Abstract
Streptococcus dysgalactiae (S. dysgalactiae ) is a common pathogen of humans and various animals. However, the phylogenetic position of animal S. dysgalactiae isolates and their zoonotic potential remain unclear. Most molecular epidemiological studies explicate beta-hemolytic streptococci according to their MLST and M protein gene (emm) types. Although human S. dysgalactiae isolates are relatively well characterized, the data concerning animal isolates are scarce. Here, we report the molecular characteristics and antimicrobial resistance of S. dysgalactiae strains recovered from sheep and their genetic relationship with isolates from other animal hosts and humans. Overall, 11 PFGE pulsotypes, five MLST sequence types (STs), and two emm types were distinguished, with ST248 and stL1376 being the most prevalent, indicating genetic diversity among tested 17 ovine isolates. Some isolates exhibited resistance to doxycycline (59%), erythromycin (6%), ciprofloxacin (6%), and trimethoprim/sulfamethoxazole (6%), harboring various resistance determinants. Phylogenetic analysis showed that studied ovine isolates grouped together with human S. dysgalactiae isolates from the cases of zoonotic infections. Moreover, some ovine isolates shared identical STs and emm gene sequences with human non-invasive and invasive S. dysgalactiae strains. These findings suggest a possible link between human and ovine isolates and indicate the zoonotic potential of this pathogen.
Similar content being viewed by others
Introduction
The species Streptococcus dysgalactiae (S. dysgalactiae) belongs to beta-hemolytic streptococci. Generally, these bacteria are considered opportunistic pathogens that can colonize various host species and cause various human and animal infections1,2,3. Molecular characterization of S. dysgalactiae strains showed that identical or closely related strains were isolated from humans and animals and supported the zoonotic transmission4,5,6. However, insufficient diagnostics of S. dysgalactiae infections, frequently without complete identification of beta-hemolytic streptococci to the species level, makes difficult the assessment of the pathogenicity as well as the zoonotic potential of these bacteria.
S. dysgalactiae consists of two subspecies, S. dysgalactiae subsp. dysgalactiae (SDSD) and S. dysgalactiae subsp. equisimilis (SDSE), but confusion about the characteristics of strains in each subspecies is common in the literature2,7,8. In 1996, Vandamme et al. defined subspecies and proposed the first classification9. According to this proposal, SDSE strains were associated with human diseases, while SDSD included strains of animal origin. Two years later, Vieira et al.1 restricted the SDSD subspecies only to bovine alpha- or gamma-hemolytic Lancefield group C strains, while the SDSE subspecies included all beta-hemolytic Lancefield group C, G, or L strains, isolated from both, humans or animals1. Although this classification has been widely used for years, many studies indicate that the proposed taxonomic division needs to be verified and more accurate2,8,10,11. The studies of the genetic relationship between S. dysgalactiae strains based on the multilocus sequence analysis (MLSA) showed that SDSE is beta-hemolytic and exclusively associated with humans, while SDSD strains demonstrating all types of hemolysis and are associated with various animal species2,7. Interestingly, the results of phylogenetic studies based on whole-genome sequencing of S. dysgalactiae strains from animal and human origins are inconsistent, and the subspecies classification of isolates from animals other than cattle is not established. Pinho et al.7 showed higher ANI (average nucleotide identity) values when comparing the genomes of equine isolates with human isolates than with the genome of the bovine strain SDSD ATCC® 279577. Another ANI analysis using over 150 S. dysgalactiae isolates derived from human and various animal hosts indicated that bovine and ovine isolates formed one separate cluster and were classified as SDSD, while isolates from other animal hosts and humans formed a second cluster and should, therefore, be classified as SDSE12. In addition, the SDSE clade was divided into two subclusters, a human subcluster and a heterogeneous animal subcluster, which could be delineated in accordance with host species12. In contrast, the phylogenomic analysis of S. dysgalactiae strains conducted by Alves-Barroco et al.8 divided human and animal isolates into two clearly separated groups, corresponding to the subspecies SDSE and SDSD, respectively. Bovine SDSD isolates were grouped into a unique, distinct clade among animal cluster, while ovine strains were not analyzed8. All these observations show how crucial further research on the molecular characterization of strains of various origins is to clarify the classification of streptococci isolated from animals2,7. Given this taxonomic discordance, in order to avoid further confusion, in our paper we use the name S. dysgalactiae for strains isolated from animals other than cattle, without distinguishing between subspecies.
In sheep, S. dysgalactiae is one of the most important causative agents of outbreaks of infectious polyarthritis in lambs, which presents a high mortality rate and an important animal health issue in many countries. S. dysgalactiae infections in sheep can also be associated with clinical and subclinical mastitis, bacteriemia and meningoencephalitis13,14,15,16,17. These bacteria were among the two most common pathogens isolated from purulent or caseous lymphadenitis in sheep in Poland (34.7%, CI 95%: 21.7%-49.6%)18. S. dysgalactiae is also a significant human pathogen increasingly isolated from patients with invasive diseases, such as severe skin and soft tissue infections, necrotizing fasciitis, toxic shock syndrome, bacteremia, endocarditis, pneumonia, meningitis, and osteoarticular infections3,4,6,8. The clinical manifestations and incidences of invasive disease are comparable to those of the closely related species Streptococcus pyogenes (group A Streptococcus, GAS). Among the virulence factors associated with the pathogenesis of streptococcal infections, the cell surface M protein, encoded by the emm gene, is a significant factor related to invasive disease, allowing the bacteria to resist phagocytosis and persist in infected tissues. The emm genes are also utilized in molecular epidemiology as the most widely used method for typing streptococcal isolates3. The emm gene typing, multilocus sequence typing analysis (MLST), and pulsed-field gel electrophoresis (PFGE) have been extensively used to characterize and distinguish streptococci of different origins. Accurate characterization and comparison of strains isolated from different hosts are crucial for determining their taxonomic position and for better understanding the pathogenic and zoonotic potential of these bacteria.
Our study aimed to provide a detailed characterization of S. dysgalactiae isolates collected from sheep, focusing on their phylogenetic comparison with strains isolated from other animals and humans, as well as the determination of their phenotypic and genotypic antimicrobial resistance (AMR). This research enhances our knowledge of the zoonotic potential and AMR of S. dysgalactiae isolates, supporting the One Health concept.
