference suggests that the porosity, chemical composition, and surface characteristics of coal responded differently to acidic and alkaline solutions, thus leading to differing scattering intensity. However, the specific changes require further analysis. Figure 7 displays the porosity deviations of the three samples and their calibration curves. It can be observed that there are regions of uneven electron density between the pores and solid particles in the coal samples, leading to the coal exhibiting a non-ideal two-phase system of solids and gases.
Changes in pore size distribution
Coal is a complex porous material composed of a matrix and internal pores and fissures. Based on SAXS experiments, the porosity of coal can be calculated using the following equation33:
where re is the Thomson electron radius, approximately equal to 2.8179 × 10–13 cm, Δpe is the electron density difference between the solid matrix and the pore of the scattering body (e/Å3), p is the porosity of the scattering body (%), \(\left( \partial \Sigma/\partial \Omega\right)\left( q \right)\) is the absolute scattering intensity of the scattering body (cm-1). The absolute scattering intensity and the relative scattering intensity can be calculated using the following equation34:
where A is the irradiated area of the sample (cm2), ΔΩ is the solid angle corresponding to a single pixel on the detector, η is the efficiency of the detector, T is the transmission rate of the sample, D is the thickness of the scattering body being measured (cm).
The pore size distribution in coal can be calculated using the maximum entropy probability method1,35. Figure 8 shows the pore size distribution of nanopores in coal after treatment with distilled water, HCl solution, and NaOH solution. It can be seen that the pore size distributions of the B and C samples show a unimodal distribution. The peak of the former is higher than that of the latter, and its post-peak decline is faster. The pore size distribution of B shows a bimodal distribution, with a rapid decline after the second peak. Figure 9 shows the distribution of nanopores in different pore size ranges after treatment with the three solutions, excluding the nanopores distributed in the 1 ~ 10 nm and 80 ~ 100 nm ranges due to their low quantity. It can be observed that nanopores in different size ranges have increased or decreased to varying degrees, indicating that acidic and alkaline solution treatments have affected the internal pore structure of coal.
The pore size distribution of coal sample B (Fig. 8b) begins to increase at 8 nm, starts to rise rapidly at 11 nm, reaches its peak at 31 nm, then decreases swiftly, and drops to its minimum value around 74 nm. This indicates that the nanopores of this sample are mainly distributed between 11 and 74 nm, but there are more nanopores distributed near a pore size of 31 nm. The pore size distribution of coal sample A (Fig. 8c) begins to increase at 5 nm, starts growing at 9 nm, reaches the first peak at 27 nm, then decreases, attains a second peak at 51 nm, followed by a rapid decline, and drops to its minimum value around 84 nm. This indicates that the nanopores of this sample are primarily distributed between 5 and 84 nm, but there are more nanopores distributed near pore sizes of 27 nm and 51 nm. The pore size distribution of coal sample C (Fig. 8d) begins to increase at 3 nm, starts to grow rapidly at 9 nm, reaches its peak at 30 nm, then decreases swiftly, and drops to its minimum value around 77 nm. This indicates that the nanopores of this sample are mainly distributed between 3 and 77 nm, but there are more nanopores distributed near a pore size of 30 nm.
From the Fig. 9, it can be seen that a large number of new nanopores structures appear in coal after treatment with acidic and alkaline solutions, and there are significant changes in the distribution of existing nanopores in the 20 ~ 70 nm pore size range. After soaking in acidic solution, the distribution of nanopores in the coal samples gradually decreases in the 20 ~ 50 nm pore size range, and increases in the 50 ~ 80 nm range. After soaking in alkaline solution, the distribution of nanopores in the coal samples mainly increases in the 10 ~ 30 nm and 50 ~ 80 nm pore size ranges, while decreasing in the 30 ~ 50 nm range. Combining the experimental results from Figs. 8 and 9 indicates that soaking in chemical solutions with different pH values causes varying degrees of change in the nanopore structure in coal, leading to different levels of changes in the internal pore distribution across various pore sizes in the coal.
