Abstract
Many studies have demonstrated hydrogen’s therapeutic and preventive effects on various diseases. Its selective antioxidant properties against hydroxyl radicals, which are responsible for the indirect effects of ionizing radiation, may make it worthy of attention as a new radio-protector. We recently developed new hydrogen water that is more stable and has higher antioxidant activity by using ultra-fine bubbles. In this study, female C57BL/6J mice given ad libitum access to ultra-fine bubble hydrogen water (UBHW) were subjected to whole-body irradiation (WBI) with X-rays, and the radio-protective effect of UBHW was evaluated. WBI with 6.0 Gy (sub-lethal dose) resulted in a 30-day survival rate of 100% in UBHW-fed mice, compared with 37% in control mice. In the case of WBI with 6.5 Gy (lethal dose), while the control mice died out in about 3 weeks, the 30-day survival rate improved to 40% by UBHW due to the high scavenging activity of hydroxy radicals. Twenty-six serum proteins involved in inflammatory and immune responses were significantly identified in UBHW-fed mice by proteomics, and UBHW may enhance and regulate these functions, resulting in reduced damage in mice exposed to WBI. We conclude that UBHW has good potential in radio-protection, with evidence that warrants further research efforts in this field.
Similar content being viewed by others
Introduction
The main biological effects of ionizing radiation can be broadly divided into direct effects, which cause not only DNA strand breakage but also alterations to the DNA bases and sugar-phosphate backbone, and indirect effects, in which reactive oxygen species (ROS) and free radicals, such as hydroxy radical (\(\bullet{\rm OH}\)) superoxide anion (\({\rm O_{2}}^{\bullet-}\))and hydrogen peroxide (H2O2) generated by ionizing and exciting water molecules in the organism are involved1,2. About 70% of radiation-induced DNA damage is caused by indirect effects. Among the molecular species of ROS and free radicals, hydroxy radicals are the most reactive, nonspecifically oxidizing and modifying nucleic acids, proteins, and lipids, and exerting toxicity.
Molecular hydrogen is an antioxidant that diffuses easily in vivo and selectively reduces highly toxic radicals. Ohsawa et al. reported that inhalation of hydrogen gas could alleviate cerebral ischemia-reperfusion injury3. They showed that hydrogen is an antioxidant, selectively reducing the highly oxidizing hydroxy radicals, and peroxynitrite (ONOO-) formed by the direct reaction of superoxide anion and nitric oxide (NO), respectively, but does not react with other ROS such as superoxide anion or hydrogen peroxide. Subsequently, a wide range of applications of hydrogen gas therapy has been reported in clinical and preclinical studies, including brain diseases, inflammatory bowel diseases, and vascular diseases4,5,6,7,8. Furthermore, as hydrogen reduces hydroxy radicals produced by radiation, several radiation damage-reducing effects of hydrogen have been reported9,10. Hirano et al. summarized the protective effects of hydrogen in animal models against various radiation injuries, including cognitive function, immune system, lung, cardiac, gastrointestinal, hematopoietic, testicular, skin, and cartilage disorders10. In most of these reports, hydrogen-rich solutions were used, which are easy to handle. Under normal atmospheric pressure, hydrogen is slightly soluble in water up to 0.8 mM (about 1.6 ppm, wt/vol); hydrogen gas is so small in size and molecular weight that it rapidly permeates the walls of glass and plastic containers, while aluminum walls can retain hydrogen gas for a relatively long time9. Technological advances to develop water with hydrogen trapped in smaller particles have enabled higher dissolved hydrogen concentrations and longer dissolution periods11,12. Recently, we have developed a method to produce more stable hydrogen water by dispersing hydrogen in water using ultra-fine bubbles with a diameter of less than 1 μm13,14. These studies have reported the functional characteristics of long-life ultra-fine bubble hydrogen water (UBHW), which has excellent antioxidant activity and storage stability. UBHW is also expected to be used in medical applications to treat the onset and complications of lifestyle-related diseases, such as aging, arteriosclerosis, and diabetes, caused by oxidative stress, as well as radiation damage induced by ionizing radiation exposure. Its selective antioxidant properties against hydroxyl radicals, which are responsible for the indirect effects of ionizing radiation, may make it worthy of attention as a new radio-protector. However, the details of the action of UBHW in reducing radiation damage are not known, nor has a detailed assessment of the effects on biological components been carried out. In this study, we used a mouse model of severe acute radiation syndrome (ARS) that had been subjected to whole-body irradiation (WBI) with a lethal dose of X-rays and allowed them to consume UBHW ad libitum during feeding. The effectiveness of UBHW in protecting against radiation-induced damage was evaluated by 30-day survival rate and proteome analysis of the serum of surviving mice using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Materials and methods
Experimental design