Materials and methods
Bacteria used in the study
In total, 17 S. dysgalactiae isolates derived from sheep (n = 17) were included in this study. The isolates were recovered from specimens collected during post-mortem examination and preliminary identified to the species level by 16S rRNA gene sequencing as described in the paper by Didkowska et al.18. The animals originated from herds in the Małopolska region in Poland. The sources of isolates were lung (n = 11) or mediastinal and tracheobronchial lymph nodes (n = 6). Previously isolated bacteria were stored frozen at -20 °C in tryptic soy broth (Graso Biotech, Poland) supplemented with 20% glycerol (v/v) (Sigma-Aldrich, Steinheim, Germany). For this research purpose, all isolates were cultured on Columbia agar supplemented with 5% (v/v) defibrinated sheep blood (CA, Graso Biotech, Poland) at 37 °C for 24–48 h under aerobic conditions. The Lancefield serological groups were determined by the latex agglutination MICROGEN®Strep test (Graso Biotech, Poland). The reference strain SDSE ATCC®12394 and Staphylococcus aureus ATCC®29213 were also used.
DNA extraction
Genomic DNA was extracted from the 18-20 h-old bacteria culture on CA. The GenElute™ Bacterial Genomic DNA kit (Sigma-Aldrich, Steinheim, Germany) was used with some modifications to the protocol for Gram-positive bacteria. In the first step, several colonies were picked from a CA and suspended into 200 μL of the Gram-Positive Lysis Solution supplemented with lysozyme (60 mg/mL), followed by incubation for 30 min at 37 °C with shaking. Next isolation steps were performed according to the manufacturer’s instruction with a minor modification of the elution step (40 µL of the Elution buffer heated to 65 °C was used). The DNA concentration was estimated spectrophotometrically (NanoDrop, 1000 Spectrophotometer, Thermo Fisher Scientific, USA), and the DNA samples were stored at -20 °C until further analysis.
Pulsed field gel electrophoresis (PFGE) typing
The PFGE was performed according to the protocol described by Kaczmarkowska et al.19. The separation of restriction fragments was carried out in a 1.1% (w/v) agarose gel under the following conditions: a running time of 21 h, a temperature of 14 °C, a voltage gradient of 6 V/cm, an initial pulse time of 1 s and a final pulse time of 30 s. The reference strain, S. dysgalactiae subsp. equisimilis ATCC®12394, was included in the study as a marker of DNA molecular size. The molecular size of restriction fragments obtained for SDSE ATCC®12394 was assessed by comparison with fragments obtained by in silico digestion (http://insilico.ehu.es/digest/). The Gel Doc™ EZ Imaging System with Image Lab Software (version 5.2.1) (Bio-Rad, Hercules, CA, USA) was used to visualize and photograph agarose gels. Gel images were analysed using the BioNumerics software version 7.6 (Applied Maths, Sint-Martens-Latem, Belgium). The cluster analysis was performed by Unweighted Pair Group Method with Arithmetic Mean (UPGMA) using the Dice similarity coefficient (optimization and position tolerance set at 1%). Isolates were clustered using an 85% homology cut-off, above which isolates were considered closely related and assigned to the same PFGE cluster20.
Multilocus sequence typing (MLST)
All isolates were submitted to MLST analysis. PCR was performed for seven housekeeping genes (atoB, gki, gtr, murI, mutS, recP, and xpt) using primers described by McMillan et al.21. The reaction mixture (50 µL total volume) contained 25 µL of Phusion High-Fidelity PCR Master Mix with HF Buffer (2 ×) (Thermo Fisher Scientific, USA), 20 pmol of each primer (Eurofins Genomics Germany GmbH, Germany), and 40 ng of DNA template. PCR was carried out using the following conditions: initial denaturation at 95 °C for 5 min, 28 cycles of denaturation at 95 °C for 45 s, annealing at 55 °C (atoB, gki, murI, mutS and recP) or 52 °C (gtr and xpt) for 1 min and extension at 72 °C for 1 min. The final extension step at 72 °C for 2 min follows the last PCR cycle. The obtained amplicons were purified using GenElute™ PCR CleanUp Kit (Sigma-Aldrich, Steinheim, Germany) and sequenced in both the forward and reverse directions (Eurofins Genomics Germany GmbH). The sequence data were analyzed using the Chromas Lite version 2.33 (Technelysium Pty Ltd., Australia). Comparing the loci DNA sequences with the Streptococcus dysgalactiae PubMLST reference database22, a seven-digit allele code and the strain’s sequence types (STs) were determined.
The emm gene typing
The primers and PCR conditions followed the protocol for typing the emm gene encoding the M protein23. Amplicons separated by electrophoresis in 1.0% (m/v) agarose gel were purified using GenElute™ Gel Extraction Kit (Sigma-Aldrich, Steinheim, Germany) and sequenced using the Sanger technique. The sequence data were analyzed using the Chromas Lite version 2.33 (Technelysium Pty Ltd., Australia). The emm type was assigned using the Streptococci Group A Subtyping Request form Blast 2.0 server available on the CDC website24. For one representative isolate of each emm type (isolate 147o and 168o), sequences of almost the entire emm gene were obtained and analysed using BLASTN 2.16.0 + 25,26. DNA sequence was translated into amino acid sequence using Expasy translating tool (https://web.expasy.org/translate/). The nucleotide and predicted amino acids sequences were compared with the sequences from selected (blast searching) S. dysgalactiae strains, including SDSD DB49998-05 (CP033163, QGG97815), SDSE GCS2 (CP117289, ABF82013), SDSE UT10236 (DQ522163) and SDSE 74MP large (EU195123). Multiple sequence alignment was performed using the Muscle program. The emm gene sequences obtained in the current study were deposited in the GenBank database (accession numbers OR051764 and OR551293 for 168o and 147o isolates, respectively).
Phylogenetic analysis based on the ppaC and pfl genes
The ppaC (encoding inorganic pyrophosphatase) and the pfl genes (encoding pyruvate formate lyase) from the multilocus sequence analysis (MLSA) scheme developed by Bishop et al.27 were used for phylogenetic analysis and comparisons with S. dysgalactiae strains isolated from human and animal infections. The obtained amplicons were purified using GenElute™ PCR CleanUp Kit (Sigma-Aldrich, Steinheim, Germany) or ExoSAP-IT reagent (Thermo Fisher Scientific, USA) and were sequenced using the Sanger technique (Eurofins Genomics Germany GmbH). The sequence data were analyzed using the Chromas Lite version 2.33 (Technelysium Pty Ltd., Australia). The phylogenetic comparison was based on the concatenated nucleotide sequences of the ppaC and pfl genes of representative isolates belonging to different pulsotypes and sequences of various streptococcal strains retrieved from the GenBank database. The sequences were trimmed to a region consisting of 552 (ppaC) and 351 (pfl) base pairs. Sequence alignments were performed using ClustalX 2.1 software. Evolutionary analyses were conducted in MEGA X28. A phylogenetic tree was built using the Maximum Likelihood method and General Time Reversible model29, and a bootstrap test was performed with 500 replicates30.