Changes in the mechanical properties of coal samples
The changes in the microstructure of coal influence its mechanical properties16,36. In the mechanical experiments, the stress–strain curves of the coal samples under loading and the corresponding AE characteristics were obtained, as shown in Figs. 10 and 11. It is observed that the time required for the compaction phase of coal samples follows the trend C > A > B > Raw coal, with samples soaked in NaOH and HCl solutions exhibiting longer compaction times compared to those soaked in distilled water and the untreated raw coal. The uniaxial compressive strength of the coal samples shows a trend of Raw coal > B > A > C. After soaking in distilled water, NaOH solution, and HCl solution for 7 days, the uniaxial compressive strength of the coal samples decreased by 24.39%, 39.19%, and 47.26%, respectively. This indicates that alkaline solutions have the largest weakening effect on coal, followed by acidic solutions and distilled water. Statistical analysis using one-way ANOVA revealed significant differences between the groups (p < 0.05), confirming that the type of solution has a notable impact on the weakening of coal strength. These differences were further supported by standard deviation and variance analysis, which demonstrated the reliability of the compressive strength reduction trends.
From Fig. 11, it can be seen that for the coal samples soaked in distilled water (Fig. 11c), the AE ringing count shows multiple peaks as the stress increases, indicating sudden structural changes or rapid crack propagation in the coal at corresponding stress levels. For the coal samples soaked in HCl solution (Fig. 11b), the AE ringing count reaches a peak in the early stages of the stress–strain curve. This indicates that even under lower stress levels, the acidic solution has already caused changes in the microstructure of the coal, making it more brittle. In the experiment, coal samples soaked in NaOH solution exhibited a lower AE ringing count, which may be due to the formation of numerous defects inside the coal samples during the soaking process. This is consistent with the results of the SAXS experiment mentioned earlier, where these defects are compacted in the initial stage of stress application. However, as the stress increases, these defects begin to connect, leading to a sharp rise in the stress–strain curve at high strain stages (Fig. 11d). This suggests that coal samples soaked in alkaline solution may suddenly become unstable and rapidly fail after reaching a certain level of strain. The slow increase in the cumulative ringing count also reflects the process of gradual accumulation of what leading to sudden release. The AE ringing count data were statistically analyzed, revealing significant differences in AE activity patterns among the groups. Coal samples soaked in alkaline solution exhibited a significantly lower average ringing count during the early stages of loading compared to those soaked in distilled water and acidic solutions, with p-values < 0.05.
In summary, coal samples soaked in distilled water exhibit typical mechanical and AE characteristics. Samples soaked in acidic solution show early structural weakening, while those soaked in alkaline solution, despite a lower frequency of AE activity initially, may ultimately fail suddenly due to the accumulation of internal defects. The differences in compressive strength among the groups are attributed to the distinct chemical interactions between the coal and the solutions. Alkaline solutions promote the propagation and connection of microcracks, resulting in more severe structural damage under stress. Acidic solutions, on the other hand, primarily dissolve mineral components, increasing brittleness at lower stress levels. Distilled water causes less significant changes, maintaining relatively stable mechanical characteristics. The findings highlight the risks associated with coal soaked in alkaline solutions, where the accumulation of internal defects can lead to sudden failure, posing significant challenges in engineering applications. This underscores the need for careful monitoring and preventive measures in coal mining operations involving chemical interactions.
Micro-macroscopic damage evolution equation of coal under the synergic action of chemical solution corrosion and stress
Micro-macroscopic damage evolution of coal under stress
Research indicates that coal contains a large number of original defects such as pores and fractures37. These original defects are randomly distributed in coal, leading to a random distribution of damage when the coal is subjected to stress. Therefore, the differences in the distribution of original defects lead to variations in the mechanical properties of coal38,39. Many scholars have divided coal into numerous micro-elements with defects40, and have demonstrated that the strength variation of these micro-elements under stress follows the Weibull probability distribution function. The Weibull probability distribution function is widely recognized for its effectiveness in describing the strength variation and damage evolution of brittle materials, such as coal, which contain random initial defects like pores and fractures. These defects are statistically distributed within the material, leading to heterogeneous mechanical responses under stress. The Weibull function accounts for this randomness through its shape parameter k, which characterizes the degree of material homogeneity, and the scale parameter λ, which reflects the critical stress threshold for damage initiation. Previous studies have successfully applied the Weibull distribution to model damage in porous media and brittle materials, demonstrating its robustness in capturing micro-to-macro damage evolution16,41.
where \(\varphi (\varepsilon )\) is the Weibull distribution variable representing the damage to the micro-element structures in coal when subjected to stress. \(\varepsilon_{A}\) is the Weibull distribution parameter value corresponding to the damage function of the micro-element structures in coal. k is the shape factor parameter of the distribution function. \(\varepsilon\) is the strain measure of the coal.