All procedures pertaining to animals were reviewed and approved by the animal experiment committee of Hirosaki University (approval number: G17001) and performed in accordance with relevant guidelines and regulations. Animal studies were conducted in compliance with ARRIVE guidelines. All efforts were made to minimize the number of animals used and their suffering in this study, which aligns with current animal welfare regulations. The corresponding figure legends indicate the number of mice used in each experiment. Seven-week-old female C57BL/6JJcl mice were delivered from the breeding facilities of CLEA Japan, Inc. (Tokyo, Japan). The mice were housed in the conventional clean vivarium. Experimental animals were identified by tail tattoo and housed up to 4 per sterile polycarbonate cage with stainless wire cover with paper bedding. The animal room was maintained at an ambient temperature of 23 °C with 50% relative humidity, and a 12 h light/dark cycle. These mice had ad libitum access to sterilized standard laboratory mouse chow CE-2 and drinking water and were acclimatized for a week before the start of each study. Cages, chow, and drinking water were replaced with new ones once a week. We randomly divided the mice into three groups that received different types of drinking water from each water bottle immediately after delivery of these mice: tap water (TW) in Hirosaki City, UBHW, or ultra-fine bubble oxygen water (UBOW). For the evaluation of 30-day survival rate, 8 mice were prepared in the TW group, 10 in the UBHW group, and 10 in the UBOW group. The mouse was monitored at fixed times twice daily to determine general health, including weight fluctuations, food and water intake, animal activity, gasping, and fur condition. A humane endpoint was used to exclude animals during the study, and only mice surviving 30 days were included in the analysis. However, the 30-day survival analysis did not include a humane endpoint in order to assess the exact date of death. Different researchers were involved in raising the mice, X-irradiating the mice, collecting samples from the mice, and analyzing the samples.
Preparation of ultra-fine bubble water
According to our previous reports, ultra-fine bubble water was prepared from deionized water using a production system with resonant foaming and vacuum cavitation13,14. Briefly, the resonance bubble-forming is performed such that water from the primary pump is transferred to the resonance ejector, gas is sucked to be mixed with the water, the pressure conditions are adjusted using a vacuum gauge and a needle valve, and the gas-liquid mixture is subjected to resonance in the resonance bubble-forming device to form fine bubbles. The produced UBHW and UBOW were stored in water containers at 4 °C in the dark during the measurement period, and water was taken from the container each time water bottles were refilled.
In vivo WBI with X-rays
Eight-week-old mice were subjected to WBI with 6.0 Gy (sub-lethal dose) or 6.5 Gy (lethal dose) of X-rays (160 kV, 3 mA, 1.0 mm aluminum filter) at a dose rate of 0.622 Gy/min using an MX-160Labo (MediXtec, Chiba, Japan) with a distance of 300 mm between the focus and the target.
In vitro irradiation with X-rays and electron spin resonance (ESR) spectroscopy
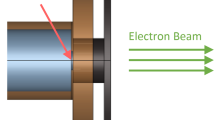
Immediately after opening (D0), 3 days (D3), 8 days (D8), 22 days (D22), and 32 days (D32) after opening, sample solutions were prepared by adding 10 uL of 1.0 M phosphate buffer (pH 7.4) and 4.5 uL of 22 mM 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) aqueous solution adjusted with high-purity secondary distilled water (DW) to 885.5 uL of DW, TW, and UBHW, respectively. The sample is ultimately a solution of 100 µM DMPO and 1 mM phosphate buffer in each aqueous solvent. The sample solutions were placed in 3 cm diameter plastic petri dishes and irradiated with 5 Gy of X-rays (200 kV, 15 mA, 1.0 mm aluminum filter) at a dose rate of 1.37 Gy/min using an X-RAD iR-225 (Precision X-Ray, North Branford, CT, USA). The amount of hydroxy radicals produced in the aqueous solution was 1.4 µM, which was calculated using the G value (G(OH) = 2.72) for the production yield of OH radicals per 100 eV. Immediately after irradiation, the sample was transferred to a flat cell (60 × 10 × 0.25 mm) and X-band CW-EPR measurements were performed at ambient temperature using a JEOL-RE1X spectrometer (JEOL, Tokyo, Japan). The EPR parameters were as follows: incident microwave power, 10 mW; microwave frequency, 9.4335 GHz; modulation frequency, 100 kHz; field modulation amplitude, 0.1 mT; time constant, 0.1 min; scan range, 335.94 ± 5.0 mT; sweep time, 2 min/10 mT; receiver gain, 2.0 × 103. Relative EPR signal intensity was estimated using the signal of a co-mounted Mn2+ digital marker (ES-DM1, JEOL) in the cavity and Win-Rad software (Radical Research, Tokyo, Japan).
Serum collection
Peripheral blood was harvested on day 30 after WBI from the orbital venous plexus of mice following anesthesia using isoflurane (Powerful Isoful; Zoetis, London, UK) by a capillary tube, and the animal was then euthanized by cervical dislocation. Samples were left at room temperature for at least 30 min to allow for clotting. Serum was collected by centrifugation at 1200× g for 10 min and stored at − 80 °C until the analysis.