Antimicrobial susceptibility testing
All strains were tested for antimicrobial susceptibility using a strip diffusion method with the Liofilchem® MIC Tests Strips (MIC strips) (Liofilchem, Via Scozia, Italy). The following antimicrobials, belonging to six different functional classes, were tested: penicillin G (PEN), amoxicillin with clavulanic acids (AMC) and cefotaxime (CTX) (beta-lactams), gentamicin (GEN) (aminoglycosides), doxycycline (DXT) (tetracyclines), erythromycin (ERY) (macrolides), ciprofloxacin (CIP) (fluoroquinolones) and sulfamethoxazole with trimethoprim in the ratio 1:19 (SXT). The range of concentrations tested for each antimicrobial agent is shown in Table 1. Müeller-Hinton agar supplemented with 5% (v/v) of sheep blood (MHB, Graso Biotech, Starogard Gdański, Poland) were used. A bacterial inoculum with turbidity adjusted to the 0.5 McFarland standard was prepared in NaCl 0.85% medium (BioMerieux) using the colonies obtained from culture on CA plates, incubated at 37 °C for 18–20 h. The results were read as the point of intersection of the elliptical inhibition zone with the MIC scale value on the test strip. The isolates were considered susceptible or resistant according to interpretive criteria defined by the current Antibiogram Committee of the French Microbiology Society (CA-SFM) guideline Vet2023 or Clinical and Laboratory Standards Institute (CLSI) guideline VET01S ED7:2024 (Table 1)31,32. Staphylococcus aureus ATCC®29213 was used as a quality control strain. The antimicrobial concentrations required to inhibit the growth of 50% (MIC50) and 90% (MIC90) of the isolates were determined for each antimicrobial agent tested.
Antimicrobial resistance genetic determinants
Resistant isolates were screened for the presence of horizontally transferable genes commonly associated with tetracycline resistance (tet(M), tet(O), tet(T), and tet(K/L)), MLSB resistance (erm(A), erm(B), erm(C), ermA(TR)) and trimethoprim resistance (dfr(F)). The used primer sets are listed in Additional file 1. The protocols were described in our previous study33. The strains with the tet(M) gene were also screened for transposons Tn916, Tn5801, and Tn5397, according to previously described33. The strains used as positive controls originated from the collection of the Division of Microbiology, Institute of Veterinary Medicine (Warsaw University of Life Sciences, Poland) (Streptococcus, Enterococcus and Staphylococcus species).
Statistical analysis
Confidence intervals were calculated using the online Sample Size Calculator tool34.
Results
All isolates used in the study were beta-hemolytic on sheep blood agar. Ten out of 17 isolates (58.8%, CI95%: 32.9%-81.6%) presented Lancefield group A antigens, while seven isolates (41.2%, CI95%: 18.4%-67.1%) belonged to Lancefield group C (Table 2). The isolates were then subjected to accurate molecular characterization.
PFGE
PFGE of chromosomal DNA digested with SmaI yielded 12 to 16 fragments in the approximately 20–690 kb size range. Seventeen ovine S. dysgalactiae isolates were distributed among 11 distinct PFGE pulsotypes (P1 to P11), and the similarity was between 96.8% and 53.2%. The pulsotype P4 was predominant and was observed in four isolates (23.5%, CI95%: 6.8%-49.9%) (Fig. 1). Pulsotypes P3 and P7 included three (17.6%, CI95%: 3.8%-43.4%) and two isolates (11.8%, CI95%: 1.5%-36.4%), respectively. A single isolate represented pulsotypes P1, P2, P5, P6, P8-P11. The cluster analysis of pulsotypes distinguished three clades, grouping eight (47.1%, CI95%: 23.0%-72.2%) (cluster 2) and three (17.6%, CI95%: 3.8%-43.4%) (cluster 1 and 3) isolates, which shared at least 85 similarities (closely related strains) (Fig. 1). Strains 142o, 147o and 157o had distinct pulsotypes and were not grouped in any distinguished cluster (P1, P2 and P8 pulsotype, respectively). The same pulsotypes were found in the strains of different Lancefield groups (P3, P4, P7). Distinct pulsotypes were observed among isolates belonging to the same Lancefield group, the emm type as well as ST (Table 2).
Genetic relatedness of the S. dysgalactiae isolates analyzed by PFGE. Dendrogram showing the degree of similarity among 17 tested ovine S. dysgalactiae isolates based on the results of PFGE analysis. Red lines indicate the obtained pulsotypes marked P1-P11. Three clusters (colored squares) were defined from groups of closely related isolates sharing at least 85% similarity.
MLST
Five STs were identified among tested isolates (Table 2). The main ST was ST248, shown in eleven strains (64.7%, CI95%: 38.3%-85.8%). Three strains (17.6%, CI95%: 3.8%-43.4%) belonged to ST564, and one strain to ST338. Moreover, two novel STs were defined. In the case of the 142o strain, analyses showed the presence of a novel allele in the xpt locus and the closest match to the 13 allele with two differences, including substitutions at C282 by G and G357 by A. The strain 151o showed novel alleles in the gki locus since sequencing chromatograms indicate the closest match gki35 (substitutions at C168 by T).
The emm typing
Thirteen of the 17 isolates (76.5%, CI95%: 50.1%-93.2%) were typeable for the antiphagocytic M protein gene, and two different emm types were identified, stL1376 and stL2764 (Table 2). The type stL1376 was the most common and was observed in 12 isolates (70.6%, CI95%: 44.0%-89.7%), while stL2764 was detected in only one isolate (5.9%, CI95%: 0.15%-28.7%) (147o). The type stL1376 was shared between group A and C isolates. The nucleotide sequences of almost the entire emm gene showed the highest similarity to the gene encoding YSIRK-type signal peptide-containing protein in the SDSD DB49998-05 strain (100% identity with 168o isolate) and to the M-protein genes of SDSE strains of human and animal origin (Additional file 2). The alignment of predicted amino acid sequences from analysed strains reveals the variation in size and the number of repeat blocks, as shown in Additional file 3. The first 20 amino acid residues represent the signal peptide. Only sporadic amino acid identity was observed throughout the A-repeat regions, however, a high similarity was observed in the regions encoding the B, C, and D repeats. The M proteins of 147o and 168o isolates have three B-repeats, which show 98.1% and 97.1% identity, respectively, with the corresponding amino acid region of the M protein from the SDSE GCS2 strain. The 168o isolate has two C-repeat segments with 97.1% identity with the SDSE GCS2 strain, whereas in the 147o isolate, the C region is deleted for only one C repeat (Additional file 3). Most isolates showed two fragments of different sizes (approximately 1400 and 1200 bp), however, sequencing of both fragments showed the same emm type. In the case of the remaining four isolates, although PCR for the emm gene was positive, the chromatograms obtained in sequencing, despite repetitions, were unreadable.