Assuming that the strain variable of the coal body under stress is \(\varepsilon_{1}\), the total random fracture damage of the entire coal body can be considered as the integral sum of the damage of all micro-element structures. Therefore, the quantitative relationship between micro-macroscopic damage accumulation can be represented by the following formula:
where D1 represents the total amount of damage incurred in the coal body under uniaxial stress; \(\varepsilon_{1}\) is the strain measure of the coal body under uniaxial stress.
According to the generalized Hooke’s law and the principle of equivalent strain, the cumulative strain \(\sigma_{1}\) caused by damage in a material under the action of stress \(\varepsilon_{1}\) is equivalent to the sum of strains produced under effective stress42. Assuming that coal under uniaxial stress satisfies the following conditions:
From this, the constitutive model of coal damage under uniaxial stress can be derived as follows:
By substituting Eq. (10) into Eq. (12), the micro-macroscopic damage mechanical constitutive model for coal under uniaxial loading conditions can be obtained as follows:
From Fig. 10, the boundary conditions of this model can be determined: when the coal is not subjected to stress loading, it is known that \(\sigma_{1} = 0,\varepsilon = 0,D_{1} = 0\). When the stress is loaded to the peak of the compressive strength, let the peak stress and peak strain be \(\sigma_{p}\), \(\varepsilon_{p}\), and according to the extreme value characteristics of the curve, it can be determined that \(\left. {\frac{{\partial \sigma_{p} }}{{\partial \varepsilon_{p} }}} \right|_{\begin{subarray}{l} \sigma_{1 = } \sigma_{p} \\ \varepsilon_{1 = } \varepsilon_{p} \end{subarray} } = 0\).
By incorporating the aforementioned known quantities and following the solution process of the Drucker-Prager failure criterion, the shape factor parameter k of the Weibull distribution function for coal damage under stress can be calculated, as well as the Weibull distribution parameter value \(\varepsilon_{0}\) corresponding to the damage function of the micro-element structures in coal at the time of failure, as shown below:
where \(\mu\) is the Poisson’s ratio; \(\sigma_{s}\) is the radial stress (MPa); \(\sigma_{p}\) and \(\varepsilon_{p}\) respectively represent the peak values of axial stress and axial strain (MPa).
Since the experimental process involves uniaxial loading, it is possible to set the confining pressure \(\sigma_{s} = 0\) in Eq. (12), immediately obtain the value of k under uniaxial loading as follows:
By combining Eqs. (11), (14), and (15), the micro-macroscopic evolution equation for damage evolution in coal under uniaxial loading can be represented as follows:
Damage to coal from soaking in chemical solutions of different pH values
Sections "Changes in the Microstructure of Coal" and “Changes in the mechanical properties of coal samples” have shown that soaking in acidic and alkaline solutions affects the micro-porous structure and macroscopic mechanical characteristics of coal. Therefore, it is necessary to study the micro-macroscopic damage evolution process in coal caused by treatment with solutions of different pH values. Combining the analysis from the previous sections, the damage evolution in coal due to soaking in acidic and alkaline solutions can refer to the strain equivalence principle of Eq. (11). Consequently, the micro-macroscopic damage constitutive relationship of coal during the acid–alkali solution soaking process can be transformed as follows:
Let the elastic modulus of coal after damage due to chemical solution be \(E_{s} = {\sigma \mathord{\left/ {\vphantom {\sigma \varepsilon }} \right. \kern-0pt} \varepsilon }\). Thus, the micro-macroscopic evolution equation for coal damage evolution due to acid–alkali solution soaking can be obtained as follows:
where Ds is the chemical damage variable of coal during the soaking process in solutions of different pH values; Es is the elastic modulus of coal during the soaking process in chemical solutions (GPa).
Mechanical constitutive model of micro-macroscopic damage in coal under the synergic action of chemical solution corrosion and stress
Considering the complex spatial environment of coal seams, it is known that in situ coal layers are affected not only by the hydrochemical environment within the pore structure but also by the stress disturbances generated by mining. The dynamic evolution process of micro-macroscopic damage in raw coal layers is a result of the coupled effects of hydrochemical damage and stress damage. Therefore, the analysis from the previous sections allows us to combine Eqs. (11) and (18) and rewrite them as the following Eq. (19), to represent the damage constitutive relationship of coal under the combined influence of chemical solution corrosion and stress disturbances.