Quantitative analysis of 8-hydroxydeoxyguanosine (8-OHdG)
The concentration of 8-OHdG in serum was analyzed using highly sensitive 8-OHdG check enzyme-linked immunosorbent assay (ELISA) monitoring kits (Jaica, Shizuoka, Japan) according to the manufacturer’s protocols. Each assay was performed immediately after thawing of the serum sample. To remove high-molecular-weight proteins, which interfered with the analysis, each serum sample was filtered through an ultrafiltration membrane (molecular weight cut-off, 10 kDa; Nihon Pall, Ibaraki, Japan) before ELISA assay. The spike recovery rate was within the range recommended by the kit.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS)
In order to match the number of mice based on the survival rate on day 30, 14 mice were prepared in the TW group and 10 mice in the UBHW group at the time of WBI. The detailed measurement methods were described in our previous report15. In brief, serum was diluted with ammonium bicarbonate. The serum proteins were precipitated with acetone and resuspended in ammonium bicarbonate, at this point, they were denatured with trifluoroethanol and dithiothreitol. Free cysteine residues were alkylated with iodoacetamide, which was quenched with dithiothreitol. The samples mixed with ammonium bicarbonate were incubated before trypsinization. These peptides were analyzed by LC-MS/MS using a nanoLC Eksigent 400 system coupled online to a TripleTOF 6600 mass spectrometer (AB Sciex; Framingham, MA, USA). A non-labeled quantitative method (SWATH) was used for the serum proteome analysis. Peptide peak areas were normalized to the sum of the peak areas of all measured peptides.
Identification of differentially expressed proteins and enrichment analysis
The principal component analysis (PCA) and orthogonal partial least square-discriminant analysis (OPLS-DA) was performed using the Simca software program (Infocom Corp, Tokyo, Japan). Results are visualized with the help of a volcano plot and heat map with dendrograms. We used SRplot (https://www.bioinformatics.com.cn/srplot, last accessed 5 September 2024), which is a freely accessible easy-to-use web server that integrates all of the commonly used data visualization and graphing functions together. The volcano plot was used to visualize the relationship between fold change and statistical significance, which showed that protein expression levels change as each plot moved away from the center; blue, red, and gray colors represent down-regulation, up-regulation, and no significant change, respectively. The heat maps show color-coded expression levels; color gradation from green to red indicates low to high expression levels, respectively. Protein trees were drawn horizontally, and sample trees were drawn vertically. Gene ontology (GO) enrichment analyses and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses of the differentially expressed proteins were performed using the WEB-based GEne SeT AnaLysis Toolkit (WebGestalt), which is a suite of tools for functional enrichment analysis in various biological contexts (https://www.webgestalt.org/, last accessed 5 September 2024).
Statistical analyses
The levels of significance were calculated using the Excel 2016 software program (Microsoft, Redmond, WA, USA) with the Statcel 3 add-on (OMS, Saitama). P values of < 0.05 were considered to indicate statistical significance by one-way ANOVA and Tukey-Kramer or Bonferroni/Dunn multiple comparison tests. A Kaplan-Meier analysis followed by a Mantel-Cox (log-rank) test was used to analyze the mouse survival rate.
Results
Survival of mice treated with ultra-fine bubble water up to day 30
After 6 Gy WBI, the 30-day survival rate of mice having ingested TW ad libitum was approximately 37% (Fig. 1A). In contrast, the UBHW-treated group significantly suppressed WBI-induced lethality and the survival rate increased to 100% (P = 0.0158 against TW-treated group and P = 0.0291 against UBOW-treated groups). The 30-day survival rate in the UBOW-treated group was 60%, but there was no significant difference between this and the TW-treated group (P = 0.3159). No differences in mouse body weight were observed among groups or on each measurement day (Fig. 1B). In the case of 6.5 Gy WBI, while the TW-treated group died out in about 3 weeks, the 30-day survival rate improved to 40% and 20% with UBHW intake or UBOW intake, respectively, but there was no significant difference compared with TW-treated group (Fig. 1A). All animals in the 6.5 Gy group lost body weight up to 3 weeks after WBI, whereas mice treated with UBHW or UBOW could recover to body weights similar to those at D0 by day 30 after WBI (Fig. 1B). Furthermore, there was no difference between the weekly intakes of each water (data not shown). 8-OHdG is a known DNA oxidative damage marker in which the 8-position of deoxyguanosine, a base constituting DNA, is hydroxylated. Therefore, 8-OHdG in the peripheral blood of surviving mice subjected to WBI with 6.0 Gy of X-rays on day 30 was estimated (Fig. 1C). When the hematocrit values at the time of blood collection were examined before 8-OHdG measurement, significant decrease was observed in all WBI groups compared to the control (47.6%), and these values recovered significantly more with UBHW intake (42.9%) than with TW (38.2%) or UBOW intake (32.3%). Serum 8-OHdG levels in mice that survived 30 days after WBI were significantly reduced by UBHW and UBOW intake compared to TW intake (Fig. 1C). It was suggested that constant intake of UBHW may reduce WBI-induced damage and chronic inflammatory response, which lead to survival of mice, and in subsequent experiments, we focused on only UBHW, which can have excellent life-saving effects on severe ARS mice.