Phylogenetic analysis based on the ppaC and pfl genes
The phylogenetic tree based on concatenated sequences of the ppaC and pfl genes is shown in Fig. 2. All analysed S. dysgalactiae isolates formed a separated cluster which was divided into two subclusters (with high bootstrap value), the first including only SDSE human isolates and the second one representing all S. dysgalactiae isolates derived from different animal hosts and the human strain DB49998-05. Two bovine strains were outgrouped from other strains of animal origin. Notably, most of the sheep-derived isolates from this study were grouped with the human strain DB49998-05, separately from other strains of animal origin. Two isolates (142o and 147o) representing unique pulsotype (P1 and P2) and MLST profile (ST newly described in this paper and ST338) were grouped closely with dog-derived strains.
Genetic relatedness of the S. dysgalactiae isolates analyzed by the ppaC and pfl gene sequences. The phylogenetic tree based on concatenated ppaC and pfl sequences shows the position of selected strains (o indicate tested ovine isolates) and other streptococci, including S. dysgalactiae strains isolated from different hosts, S. equi and S. canis. The evolutionary history was inferred using the Maximum Likelihood method and the General Time Reversible model. The tree with the highest log likelihood (-2786.14) is shown. The concatenated data set consisted of 903 base pairs. Branches clustering sequences with greater than 80% bootstrap support are indicated. Branch lengths correspond to the number of base substitutions per site. The missing isolates were not analysed as they show the same pulsotype.
Antimicrobial susceptibility testing
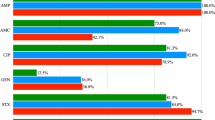
The MICs, MIC50 and MIC90 values of tested antimicrobial agents are shown in Table 3. The MIC ranges for particular antimicrobials were as follows: for PEN < 0.08–0.023 mg/L, for CTX 0.012–0.047 mg/L, for AMC 0.016–0.025 mg/L, for GEN 0.5–1.5 mg/L, for DXT 0.38- > 256 mg/L, for ERY 0.047- > 256 mg/L, for CIP 0.38–4 mg/L and for SXT 0.019- > 32 mg/L (Table 3). According to the used breakpoints (Table 1), four isolates (23.5%, CI95%: 6.8%-49.9%) were susceptible to all tested antimicrobials, including two isolates classified as intermediate to CIP. All 17 isolates were susceptible to all tested beta-lactams (PEN, CTX and AMC) and GEN. The highest frequency of resistance was noted for DXT, since ten isolates (58.8%, CI95%: 32.9%-81.6%) presented MIC values above the breakpoint (MICs > 8). Only one isolate (5.9%, CI95%: 0.15%-28.69%) was resistant to ERY, CIP or SXT. Among 17 isolates, nine displayed resistance to one antimicrobial (52.9%, CI95%: 27.8%-77.0%), and two isolates (11.8%, CI95%: 1.5%-36.4%) were resistant to two antimicrobials, DXT and SXT or DXT and ERY (Table 3). Eight isolates (47.1%, CI95%: 23.0%-72.2%) displayed intermediate resistance to CIP and one isolate to ERY.
Antimicrobial resistance genetic determinants
The distribution of the tested acquired AMR genes is presented in Table 4. Eleven isolates (64.7%, CI95%: 38.3%-85.8%) were positive for at least one of the AMR genes. The tet(M) and tet(O) genes encoding ribosomal protection proteins were found in nine (90.0%, CI95%: 55.5%-99.75%) and six (60.0%, CI95%: 26.2%-87.8%) DXT-resistant isolates, respectively. Five isolates (50.0%, CI95%: 18.7%-81.3%) carried both, the tet(O) and tet(M) genes. In four tet(M)-positive isolates (44.4%) the xis-Tn gene of the conjugative transposon Tn916 was identified. The tndX and int genes of the Tn5397 and Tn5801 conjugative elements, respectively, were not detected. In one macrolide resistant isolate the erm(B) gene was identified. All resistant isolates were negative for other tested tetracycline (tet(T), tet(K), tet(L)) and MSLB ermA(TR) resistance genes and the dfrF gene.
Discussion
Despite the emerging role of Streptococcus dysgalactiae in animal and human infections, the pathogenicity, zoonotic potential and drug resistance of this bacterium are still poorly understood. In particular, literature data regarding strains isolated from animals are scarce. To date, only two papers have undertaken in-depth studies of ovine S. dysgalactiae isolated in Norway12,15. In this research, we characterized S. dysgalactiae isolated from the lung or mediastinal and tracheobronchial lymph nodes of sheep in Poland by Didkowska et al.18. The isolates were analyzed using MLST, emm typing, and sequencing of the pfl and ppaC genes from the MLSA scheme. These methods are the most commonly used typing tools for the genetic characterization and molecular epidemiology of GAS (Group A streptococci) and S. dysgalactiae infections, enabling comparison with previously described strains from human and animal infections2,4,7,21,35,36,37,38.