Based on the functional relationship between material stress–strain and damage, substituting Eqs. (16) and (18) into Eq. (19) yields the specific expression for the micro-macroscopic damage variable of coal under the coupled action of chemical solution corrosion and stress:
By combining Eqs. (12) to (19) along with Eq. (20), the mechanical constitutive model for the micro-macroscopic damage in coal under the coupled action of chemical solution corrosion and stress can be represented as follows:
Verification and modification of the theoretical model
Based on the micro-macroscopic experimental results, combined with the Weibull distribution function, the mechanical constitutive model for micro-macroscopic damage in coal under the coupled action of chemical solution corrosion and stress was established. The stress–strain curve from the uniaxial compression experiment of the coal samples was compared with the curve calculated from the theoretical model, as shown in Fig. 12. Through comparison, it can be seen that there is a discrepancy between the theoretical calculations and experimental results for coal samples soaked in chemical solutions.
Based on the analysis of the SAXS experiment results mentioned earlier, coal develops a large number of new nanopores and microcracks after soaking in chemical solutions, indicating that the corrosive action of the chemical solutions has a significant damaging effect on coal. According to Fig. 11, at the same stress level, when pH < 7, the damage to the coal body gradually increases as the pH value decreases. When pH > 7, the damage to the coal body gradually increases as the pH value increases, which once again proves the correctness of the research results of Feng et al.43. This is due to the various mineral particles contained in coal reacting differently with the chemical solutions, leading to the random distribution of pores or fractures created during the soaking process. Therefore, the coupled action of chemical solution corrosion and stress is nonlinear and continuously changes throughout the entire stress loading process. Zhang36 and Wang16 achieved good results by introducing a thermo-mechanical coupling factor to modify the constitutive model of rock damage under the influence of high temperature or chemical corrosion as well as stress. Similarly, this paper considers introducing a chemical solution corrosion-stress coupling factor (as in Eq. 23), to modify the damage constitutive model (Eq. 22)36, thereby improving the model’s accuracy and applicability. Therefore, by transforming Eq. 22, the modified mechanical constitutive model for micro-macroscopic damage under the coupled action of chemical solution corrosion and stress is obtained.
where \(\mu_{0}\) is the lower limit of the chemical solution corrosion-stress coupling factor (determined by the semi-empirical method, where \(\mu\) follows a Gaussian distribution with changes in chemical solution corrosion and stress). A is the total area of the integral above the baseline of the Gaussian distribution curve. \(\omega\) is the standard deviation of the Gaussian distribution function, representing the concentration degree of the chemical solution corrosion-stress coupling effect. These related parameters are obtained as specific numerical values through fitting of experimental data.
As shown in Fig. 12, where the blue solid line represents the revised theoretical data curve, it can be observed that when considering the chemical solution corrosion-stress coupling factor H, the theoretical calculation curve of the revised mechanical constitutive model aligns well with the experimental curve. This is especially true in describing the compression, elastic, and yielding stages of the coal samples’ behavior. However, there is a discrepancy in the post-peak failure stage. This is because the parameters \(\varepsilon_{A}\) and k in the Weibull distribution function are quite sensitive to changes in the mechanical parameters of the micro-element structures in coal[47]. When the coal sample is subjected to uniaxial stress and enters the post-peak failure stage, a large number of defects in the coal’s micro-element structures develop randomly and interact with each other. This leads to an increased probability of random changes in the mechanical parameters of these micro-structures, ultimately affecting the overall macroscopic mechanical behavior of the coal sample. Therefore, for the description of the post-peak failure stage of coal under the combined influence of chemical solution corrosion and stress, it is necessary to explore new damage factors to compensate for this deficiency. The current model is more suitable for analyzing the pre-peak change characteristics of coal after soaking in chemical solutions. However, the derivation process of this coupled model also has certain referential significance for the establishment of constitutive models under other comprehensive conditions. For coal-rock materials with strong micro-macroscopic discreteness, using a mechanical constitutive model that incorporates semi-empirical correction factors is also more applicable to practical engineering.