Radio-protective effect of each ultra-fine bubble water on WBI mice. (A) A Kaplan-Meier plot for the survival and (B) mouse body weight of 6 Gy or 6.5 Gy X-irradiated C57Bl/6JJcl female mice treated with UBHW (n = 10), UBOW (n = 10), or TW in Hirosaki City (n = 8), respectively. In addition, mice that were not subjected to WBI and only having ingested TW were used as controls (n = 8). The statistical significance (P values of < 0.05 (*)) of the difference was determined by a log-rank test. Each point and vertical bar represent the mean ± standard deviation. (C) Hematocrit values (%) and serum 8-OHdG levels (ng/ml) were shown. Box plots show the 25%, 50% and 75% percentiles. Statistically significant differences were evaluated by a one-way ANOVA and the multiple comparison tests; P values of < 0.05 (*) or 0.01 (**).
Measurement of scavenging ability of UBHW against hydroxy radicals by ESR and spin-trapping technique
In water radiolysis, when DW is exposed to ionizing radiation, water molecules (H2O) are known to decompose to form hydroxy radicals, hydrated electrons (eaq-), and hydrogen (H). Among these water radicals, hydroxy radicals are particularly reactive. When aqueous solutions containing the spin-trapping agent DMPO are irradiated with X-rays, the spin adduct DMPO-OH is specifically formed as shown in Fig. 2A. This spin adduct has already been reported by an X-band electron spin resonance system to give a characteristic ESR spectrum with an intensity ratio of 1:2:2:1 due to the nuclear spins of the nitrogen atom (AN = 1.49 mT) and hydrogen (AHβ= 1.49 mT) at the β position of the trapping agent itself16. Suppose substance X in UBHW (XUBHW), which competitively scavenges hydroxy radicals in this aqueous solution against DMPO, is present. In that case, a competitive reaction of the following reaction equation occurs and the intensity of the ESR spectrum in the absence of XUBHW is attenuated depending on the concentration of X and the reaction rate. k(DMPO+OH) was reported as 3.4 × 109 M−1∙s−117.
ESR spectra obtained from similar irradiation experiments with TW are shown in the black ESR spectrum of Fig. 2A. The signal intensity showed little change compared to that obtained with DW (the green ESR spectrum). On the other hand, the signal intensity in similar irradiation experiments with UBHW, shown in the red ESR spectrum, was significantly reduced to about half that of DW. The relative signal intensities of TW and UBHW were plotted, with the signal intensity of the OH adduct as Hs shown in the green ESR spectrum of Fig. 2A, standardized by the signal intensity HMn2+ of the Mn2+ digital marker, which was always set to the same condition, with DW on each day as 1.0. In comparison with the signal intensity of DW, that of TW did not show any significant decrease in signal intensity from the beginning of the experiment to 32 days, while that of UBHW was found to have decreased from 50 to 40% as shown in Fig. 2B. During this experimental period, all samples were stored in the same refrigerator at 4 °C under identical conditions. These results suggest that UBHW contains substances that scavenge hydroxy radicals. The most likely candidate is hydrogen gas, but the reaction rate between hydrogen gas and hydroxy radicals is already known to be k(H2+OH) = 4.0 × 107 M−1∙s−118. From the reaction kinetics equation, k(DMPO+OH) [DMPO][OH] = k(H2+OH) [H2][OH] for 50% [OH] to react with hydrogen gas, which should be [H2] = 8.5 mM. The solubility of hydrogen gas in water at 20 °C is about 0.81 mM9. By creating ultra-fine bubbles, it is possible to confine high concentrations of hydrogen to an order of magnitude higher concentration, which is thought to increase the scavenging activity of hydroxy radicals. Our previous paper showed that the low level of the oxidation-reduction potential and the high scavenging capacity of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals in UBHW were maintained for as long as three months14, and the present experiments also showed that the radiation-induced hydroxy radical scavenging ability was maintained for more than one month. This means that the ultra-fine bubbles remain in the bottle for more than a month after they are produced. We evaluated how long the ultra-fine bubbles would remain in the bottle by irradiating the sealed polyethylene terephthalate container in which the sample was stored with green laser light (532 nm) with 1 mW power, which is commonly used as a pointer, and visually observing the scattered light in a dark room, as shown in Fig. 2C. The images of the laser irradiation of DW and UBHW shown in Fig. 2D clearly show that the scattered light originating from the ultra-fine bubbles is present in UBHW for at least one month after its production, at least as long as it was present immediately after production. Nirmalkar et al. have already reported that nanobubbles maintain their bubble size and number for 6 months19, and the present results reaffirm this property of nanobubbles.