The studied isolates belonged to the Lancefield groups A and C. According to literature data, group C is the most predominant serogroup in swine (100%)35 and equine S. dysgalactiae isolates (88.9%)7, while group L was found in 11.1% of equine isolates7. Also, the PubMLST database for S. dysgalactiae indicates that group C is the most common among S. dysgalactiae isolates of animal origin39. In S. dysgalactiae strains with specified serogroup, C antigen was present in 100% (23/23), 89% (99/111), 89% (24/27), and 79% (19/24) of strains isolated from cattle, horses, pigs, and dogs, respectively. The antigen L was noted in 17% (4/24), 11% (12/111), and 11% (3/27) of strains isolated from dogs, horses, and pigs, respectively. Group A has been previously recorded only in the case of one canine S. dysgalactiae isolate39. The criteria described by Vieira et al. do not include S. dysgalactiae strains with the Lancefield group A surface antigen since S. dysgalactiae subspecies equisimilis strains have been classified to Lancefield groups C, G, or L, and S. dysgalactiae subspecies dysgalactiae strains to serogroup C1. It has been assumed that S. dysgalactiae received the group A antigen through homological recombination between the ancestral group C SDSE strain and Streptococcus pyogenes belonging to group A (GAS)40. As a result, a chromosomal segment including several structural genes contained in the 12-genes group A carbohydrate biosynthetic cluster (gacA-L cluster) was transferred40. It should be highlighted that the Lancefield group A carbohydrate is not only a structural component of the cell wall but also an important virulence determinant of streptococci. Van Sorge et al.41 showed that the Lancefield group A antigen is a polyrhamnose backbone with N-acetylglucosamine side chains (GlcNAc), which play a crucial role in GAS pathogenesis, increasing bacterial survival and resistance to host innate immunity41. Thus, finding the group A antigen in 10 out of 17 tested isolates suggests the presence of the gac locus containing genes directly involved in the biosynthesis of GlcNAc affecting streptococcal virulence. SDSE strains belonging to the A serogroup, isolated from invasive human infections, are increasingly described in the literature40,42. To the best of our knowledge, among the animal-derived S. dysgalactiae strains, Lancefield’s group A has been previously noted only in S. dysgalactiae isolates from dog22 and pigs19, thus this is the first study reporting ovine S. dysgalactiae isolates belonging to the serogroup A.
Another factor that plays an important role in the pathogenesis of streptococcal infections is the surface M-protein, a multifunctional molecule with antiphagocytic activity, affecting the ability to host cell adhesion and invasion and evasion of the host immune system43,44. The M-protein is a key virulence determinant of S. pyogenes and is present in all isolates45. Homologs of this protein are also consistently found in human SDSE strains. In contrast, the emm gene encoding the M-protein is recognized less frequently in animal-associated strains. Specifically, the emm gene was amplified in only one (9%) swine isolate and 60 (56%) equine S. dysgalactiae isolates7,35. However, the same emm type was recognized in both animal and human S. dysgalactiae or even S. pyogenes strains, indicating the possibility of gene acquisition through horizontal gene transfer7,46. The emm typing has proved to be of particular importance in analyzing the molecular epidemiology of GAS and SDSE human infections3,36,37,38,40,46.
In the previous study, including ovine S. dysgalactiae isolates, emm-like genes with very high homology to SDSE and S. pyogenes M-protein genes were identified. However, they were assigned to different new emm-types12. In contrast, the emm genes detected in our isolates were typed as stL2764 and stL1376. Both these emm types had been previously identified among animal as well as human S. dysgalactiae strains6,7,35,47. Almost the entire sequence of the stL1376 emm gene from the 168o isolate showed 100% identity to the gene encoding YSIRK-type signal peptide-containing protein of the SDSD DB49998-05 strain. In fact, the arrangement of the protein encoded by this gene (QGG97815) closely resembles that found in the SDSE M protein. It includes the YSIRK signal peptide, regions representing the A, B, C, and D repeats, and a transmembrane LPxTG anchor domain (Additional file 3). Interestingly, the SDSD DB49998-05 strain was isolated from a human blood sample and described as the first case of SDSD zoonotic infection6,48. Moreover, the stL1376 type was also recognized in other S. dysgalactiae strains isolated from human blood, acute glomerulonephritis, and skin6,36,37,38. In contrast, the stL2764 was shown previously in S. dysgalactiae strains isolated from humans, horses, pigs, dogs, and lizard2,7,35,46,47.
The ovine strains characterized by Porcellato et al.12 and Smistad et al.15 represented five MLST types (ST454, ST531, ST245, ST298 and ST306), some of which (ST245, ST298, ST306) were previously exclusively identified in isolates of bovine origin. Two STs (ST454 and ST531) dominated among ovine isolates. In contrast, the STs found in most of our isolates were previously described in human and swine strains. Most of the isolates belonged to ST248, detected previously in the non-invasive SD22 strain recovered from the skin and soft tissue in humans37. Interestingly, the pan-genome analysis showed that the SD22 strain groups within a clade of different strains of animal hosts and separates from the clade grouping most strains recovered from human infection. It suggests a possible case of zoonotic infection37. Moreover, ST248 shares six allelic loci with ST341, previously ascribed to the human-derived SD DB49998-05 and DB60705-15 strains, indicating their close relationship. The other detected ST338 and ST564 were found previously in S. dysgalactiae strains from the peritoneal cavity and joint of pig and chicken, respectively. This analysis is in line with the results of phylogenetic analysis based on the ppaC and pfl gene sequences. Our isolates clustered in a clade containing strains derived from different animal hosts (dogs, pigs, fish, and horses), however they clearly separated from the bovine strains and from the human strains clade. Moreover, the analysed ppaC and pfl sequences of most tested isolates had 100% identity with human S. dysgalactiae invasive strain DB49998-05 sequences. Other Authors obtained similar results based on the MLSA scheme2,7. Moreover, Jensen and Kilian2 showed that phylogenetic trees based on concatenated seven housekeeping genes, as well as only ppaC/pfl genes, were consistent.
S. dysgalactiae subspecies’ definition remains unclear and is the subject of ongoing debate. Based on genetic comparisons, recent studies have shown that S. dysgalactiae species strains segregate into two clearly distinct clades, which correspond to both subspecies. The subspecies equisimilis includes only strains of human origin, while subspecies dysgalactiae includes strains from various animal hosts, including more phylogenetically distinct bovine strains2,7,8. The ovine strains presented in our study are clearly grouped in a clade that includes strains from animals other than cattle. In contrast, Porcellato et al.12 showed, based on a pangenome analysis, that bovine and the majority of ovine isolates were almost identical and formed a tight phylogenetic clade, clustered separately from strains of other hosts12. Only one ovine strain clustered phylogenetically with porcine isolates12. It indicates that sheep isolates are more heterogeneous and may be adapted to different host species, representing a bovine clade as well as a clade of other animal hosts. Nevertheless, further characterization of a larger number of S. dysgalactiae isolates from various animal hosts, including whole-genome sequencing, is required for accurate taxonomic delineation of S. dysgalactiae species, as well as a better understanding of the host adaptation and clarify the zoonotic potential of this pathogen.