Discussion
Our experimental results and analyses clearly show that soaking coal in chemical solutions of varying pH values can incur damage, affecting its mechanical parameters such as uniaxial compressive strength. To describe this factor, a mechanical constitutive model considering micro-macroscopic damage under the coupled action of chemical solution corrosion and stress was established based on the Weibull probability distribution function. This effectively integrates the damage to the micro-element structures in coal caused by chemical solution corrosion with the overall damage of the coal sample. A chemical solution corrosion-stress coupling factor was introduced to modify the established model, and it was validated with experimental data. The research outcomes provide useful insights for managing the stability of coal situated in water chemical environments, affected by the stress from mining disturbances, thus aiding in the safe production of coal mines.
The SAXS experiments and uniaxial compression tests also suggest that acidic and alkaline solutions have a greater impact on the microstructure and macroscopic mechanical properties of coal than distilled water. This is due to the chemical reactions that occur between the ionized hydrogen ions and hydroxide ions in acidic and alkaline solutions and the minerals in coal, namely the erosive action of the chemical solutions on coal. These reactions lead to the formation of a large number of new pores and microcracks in the coal, and even an increase in acidity or alkalinity can intensify this erosive effect11. The uniaxial compression tests showed that coal soaking in chemical solutions incur greater reduction in mechanical parameters, such as elastic modulus and peak strength than soaking in distilled water. Soaking in acidic and alkaline solutions not only causes changes in the nanopores structure of coal but also leads to alterations in parameters such as the functional groups and chemical structure of the coal itself, which greatly affect the experimental results11,42. In future research, experimental methods such as TFIR infrared spectroscopy could be employed to further analyze the experimental patterns, in order to reveal the mechanism by which changes in the inherent structure of coal during the soaking process in chemical solutions affect its mechanical properties.
The soaking of coal samples in acidic and alkaline solutions caused significant changes in the pore structure, as evidenced by the increase in porosity and specific surface area. These changes weaken the structural integrity of the coal, leading to a reduction in its compressive strength and elastic modulus. Specifically, acidic solutions primarily dissolve mineral components, increasing the connectivity of pores and reducing the material’s load-bearing capacity. Alkaline solutions, in contrast, accelerate the expansion of microcracks, causing more pronounced damage under external stress. Experimental results showed that coal samples soaked in acidic solutions experienced a 15% reduction in compressive strength, while those treated with alkaline solutions exhibited a 20% reduction. This difference can be attributed to the more aggressive microcrack propagation induced by alkaline solutions. The observed reduction in mechanical properties aligns with the SAXS-derived microstructural changes, where alkaline solutions caused a higher degree of pore expansion and connectivity compared to acidic solutions. This highlights the dominant role of microstructural damage in determining macroscopic performance.
Geological regions rich in coal often feature complex, water-containing environments, and the pH value of the groundwater environment, affected by human production activities over the long term, can change. This has varying degrees of impact on the compressive properties of the coal 14. Existing mechanical models are mostly based on macroscopic mechanical experiments or theoretical analyses to establish the correlation between micro-macroscopic levels 44. However, they often overlook the damaging effects of the water environment’s pH value on coal. This paper, based on SAXS experiments, studies the microscopic damage to coal caused by soaking in chemical solutions. Combining the Weibull probability distribution function, and considering the micro-macroscopic damage effect of the coupled action of chemical solution corrosion and stress on coal, a mechanical constitutive model for micro-macroscopic damage under the influence of chemical solution corrosion-stress coupling has been established. After validation through experimental results, the model is able to effectively reflect the strain process of coal from micro to macroscopic levels under the combined action of chemical solution corrosion and external stress. The experimental results show that the pH value of the soaking water environment has a significant impact on the compressive strength of coal, with different pH values causing varying degrees of damage to the coal. Acidic solutions tend to alter the coal’s internal pore structure and promote the formation of microfractures, while alkaline solutions significantly accelerate microcrack propagation. These microstructural changes can result in reduced compressive strength and increased brittleness of coal.
In mining production activities, especially after chemical permeability enhancement of coal seams, it is critical to consider environmental factors such as solution pH to mitigate risks. For instance, the corrosive effects of acidic and alkaline solutions on bolts and anchoring systems should be addressed by adopting corrosion-resistant materials. Additionally, leaving coal pillars of sufficient width and enhancing support strength are essential for maintaining structural stability. The findings of this study provide practical guidance for optimizing mining operations and ensuring safety in coal mines under chemically altered conditions.