Measurement of scavenging ability of UBHW against hydroxy radicals. (A) The representative ESR spectrum immediately after DW, TW, and UBHW containing 100 μM DMPO and 1 mM potassium phosphate were irradiated with 5 Gy, respectively. (B) Time course of the relative signal intensity of the ESR spectra, where the relative values of Hs/HMn2+ obtained in TW and UBHW are plotted when the Hs/HMn2+ in DW is standardized to 1.0. Each water sample was stored in a cool, dark place and analyzed after exposing it to X-rays on each analysis day. Three independent experiments were performed. Each point and vertical bar represent the mean ± standard deviation. Statistical treatment was performed by Student’s t-test for DW data, indicated by * when P < 0.05 and ** when P < 0.01. (C and D) The lifetime of the bubbles in the UBHW was conventionally confirmed using a laser pointer (532 nm) with 1 mW. The bottles were irradiated under the same conditions as shown in C at the time of the date noted from the first experiment to visualize the side scattering light. The photographs were taken in a dark room as shown in D.
Proteome analysis of mice treated with UBHW
LC-MS/MS was used to examine the expression of serum proteins of mice consistently having ingested UBHW or TW, respectively, on day 30 after WBI with a sub-lethal dose of X-rays, because lethal WBI of TW-fed mice results in the death of all mice. In order to elucidate the mechanism through which UBHW alleviates radiation damage, the full dataset from all mouse serum samples was subjected to a PCA to obtain an overview of the data. The first and second principal component scores were 34.0% and 25.4%, respectively, in Fig. 3A (the ellipse represents a 95% tolerance region for the scores based on Hotelling’s T2). An OPLS-DA was applied to the samples to visualize class separation. The OPLS-DA model concentrates all of the discriminating information into the first component. The score scatter plots of the OPLS-DA model in Fig. 3B demonstrated satisfactory separation between mice constantly having ingested UBHW or TW, respectively, using one predictive component and one orthogonal component, which were completely separated along the first predictive component. These results indicate that the serum proteomic profile can be used to distinguish UBHW-treated from TW-treated WBI mice.
Proteomic analysis of serum from WBI mice constantly having ingested UBHW. (A) A PCA overview of all 16 mice serum samples (n = 8 per each group). Uncharacterized samples are plotted at the center, and those with features are plotted at a distance from the center. Similar features are plotted at close positions. (B) Score scatter plots of the OPLS-DA model based on serum proteome data. A score scatter plot (left panel) and S-plot (right panel) are represented. The ellipse in each score scatter plot indicates this model’s Hotelling T2 (0.95) range. Gray circles (H) or black circles (T) mean WBI mice treated with UBHW or TW, respectively.
This analysis revealed a total of 375 differentially expressed proteins upon radiation exposure while ingesting UBHW, including 194 up-regulated and 179 down-regulated proteins, and after univariate Student’s t-test data processing, 26 (9 were up-regulated and 17 were down-regulated) proteins with a significant change in expression in UBHW relative to TW are extracted, as represented in the volcano plot (Fig. 4A). A hierarchical cluster analysis demonstrated that proteins in the UBHW-treated mice could be distinguished from that in the TW-treated mice based on their protein expression patterns, which showed the variation in 26 responsive proteins identified by Student’s t-test with a cut-off P value of 0.05 (Fig. 4B). Twenty-six proteins were listed by UniProt ID, protein name, P value, and fold-change in Table 1. A statistical analysis with GO based on the biological process criterion revealed that 9 up-regulated serum proteins due to a daily intake of UBHW were related to the “regeneration”, “regulation of Cdc42 protein signal transduction”, “high-density lipoprotein particle assembly”, “very-low-density lipoprotein particle remodeling”, “triglyceride-rich lipoprotein particle remodeling”, “high-density lipoprotein particle remodeling”, “regulation of phospholipid transport”, “positive regulation of phospholipid transport phospholipid efflux”, and “Cdc42 protein signal transduction” (Fig. 4C). These results suggest that these processes may be related to a response to UBHW. On the other hand, a statistical analysis with GO based on the biological process criterion revealed that 17 down-regulated serum proteins due to a daily intake of UBHW were related to the “inflammatory response”, “biological process involved in interspecies interaction between organisms”, “defense response”, “response to other organisms”, “response to external biotic stimulus”, “response to biotic stimulus”, “humoral immune response”, “antimicrobial humoral immune response mediated by antimicrobial peptide”, “disruption of the anatomical structure in another organism”, and “acute-phase response” (Fig. 4D).