Given that S. dysgalactiae strains derived from animals are potential zoonotic agents, it is essential to determine their phenotype and genotype of antimicrobial resistance. Nevertheless, data on antimicrobial susceptibility, including genetic resistance determinants of streptococci, are limited. In this study, beta-lactam antibiotics were highly active among antimicrobial agents against tested isolates, and similar results were obtained by other Authors4,19,35,49,50,51. However, some studies have reported a high rate of beta-lactam resistance in S. dysgalactiae isolates associated with the gene blaZ, blaTEM, blaIMP, and blaSPM-117,52. In contrast, DXT showed the highest MIC50 and MIC90 values, and these results were consistent with previous studies that revealed high tetracycline resistance among S. dysgalactiae strains worldwide, ranging between approximately 30%-90%4,19,35,49,50,51,52,53. Moreover, high tetracycline resistance has also been observed in other streptococci species33. In the tested S. dysgalactiae isolates, resistance to DXT was due to the presence of the tet(M) and tet(O) genes, and this is consistent with the data regarding the resistance in streptococci of both human and animal origin4,19,33,35,50,51,52,53. S. dysgalactiae also harbors other genes associated with tetracycline resistance, such as tet(K), tet(L), tet(S), and tet(T)4,17,50,51,52,53. In four isolates, the tet(M) gene was linked with the Tn916 transposon, and the acquired resistance facilitated by mobile genetic elements through horizontal gene transfer poses a greater threat due to easy dissemination.
The low MIC values were observed for ERY and SXT since only single isolates were resistant. The macrolide phenotype was associated with the presence of ermB. In other studies, the resistance of streptococci to macrolides was observed at various frequencies, from approximately 10%4,19,50 to even 70–80%35,53. Most resistant streptococci carried erm(B), erm(C), ermA(TR) variant, or mef(A/E) genes4,17,35,49,50,52. Nikolasein et al. reported a high rate of susceptibility to SXT [49,]. On the other hand, other Authors noted the higher frequency of resistance to SXT/sulfonamide in human SDSE isolates and SDSD isolates from cases of bovine mastitis, approximately between 9.5% and 83%, respectively51,52,54. The resistance phenotype was due to dfrF or sul1 and sul2 genes51,52. Moreover, the dfrG gene has also been detected in S. pyogenes55.
The MICs of CIP indicated a lower sensitivity of the isolates to fluoroquinolones (FQs). Reduced susceptibility to CIP (MIC 1 µg/mL) was also noted in 10 out of 30 (33%) tested mink isolates49. Furthermore, for enrofloxacin, most porcine isolates (10 out of 11, 91%) showed MIC values ranging from 1 to 2 µg/mL35. Pinho et al.56 reported a high proportion of levofloxacin-resistant (MICs > 2 µg/mL) SDSE human isolates (42 out of 314, 13%), which was associated with spontaneous mutations in gyrA, parC, or both genes. Generally, the results of antimicrobial susceptibility testing for FQs are challenging to interpret because various agents and breakpoint interpretative criteria are used. Moreover, studies reporting on FQs resistance mechanisms in streptococci are scarce. Fluoroquinolone resistance is multifactorial and complex to clarify, requiring screening for alterations (point substitutions) in the quinolone resistance determining region (QRDR) of target genes. Additionally, the expression of a quinolone efflux pump can confer low-level resistance to FQs in some streptococci56,57. However, data concerning the prevalence of mutations in S. dysgalactiae species are scarce35,56.
Conclusion
Most of the literature has focused on the characteristics of S. dysgalactiae animal strains isolated from cattle, horses, and pigs. Our study and previous data from Porcellato et al.12 indicate that ovine isolates constitute a heterogeneous group and may be adapted to different host species, clustering within a bovine clade and a clade associated with other animal hosts. Regarding the ongoing confusion over the taxonomic division of S. dysgalactiae into subspecies, the presented genetic analysis based on ppaC and pfl genes indicates that the subspecies SDSE is related to human infections, while isolates from animals belong to the subspecies SDSD. For the first time, in this study, we report that ovine isolates display an MLST type identical to those previously found only in human isolates (ST248)22,37. Moreover, these ovine isolates showed close relatedness to the human invasive SDSD strain DB49998-05, as indicated by a similar ST (sharing six out of 7 alleles) and identical sequences of the ppaC, pfl, and emm genes. These methodologies have proven particularly important and are widely used in the molecular epidemiology of beta-hemolytic streptococcal infections. The genetic relatedness of the tested isolates to strains causing human infections suggests that sheep S. dysgalactiae isolates should be considered potential zoonotic pathogens.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Vieira, V. V. et al. Genetic relationships among the different phenotypes of Streptococcus dysgalactiae strains. Int. J. Syst. Bacteriol. https://doi.org/10.1099/00207713-48-4-1231 (1998).
Jensen, A. & Kilian, M. Delineation of Streptococcus dysgalactiae, its subspecies, and its clinical and phylogenetic relationship to Streptococcus pyogenes. J. Clin. Microbiol. https://doi.org/10.1128/JCM.05900-1 (2012).
Baracco, G. J. Infections caused by group C and G Streptococcus (Streptococcus dysgalactiae subsp. equisimilis and others): epidemiological and clinical aspects. Microbiol. Spectr. 7, https://doi.org/10.1128/microbiolspec.GPP3-0016-2018; https://doi.org/10.1128/microbiolspec.GPP3-0016-2018 (2019).
Silva, L. G. et al. Group C Streptococcus dysgalactiae subsp. equisimilis in south-east Brazil: genetic diversity, resistance profile and the first report of human and equine isolates belonging to the same multilocus sequence typing lineage. J. Med. Microbiol. https://doi.org/10.1099/jmm.0.000052 (2015).
Jordal, S., Glambek, M., Oppegaard, O. & Kittang, B. R. New tricks from an old cow: infective endocarditis caused by Streptococcus dysgalactiae subsp dysgalactiae. J. Clin. Microbiol. https://doi.org/10.1128/JCM.02437-14 (2015).
Koh, T. H., Binte Abdul Rahman, N. & Sessions, O. M. Comparative genomic analysis of Streptococcus dysgalactiae subspecies dysgalactiae, an occasional cause of zoonotic infection. Pathology https://doi.org/10.1016/j.pathol.2019.09.016. (2020).
Pinho, M. D. et al. Beta-hemolytic Streptococcus dysgalactiae strains isolated from horses are a genetically distinct population within the Streptococcus dysgalactiae taxon. Sci. Rep. https://doi.org/10.1038/srep31736 (2016).