Conclusion
This study employed SAXS experiments and uniaxial compression tests to investigate the effects of chemical solutions on the nanopore structure, microcrack development, and mechanical properties of coal. A mechanical constitutive model for micro-macroscopic damage under the combined action of chemical corrosion and stress was proposed. The key findings are summarized as follows:
-
1.
Chemical solutions with different pH values caused varying degrees of damage to the nanopore structure of coal, altering the pore size distribution and significantly impacting the scattering intensity of the samples.
-
2.
Coal samples soaked in distilled water exhibited typical mechanical and AE characteristics. Samples soaked in acidic solutions showed early structural weakening. Samples soaked in alkaline solutions initially displayed lower frequencies of AE activities in the initial period of loading and ultimately experienced sudden failure due to the accumulation of internal defects.
-
3.
The uniaxial compressive strength of coal samples decreased by 24.39%, 39.19%, and 47.26% after soaking in distilled water, NaOH solution, and HCl solution for 7 days, respectively. Alkaline solutions had the most severe impact, followed by acidic solutions and distilled water.
-
4.
A chemical solution corrosion-stress coupling factor was introduced to capture the nonlinear effects of chemical erosion and stress. The modified constitutive model demonstrated good agreement with the experimental results.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Nie, B. et al. Micro-scale mechanism of coal and gas outburst: A preliminary study. J. China Univ. Min. Technol. 51, 207–220 (2022).
Li, Z. et al. Multi-scale pore fractal characteristics of differently ranked coal and its impact on gas adsorption. Int. J. Min. Sci. Technol. https://doi.org/10.1016/j.ijmst.2022.12.006 (2023).
Zhang, N., Yao, S. & Wang, Y. Nanopore structure and mechanical properties in brittle tectonically deformed coals explored by atomic force microscopy. Front. Earth Sci. https://doi.org/10.3389/feart.2022.844120 (2022).
Ma, K., Jiang, H., Li, J. & Zhao, L. Experimental study on the micro alkali sensitivity damage mechanism in low-permeability reservoirs using QEMSCAN. J. Nat. Gas Sci. Eng. 36, 1004–1017. https://doi.org/10.1016/j.jngse.2016.06.056 (2016).
Geng, Y. et al. Experimental study on permeability stress sensitivity of reconstituted granular coal with different lithotypes. Fuel 202, 12–22. https://doi.org/10.1016/j.fuel.2017.03.093 (2017).
Balucan, R. D., Turner, L. G. & Steel, K. M. X-ray μCT investigations of the effects of cleat demineralization by HCl acidizing on coal permeability. J. Nat. Gas Sci. Eng. 55, 206–218. https://doi.org/10.1016/j.jngse.2018.05.007 (2018).
Yuan, M. et al. Effect of acidification on coal microstructure and CBM desorption and diffusion. Nat. Gas Ind. 42, 163–172 (2022).
Jiang, T. et al. Deterioration evolution mechanism and damage constitutive model improvement of sandstone-coal composite samples under the effect of repeated immersion. Phys. Fluids https://doi.org/10.1063/5.0208619 (2024).
Xia, D. et al. Experimental study on reducing outburst proneness of coal seam via alkaline solution. J. China Coal Soc. 40, 1768–1773 (2015).
Song, L. et al. Effect of alkali treatment on the pore structure of lignite. J. China Univ. Min. Technol. 41, 629–634 (2012).
Li, H. et al. A review of laboratory study on enhancing coal seam permeability via chemical stimulation. Fuel 330, 125561. https://doi.org/10.1016/j.fuel.2022.125561 (2022).
Cai, X., Zhou, Z., Zang, H. & Song, Z. Water saturation effects on dynamic behavior and microstructure damage of sandstone: Phenomena and mechanisms. Eng. Geol. 276, 105760. https://doi.org/10.1016/j.enggeo.2020.105760 (2020).
Cai, X., Zhou, Z., Tan, L., Zang, H. & Song, Z. Fracture behavior and damage mechanisms of sandstone subjected to wetting-drying cycles. Eng. Fract. Mech. 234, 107109. https://doi.org/10.1016/j.engfracmech.2020.107109 (2020).
Lv, X. & Ji, X. Investigating the effect of acid erosion on degradation and brittle fractures in coal using etching solutions. Energy Sources Part A Recov. Util. Environ. Effects https://doi.org/10.1080/15567036.2021.1961949 (2021).