Identification of differentially expressed serum proteins and GO functional annotation. (A) In the volcano plot, the vertical lines correspond to a 1.0-fold increase or decrease in an expression level, while the horizontal line represents a P value of 0.05. Gray points in the plot represent proteins with no statistical differences, and red/blue points represent significantly up-regulated/down-regulated proteins, respectively. (B) A heat map with dendrograms showed the variation in 26 responsive proteins, which were identified by Student’s t-test with a cut-off P value of 0.05, using the SRplot. GO enrichment analysis using variable serum proteins that were (C) up-regulated or (D) down-regulated by ad libitum intake of UBHW. Bubble plots of the GO terms in biological processes based on P-values are shown. Enrichment is defined as the ratio between intersection size and query size. Counts refer to interaction size, i.e., the number of proteins corresponding to an ontology term.
Discussion
The present study examined the effect that ad libitum administration of UBHW had on mice treated with WBI as evaluated by a 30-day survival rate and proteomic analysis of serum from surviving individuals. The 30-day survival rate of 6 Gy WBI mice having ingested UBHW increased to 100% with lethality significantly reduced in the TW-treated group from approximately 37% of controls (Fig. 1A). In contrast, the UBOW intake group improved to 60% (no significant difference). The main cause of radiation-induced biological effects is likely ROS and free radicals, such as hydroxy radicals, superoxide anion, and hydrogen peroxide, which are generated by ionization and excitation of water molecules in living organisms. This is called indirect action, as distinguished from the direct action of radiation on biomolecules. Previous studies have consistently demonstrated the radiation damage-reducing effects of hydrogen. Qian et al. examined the radioprotective properties of hydrogen water and demonstrated its ability to reduce radiation-induced oxidative stress20. Guo et al. found that hydrogen treatment diminished the detrimental effects of low-dose long-term radiation in mice21, while Qiu et al. showed that hydrogen attenuated radiation-induced intestinal damage by reducing oxidative stress and inflammatory response22. Furthermore, Ohsawa et al. reported that the inhalation of hydrogen gas ameliorated ischemia-reperfusion injury in a rat model with cerebral infarction3. This report showed that hydrogen is an antioxidant that selectively reduces highly oxidative ROS and reactive nitrogen species, such as hydroxy radicals and peroxynitrite, but does not react with other ROS, such as superoxide anion and hydrogen peroxide. Therefore, it is suggested that UBHW may have shown a survival effect in mice treated with sub-lethal WBI due to the selective reduction of hydroxy radicals and peroxynitrite by hydrogen. In addition, the present study used the ESR method to show that the amount of hydroxy radicals produced by X-irradiation of UBHW is significantly lower than in control TW (Fig. 2). In particular, the hydroxy radical scavenging capacity of UBHW was also demonstrated by the ESR methods to be maintained for at least one month after its production during the periods in survival assessment of WBI mice (Fig. 1A), suggesting that UBHW contains hydrogen that scavenge hydroxy radicals and these activities are maintained for a long period.
Accidental exposure to high doses of radiation, such as nuclear disasters and radiation accidents, can result in death from ARS due to myelosuppression and intestinal disorders21,22. Appropriate medical treatment must be given immediately after radiation exposure, but emergency medical responders who treat victims during a radiological emergency are likewise at risk of radiation exposure23. With the development and implementation of nuclear technology, the risks of internal and external exposure to ionizing radiation are increasing, so studies focusing on radiological prevention and medical countermeasures from a security perspective have been discussed on numerous occasions. Radioprotective drugs are particularly important components of emergency medical preparedness strategies for the clinical management of radiation-induced injuries24. Radioprotective agents are generally classified into four categories based on their mechanism of action: radical scavengers, agents that activate biological defense mechanisms, agents that prevent systemic absorption and deposition, and agents that promote the excretion of radionuclides24; the UBHW introduced here is thought to function mainly as a radical scavenger. Amifostine (WR2721) has been developed as a radioprotective agent with free radical scavenging properties, such as against hydroxy radicals. It is the only radioprotective agent approved by the U.S. Food and Drug Administration for clinical use25,26,27,28,29,30. However, this drug has not been widely considered a useful radioprotective agent of choice because of its dose-dependent side effects such as hypotension, nausea, and vomiting28. Hirano et al. recently showed that although hydrogen is an inactive substance, compared to other antioxidants, it is the only molecule with mitochondrial permeability and the ability to reduce hydroxy radicals, which is promising for future medical applications31,32. They suggested that selective hydroxy radical scavengers may have potential medical applications as radioprotective agents.