Alves-Barroco, C., Brito, P. H., Santos-Sanches, I. & Fernandes, A. R. Phylogenetic analysis and accessory genome diversity reveal insight into the evolutionary history of Streptococcus dysgalactiae. Front. Microbiol. 13, 952110; https://doi.org/10.3389/fmicb.2022.952110 (2022).
Vandamme, P., Pot, B., Falsen, E., Kersters, K. & Devriese, L. A. Taxonomic study of Lancefield streptococcal groups C, G, and L (Streptococcus dysgalactiae) and proposal of S dysgalactiae subsp equisimilis subsp. nov. Int. J. Syst. Bacteriol. https://doi.org/10.1099/00207713-46-3-774 (1996).
Vela, A. I. et al. Neonatal mortality in puppies due to bacteremia by Streptococcus dysgalactiae subsp dysgalactiae. J. Clin. Microbiol. https://doi.org/10.1128/JCM.44.2.666-668.2006 (2006).
Ciszewski, M., Zegarski, K. & Szewczyk, E. M. Streptococcus dysgalactiae subsp. equisimilis isolated from infections in dogs and humans: are current subspecies identification criteria accurate?. Curr Microbiol. https://doi.org/10.1007/s00284-016-1113-x (2016).
Porcellato, D. et al. Whole genome sequencing reveals possible host species adaptation of Streptococcus dysgalactiae. Sci. Rep. https://doi.org/10.1038/s41598-021-96710-z (2021).
Rutherford, S.-J., Jeckel, S. & Ridler, A. Characteristics of sheep flocks affected by Streptococcus dysgalactiae arthritis. Vet. Rec. https://doi.org/10.1136/vr.102781 (2015).
Ridler, A., Hickson, R., Griffiths, K., Pettigrew, E. & Kenyon, P. Effects of Streptococcus dysgalactiae polyarthritis on lamb growth and mortality and risk factors for disease. Small Rumin. Res. https://doi.org/10.1016/j.smallrumres.2019.06.008 (2019).
Smistad, M. et al. Molecular detection and genotype characterization of Streptococcus dysgalactiae from sheep flocks with outbreaks of infectious arthritis Vet. Microbiol https://doi.org/10.1016/j.vetmic.2021.109221 (2021).
Lacasta, D., Ferrer, L. M., Ramos, J. J., Loste, A. & Bueso, J. P. Digestive pathway of infection in Streptococcus dysgalactiae polyarthritis in lambs. Small Rum. Res. https://doi.org/10.1016/j.smallrumres.2008.06.001 (2008).
Ozavci, V., Dolgun, H. T. Y., Kirkan, S., Seferoglu, Y., Semen, Z., & Parin, U. Evaluation of Streptococcus species isolated from subclinical sheep mastitis by molecular methods and determination of virulence factors and antimicrobial resistance genes. Vet. Med. (Praha), https://doi.org/10.17221/42/2023-VETMED (2023)
Didkowska, A. et al. Microbiological assessment of sheep lymph nodes with lymphadenitis found during post-mortem examination of slaughtered sheep: implications for veterinary-sanitary meat control. Acta Vet. Scand. 62, 48; https://doi.org/10.1186/s13028-020-00547-x (2020).
Kaczmarkowska, A. et al. The genetic diversity and antimicrobial resistance of pyogenic pathogens isolated from porcine lymph nodes. Antibiotics (Basel) https://doi.org/10.3390/antibiotics12061026 (2023).
Tenover, F. C. et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. https://doi.org/10.1128/jcm.33.9.2233-2239.1995 (1995).
McMillan, D. J. et al. Population genetics of Streptococcus dysgalactiae subspecies equisimilis reveals widely dispersed clones and extensive recombination. PLoS One https://doi.org/10.1371/journal.pone.0011741 (2010).
Jolley, K. A., Bray, J. E. & Maiden, M. C. J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Research https://doi.org/10.12688/wellcomeopenres.14826.1 (2018).
Frost, H. R. et al. Updated emm-typing protocol for Streptococcus pyogenes. Clin. Microbiol. Infect. 26(946), e5-946.e8. https://doi.org/10.1016/j.cmi.2020.02.026 (2020).
Streptococci Group A Subtyping Request Form Blast 2.0 Server. Available online: https://www2.cdc.gov/vaccines/biotech/strepblast.asp
Zhang, Z., Schwartz, S., Wagner, L. & Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Bio.l, 7, 203–214, (2000).
Morgulis, A. et al. Database Indexing for Production MegaBLAST Searches. Bioinformatics 24, 1757–1764 (2008).
Bishop, C. J. et al. Assigning strains to bacterial species via the internet. BMC Biol. https://doi.org/10.1186/1741-7007-7-3 (2009).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Nei, M. & Kumar (S. Molecular Evolution and Phylogenetics in Oxford University Press, 2000).
Felsenstein, J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791 (1985).
Comité de l’antibiogramme de la Société Française de Microbiologie (CA-SFM). Antibiogram Committee of the French Society of Microbiology Guidelines: Recommandations Vétérinaires 2023 [In French], (2023). Available online: https://www.sfm-microbiologie.org/wp-content/uploads/2023/06/CASFM_VET2023.pdf (2023).
Clinical and Laboratory Standards Institute (CLSI) VET01S-ED7:2024 Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals, 7th Edition, January (2024). Available online: clsivet.org/GetDoc.aspx?doc=CLSI%20VET01S%20ED7:2024&xormat=SPDF&src=BB (2024).
Stefańska, I., Kwiecień, E., Kizerwetter-Świda, M., Chrobak-Chmiel, D. & Rzewuska, M. Tetracycline, macrolide and lincosamide resistance in Streptococcus canis strains from companion animals and its genetic determinants. Antibiotics (Basel) https://doi.org/10.3390/antibiotics11081034 (2022).
Kohn, M. A. & Senyak, J. Sample Size Calculators [website]. UCSF CTSI. 12 June 2024. Available at https://www.sample-size.net/ Accessed 6 August (2024).
Oh, S. I. et al. Molecular subtyping and antimicrobial susceptibility of Streptococcus dysgalactiae subspecies equisimilis isolates from clinically diseased pigs. J. Vet. Sci. https://doi.org/10.4142/jvs.2020.21.e57 (2020).
Tewodros, W. & Kronvall, G. M protein gene (emm type) analysis of group A beta-hemolytic streptococci from Ethiopia reveals unique patterns. J. Clin. Microbiol. https://doi.org/10.1128/JCM.43.9.4369-4376.2005 (2005).