Wang, B. et al. Experimental study on water sensitivity and salt sensitivity of lignite reservoir under different pH. J. Pet. Sci. Eng. 172, 1202–1214. https://doi.org/10.1016/j.petrol.2018.09.036 (2019).
Wang, M., Guo, Q., Tian, Y. & Dai, B. Physical and mechanical properties evolution of coal subjected to salty solution and a damage constitutive model under uniaxial compression. Mathematics 9, 3264. https://doi.org/10.3390/math9243264 (2021).
Han, T. et al. Laboratory investigation of the mode-I fracture of sandstone caused by a combination of freeze-thaw cycles and chemical solutions. Bull. Eng. Geol. Environ. 79, 3689–3706. https://doi.org/10.1007/s10064-020-01762-6 (2020).
Zheng, Z., Yang, Y. & Pan, C. The nonlinear energy model and stress–strain model of sandstone. Sci. Rep. 13, 8456. https://doi.org/10.1038/s41598-023-35145-0 (2023).
Zhang, C. et al. Strength weakening and its micromechanism in water–rock interaction, a short review in laboratory tests. Int. J. Coal Sci. Technol. 10, 10. https://doi.org/10.1007/s40789-023-00569-6 (2023).
Zhang, W., Zhang, D. & Zhao, J. Experimental investigation of water sensitivity effects on microscale mechanical Behavior of shale. Int. J. Rock Mech. Min. Sci. 145, 104837. https://doi.org/10.1016/j.ijrmms.2021.104837 (2021).
Li, H., Zhong, Z., Liu, X., Sheng, Y. & Yang, D. Micro-damage evolution and macro-mechanical property degradation of limestone due to chemical effects. Int. J. Rock Mech. Min. Sci. 110, 257–265. https://doi.org/10.1016/j.ijrmms.2018.07.011 (2018).
Li, Z.-H. A program for SAXS data processing and analysis. Chin. Phys. C 37, 108002. https://doi.org/10.1088/1674-1137/37/10/108002 (2013).
Li, Z., Wu, Z., Mo, G., Xing, X. & Liu, P. A small-angle x-ray scattering station at Beijing synchrotron radiation facility. Instrum. Sci. Technol. 42, 128–141. https://doi.org/10.1080/10739149.2013.845845 (2014).
Hammersley, A. P. FIT2D: A multi-purpose data reduction, analysis and visualization program. J. Appl. Cryst. 49, 646–652. https://doi.org/10.1107/S1600576716000455 (2016).
Mitropoulos, ACh., Stefanopoulos, K. L. & Kanellopoulos, N. K. Coal studies by small angle X-ray scattering. Microporous Mesoporous Mater. 24, 29–39. https://doi.org/10.1016/S1387-1811(98)00143-7 (1998).
Vollet, D. R., Donatti, D. A. & Ibañez, R. A. Comparative study using small-angle x-ray scattering and nitrogen adsorption in the characterization of silica xerogels and aerogels. Phys. Rev. B 69, 064202. https://doi.org/10.1103/PhysRevB.69.064202 (2004).
Xie, F. et al. In-situ SAXS study of pore structure during carbonization of non-caking coal briquettes. Fuel 262, 116547. https://doi.org/10.1016/j.fuel.2019.116547 (2020).
Jia, P., Jia, J. & Nadimi, S. Nano-scale pore distribution characterisation of coal using small angle X-ray scattering. Particuology 81, 73–85. https://doi.org/10.1016/j.partic.2022.12.014 (2023).
Zhao, Y., Guo, X., Tai, Z., Gao, Y. & Li, S. Evolution of anthracite nanopores with CO2 adsorption at different pressures by synchrotron radiation small angle X-ray scattering. Fuel 345, 128261. https://doi.org/10.1016/j.fuel.2023.128261 (2023).
Pauw, B. R., Kästner, C. & Thünemann, A. F. Nanoparticle size distribution quantification: Results of a small-angle X-ray scattering inter-laboratory comparison. J. Appl. Crystallogr. 50, 1280–1288. https://doi.org/10.1107/S160057671701010X (2017).
Porod, G. Die Röntgenkleinwinkelstreuung von dichtgepackten kolloiden systemen. Kolloid-Zeitschrift 124, 83–114. https://doi.org/10.1007/BF01512792 (1951).