The present study showed that a total of 375 differentially expressed proteins were found in the analysis of serum proteins on day 30 of individuals surviving UBHW ingestion in WBI mice. Of these, 26 proteins were extracted whose expression was significantly altered in UBHW compared to TW, 9 of which were up-regulated and 17 down-regulated (Table 1). A statistical analysis with GO based on the biological processes based on 9 up-regulated serum proteins picked up the regeneration, Cdc42 protein, lipoprotein, and phospholipid transport (Fig. 4C). These proteins in the KEGG pathway analysis were involved in cholesterol metabolism, alcoholic liver disease, lipid and atherosclerosis, vitamin digestion and absorption, and African trypanosomiasis (Fig. 5A). Cdc42, a small GTPase, plays a key role in cell polarity, proliferation, and vascular repair, and endothelial Cdc42 deficiency impairs vascular regeneration after inflammatory injury33. These findings highlight the importance of Cdc42 and its lipid-mediated regulation in various cellular processes resulting from ad libitum ingestion of UBHW and suggest its potential efficacy against vascular damage induced by radiation exposure. On the other hand, a statistical analysis with GO based on the biological process revealed that 17 down-regulated serum proteins were related to the inflammatory response, acute-phase response, defense response, immune response, and disruption of the anatomical structure in another organism (Fig. 4D). These proteins in the KEGG pathway analysis were involved in cytokine-cytokine receptor interaction, mitogen-activated protein kinase (MAPK) signaling pathway, ferroptosis, and acute myeloid leukemia (Fig. 5B). MAPK signaling pathway plays a crucial role in regulating immune and stress responses in both invertebrates and vertebrates. MAPK phosphatase-1 is a key regulator of the acute innate immune response, controlling inflammation, metabolism, and acute phase response34. In addition, ferroptosis, a type of cell death driven by lipid peroxidation, is regulated by various signaling pathways and defense mechanisms, including the glutamate cystine antiporter/glutathione/glutathione peroxidase 4 axis and the NFE2L2-dependent antioxidant response35. Moreover, Morishita et al. have recently reported the antioxidant effect of H2-filled ultra-fine bubbles (H2 NanoGAS®) water against hepatic oxidative stress caused by continuous alcohol intake in rats, revealing that H2-filled ultra-fine bubbles significantly suppressed blood lipid peroxidation levels and maintained the functional activity of the mitochondrial electron transport system at normal levels36. Therefore, it is suggested that UBHW may enhance and regulate these functions, resulting in reduced damage in mice treated with sub-lethal dose WBI. However, this current study has many limitations to truly understand the radioprotective effect of UBHW, such as the sample size of mice and the number of conditions examined. Future research is essential to clarify the radioprotective effects of UBHW among dual gender, ages, and various mouse strains, the time-dependent changes in serum proteins and mitochondrial functions after radiation exposure, and the prevention effects, such as cancer, lifestyle-related diseases, and aging, and the extension of health lifespan in healthy mice. Moreover, it is necessary to investigate whether the radio-protective effect of UBHW is due solely to hydrogen, or whether the ultrafine bubbles themselves also contribute to the radio-protective effect.
Conclusion
The present study demonstrated that hydrogen water, particularly in the form of UBHW, has potential applications in reducing radiation damage. However, further research is needed to fully understand the mechanisms and potential challenges of using UBHW as a radiation protective agent.
Ethics statement
The study was approved by the animal experiment committee of Hirosaki University (approval number: G17001) and performed in accordance with ARRIVE guidelines.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Zhang, J., Shim, G., de Toledo, S. M. & Azzam, E. I. The translationally controlled tumor protein and the cellular response to ionizing radiation-induced DNA damage. Results Probl. Cell. Differ. 64, 227–253. https://doi.org/10.1007/978-3-319-67591-6_12 (2017).
Helm, J. S. & Rudel, R. A. Adverse outcome pathways for ionizing radiation and breast cancer involve direct and indirect DNA damage, oxidative stress, inflammation, genomic instability, and interaction with hormonal regulation of the breast. Arch. Toxicol. 94, 1511–1549. https://doi.org/10.1007/s00204-020-02752-z (2020).
Ohsawa, I. et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 13, 688–694. https://doi.org/10.1038/nm1577 (2017).
Dohi, K. et al. Molecular hydrogen in the treatment of acute and chronic neurological conditions: mechanisms of protection and routes of administration. J. Clin. Biochem. Nutr. 61, 1–5. https://doi.org/10.3164/jcbn.16-87 (2017).
Zhang, Y., Tan, S., Xu, J. & Wang, T. Hydrogen therapy in cardiovascular and metabolic diseases: from bench to bedside. Cell. Physiol. Biochem. 47, 1–10. https://doi.org/10.1159/000489737 (2018).
Matei, N., Camara, R. & Zhang, J. H. Emerging mechanisms and novel applications of hydrogen gas therapy. Med. Gas Res. 8, 98–102. https://doi.org/10.4103/2045-9912.239959 (2018).
Li, H., Luo, Y., Yang, P. & Liu, J. Hydrogen as a complementary therapy against ischemic stroke: a review of the evidence. J. Neurol. Sci. 396, 240–246. https://doi.org/10.1016/j.jns.2018.11.004 (2019).
Sano, M. & Tamura, T. Hydrogen Gas Therapy: from preclinical studies to clinical trials. Curr. Pharm. Des. 27, 650–658. https://doi.org/10.2174/1381612826666201221150857 (2021).