Mendes, C. I. M. Pan-genome comparison between Streptococcus dysgalactiae subsp. equisimilis isolates from human and animal sources. Master Thesis, University of Lisbon. Available online (accessed 6 August 2024): https://repositorio.ul.pt/bitstream/10451/25958/1/ulfc120728_tm_Catarina_Mendes.pdf (2016).
Srifuengfung, S., Tribuddharat, C., Sapcharoen, S. & Nitayanon, P. Prevalence of the M protein gene in group C and group G streptococci isolated from patients in Thailand. Jpn. J. Infect. Dis. https://doi.org/10.7883/yoken.JJID.2015.616 (2017).
Public databases for molecular typing and microbial genome diversity (PubMLST). Streptococcus dysgalactiae isolates database. Available online: https://pubmlst.org/bigsdb?db=pubmlst_sdysgalactiae_isolates. Accessed 6 August 2024.
Chochua, S. et al. Emergent Invasive Group A Streptococcus dysgalactiae subsp. equisimilis, United States, 2015–2018. Emerg. Infect. Dis. https://doi.org/10.3201/eid2508.181758 (2019).
van Sorge, N. M. et al. The classical Lancefield antigen of group a Streptococcus is a virulence determinant with implications for vaccine design. Cell Host Microbe https://doi.org/10.1016/j.chom.2014.05.009 (2014).
Tanaka, D. et al. Genetic features of clinical isolates of Streptococcus dysgalactiae subsp. equisimilis possessing Lancefield’s group A antigen. J. Clin. Microbiol. https://doi.org/10.1128/JCM.02188-07 (2008).
Brandt, C. M. & Spellerberg, B. Human infections due to Streptococcus dysgalactiae subspecies equisimilis. Clin. Infect. Dis. https://doi.org/10.1086/605085 (2009).
Laabei, M. & Ermert, D. Catch me if you can: Streptococcus pyogenes complement evasion strategies. J. Innate Immun. https://doi.org/10.1159/000492944 (2019).
Fischetti, V. A. M protein and other surface proteins on Streptococcus pyogenes. In: Ferretti JJ, Stevens DL, Fischetti VA, eds. Streptococcus pyogenes: Basic Biology to Clinical Manifestations. 2nd ed. Oklahoma City (OK): University of Oklahoma Health Sciences Center; June 1, 2022 (2022).
The M type-specific sequence databases. Available online (accessed 6 August 2024): https://ftp.cdc.gov/pub/infectious_diseases/biotech/tsemm/
Loubinoux J. et al. CNR-Strep Network. Adult invasive and noninvasive infections due to Streptococcus dysgalactiae subsp. equisimilis in France from 2006 to 2010. J. Clin. Microbiol. (2013).
Koh, T. H. et al. Streptococcal cellulitis following preparation of fresh raw seafood. Zoonoses Public Health https://doi.org/10.1111/j.1863-2378.2008.01213.x (2009).
Nikolaisen, N. K. et al. Antimicrobial resistance among pathogenic bacteria from mink (Neovison vison) in Denmark. Acta Vet. Scand. https://doi.org/10.1186/s13028-017-0328-6 (2017).
Alves-Barroco, C. et al. New insights on Streptococcus dysgalactiae subsp. dysgalactiae isolates. Front Microbiol. https://doi.org/10.3389/fmicb.2021.686413 (2021).
Rojo-Bezares, B. et al. Streptococcus dysgalactiae subsp. equisimilis from invasive and non-invasive infections in Spain: combining epidemiology, molecular characterization, and genetic diversity. Eur. J. Clin. Microbiol. Infect. Dis. https://doi.org/10.1007/s10096-020-04119-9 (2021).
Lu, B. et al. Molecular characterization and antibiotic resistance of clinical Streptococcus dysgalactiae subsp. equisimilis in Beijing. China. Infect. Genet. Evol. https://doi.org/10.1016/j.meegid.2016.01.030 (2016).
Zhang, S. et al. Phenotypic and genotypic characterization of antimicrobial resistance profiles in Streptococcus dysgalactiae isolated from bovine clinical mastitis in 5 provinces of China. J. Dairy Sci. https://doi.org/10.3168/jds.2017-14031 (2018).
McDougall, S., Hussein, H. & Petrovski, K. Antimicrobial resistance in Staphylococcus aureus, Streptococcus uberis and Streptococcus dysgalactiae from dairy cows with mastitis. N. Z. Vet. J. https://doi.org/10.1080/00480169.2013.843135 (2014).
Bergmann, R., van der Linden, M., Chhatwal, G. S. & Nitsche-Schmitz, D. P. Factors that cause trimethoprim resistance in Streptococcus pyogenes. Antimicrob. Agents Chemother. https://doi.org/10.1128/AAC.02282-13 (2014).
Pinho, M. D., Melo-Cristino, J. & Ramirez, M. Portuguese Group for the Study of Streptococcal Infections. Fluoroquinolone resistance in Streptococcus dysgalactiae subsp. equisimilis and evidence for a shared global gene pool with Streptococcus pyogenes. Antimicrob. Agents Chemother. https://doi.org/10.1128/AAC.01377-09 (2010).
Bast, D. J. et al. Fluoroquinolone resistance in clinical isolates of Streptococcus pneumoniae: contributions of type II topoisomerase mutations and efflux to levels of resistance. Antimicrob. Agents Chemother. https://doi.org/10.1128/AAC.44.11.3049-3054.2000 (2000).
Acknowledgements
The authors thank Alicja Grzechnik, Małgorzata Murawska, Beata Kowalkowska, and Barbara Chojnacka for excellent technical assistance.
Funding
The publication was (co)financed by Science development fund of the Warsaw University of Life Sciences – SGGW.
Author information
Authors and Affiliations
Contributions
I.S.: Conceptualization, Methodology, Validation, Software, Investigation, Data analysis and interpretation, Funding acquisition, Writing – original draft, Writing – review & editing. E.K.: Methodology, Software, Investigation, Writing – review & editing. A.D., M.K.-Ś., D.C.-C., A.S.-G.: Resources, Writing – review & editing. P.Ż., K.A.: Resources. M.R.: Resources, Funding acquisition, Project administration, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
This non-experimental study did not require official or institutional ethical approval.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stefańska, I., Kwiecień, E., Didkowska, A. et al. Genetic analysis reveals the genetic diversity and zoonotic potential of Streptococcus dysgalactiae isolates from sheep. Sci Rep 15, 3165 (2025). https://doi.org/10.1038/s41598-025-87781-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87781-3