Li, Z. H. et al. A negative deviation from Porod’s law in SAXS of organo-MSU-X. Microporous Mesoporous Mater. 46, 75–80. https://doi.org/10.1016/S1387-1811(01)00292-X (2001).
Xie, F. et al. Absolute intensity calibration and application at BSRF SAXS station. Nucl. Instrum. Methods Phys. Res. Sect. A Accelerators Spectrom. Detect. Assoc. Equip. 900, 64–68. https://doi.org/10.1016/j.nima.2018.05.026 (2018).
Dreiss, C. A., Jack, K. S. & Parker, A. P. On the absolute calibration of bench-top small-angle X-ray scattering instruments: A comparison of different standard methods. J. Appl. Crystallogr. 39, 32–38. https://doi.org/10.1107/S0021889805033091 (2006).
Zhang, Q. et al. Pore characterization of sandy silty soil in metro surrounding rock: A synchrotron small-angle X-ray scattering experiment. Nat. Resour. Res. https://doi.org/10.1007/s11053-023-10270-9 (2023).
Zhang, Z., Gao, F. & Xu, X. Experimental study of temperature effect of mechanical properties of granite. Rock Soil Mech. 32, 2346–2352 (2011).
Zhang, L. et al. Experimental study on evolution of fracture network and permeability characteristics of bituminous coal under repeated mining effect. Nat. Resour. Res. 31, 463–486. https://doi.org/10.1007/s11053-021-09971-w (2022).
Song, Z., Konietzky, H. & Herbst, M. Bonded-particle model-based simulation of artificial rock subjected to cyclic loading. Acta Geotech. 14, 955–971. https://doi.org/10.1007/s11440-018-0723-9 (2019).
Meng, Q., Zhang, M., Han, L., Pu, H. & Nie, T. Effects of acoustic emission and energy evolution of rock specimens under the uniaxial cyclic loading and unloading compression. Rock Mech. Rock Eng. 49, 3873–3886. https://doi.org/10.1007/s00603-016-1077-y (2016).
Rong, T., Zhou, H., Wang, L., Ren, W. & Ji, S. Coal permeability model for gas movement under the three-dimensional stress. J. China Coal Soc. 43, 1930–1937 (2018).
Song, S. et al. Fracture features of brittle coal under uniaxial and cyclic compression loads. Int. J. Coal Sci. Technol. 10, 9. https://doi.org/10.1007/s40789-023-00564-x (2023).
Zheng, C., Liu, S., Xue, S., Jiang, B. & Chen, Z. Effects of chemical solvents on coal pore structural and fractal characteristics: An experimental investigation. Fuel 327, 125246. https://doi.org/10.1016/j.fuel.2022.125246 (2022).
Feng, X.-T., Chen, S. & Zhou, H. Real-time computerized tomography (CT) experiments on sandstone damage evolution during triaxial compression with chemical corrosion. Int. J. Rock Mech. Min. Sci. 41, 181–192. https://doi.org/10.1016/S1365-1609(03)00059-5 (2004).
Cao, W., Zhang, C., He, M. & Liu, T. Voids change and statistical damage simulation method of the full deformation process for rocks. J. Hunan Univ. (Nat. Sci.) 44, 100–106 (2017).
Acknowledgements
This work was supported by National Natural Science Foundation of China (52274245, U23B2093), the opening project of the State Key Laboratory of Explosion Science and Technology (Beijing Institute of Technology, KFJJ22-15M), Youth Foundation of Social Science and Humanity Ministry of Education of China (19YJCZH087).
Author information
Authors and Affiliations
Contributions
Yaoyu Shi: Writing – review & editing, Writing – original draft, Formal analysis, Methodology. Xiangchun Li: review & editing, Formal analysis, Resources, Funding acquisition. Xiaowei Li: Software, Investigation. Haonan Song: Supervision, Resources. Xuefei Zhuo: Software, Resources. Jianhua Zeng: Software, Formal analysis. Zhenzhong Li: Formal analysis. Qingdong Qu: Writing – review & editing, Methodology.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shi, Y., Li, X., Li, X. et al. An experimental study on the synergic damage of chemical solutions and stress to coal. Sci Rep 15, 3009 (2025). https://doi.org/10.1038/s41598-025-87720-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87720-2