Hu, Q. et al. Molecular hydrogen: a potential radioprotective agent. Biomed. Pharmacother. 130, 110589. https://doi.org/10.1016/j.biopha.2020.110589 (2020).
Hirano, S. I. et al. Molecular hydrogen as a potential clinically applicable radioprotective agent. Int. J. Mol. Sci. 22, 4566. https://doi.org/10.3390/ijms22094566 (2021).
Liu, S. et al. Antioxidant activity of hydrogen nanobubbles in water with different reactive oxygen species both in vivo and in vitro. Langmuir 34, 11878–11885. https://doi.org/10.1021/acs.langmuir.8b02440 (2018).
Zhang, Y., Fan, W., Li, X., Wang, W. X. & Liu, S. Enhanced removal of free radicals by aqueous hydrogen nanobubbles and their role in oxidative stress. Environ. Sci. Technol. 56, 15096–15107. https://doi.org/10.1021/acs.est.2c03707 (2022).
Unites States Patent. Available online: May (2024). https://image-ppubs.uspto.gov/dirsearch-public/print/downloadPdf/10500553 (accessed on 12.
Kamimura, C., Ohba, R., Yamaguchi, M., Hosoda, M. & Kashiwakura, I. Functional characteristics of antioxidant long-life ultra-fine bubble hydrogen water. Inorganics 12, 141. https://doi.org/10.3390/inorganics12050141 (2024).
Yamaguchi, M. et al. Detection of biological responses to low-dose radiation in humans. Free Radic Biol. Med. 184, 196–207. https://doi.org/10.1016/j.freeradbiomed.2022.04.006 (2022).
Buettner, G. R. Spin trapping: ESR parameters of spin adducts. Free Radic Biol. Med. 3, 259–303. https://doi.org/10.1016/s0891-5849(87)80033-3 (1987).
Finkelstein, E., Rosen, G. M. & Rauckman, E. J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch. Biochem. Biophys. 200, 1–16. https://doi.org/10.1016/0003-9861(80)90323-9 (1980).
Farhataziz, Ross, A. B. Selected specific rates of reactions of transients from water in aqueous solution. III. Hydroxyl radical and perhydroxyl radical and their radical ions. NSRDS-NBS 59, 126 (2017).
Nirmalkar, N., Pacek, A. W. & Barigou, M. On the existence and Stability of Bulk Nanobubbles. Langmuir 34, 10964–10973. https://doi.org/10.1021/acs.langmuir.8b01163 (2018).
Qian, L., Shen, J., Chuai, Y. & Cai, J. Hydrogen as a new class of radioprotective agent. Int. J. Biol. Sci. 9, 887–894. https://doi.org/10.7150/ijbs.7220 (2013).
Guo, J. et al. Protective Effects of Hydrogen against Low-Dose Long-Term Radiation-Induced Damage to the Behavioral Performances, Hematopoietic System, Genital System, and Splenic Lymphocytes in Mice. Oxid Med Cell Longev 1947819 (2016). (2016). https://doi.org/10.1155/2016/1947819
Qiu, X., Dong, K., Guan, J. & He, J. M. Hydrogen attenuates radiation-induced intestinal damage by reducing oxidative stress and inflammatory response. Int. Immunopharmacol. 84, 106517. https://doi.org/10.1016/j.intimp.2020.106517 (2020).
Tsujiguchi, T. et al. Simulation study on radiation exposure of emergency medical responders from radioactively contaminated patients. Sci. Rep. 11, 6162. https://doi.org/10.1038/s41598-021-85635-2 (2021).
Guan, B., Li, D. & Meng, A. Development of radiation countermeasure agents for acute radiation syndromes. Anim. Model. Exp. Med. 6, 329–336. https://doi.org/10.1002/ame2.12339 (2023).
Ji, L. et al. Advances of Amifostine in Radiation Protection: Administration and Delivery. Mol. Pharm. 20, 5383–5395. https://doi.org/10.1021/acs.molpharmaceut.3c00600 (2023).
King, M. et al. Use of Amifostine for Cytoprotection during Radiation Therapy. Rev. Oncol. 98, 61–80. https://doi.org/10.1159/000502979 (2020).
Bahat, Z. et al. Could Amifostine prevent experimental Radiotherapy-Induced Acute Pericarditis? Asian Pac. J. Cancer Prev. 23, 3209–3213. https://doi.org/10.31557/APJCP.2022.23.9.3209 (2022).
Koukourakis, M. I. & Maltezos, E. Amifostine administration during radiotherapy for cancer patients with genetic, autoimmune, metabolic and other diseases. Anticancer Drugs. 17, 133–138. https://doi.org/10.1097/00001813-200602000-00003 (2006).
Kouvaris, J. R., Kouloulias, V. E. & Vlahos, L. J. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist 12, 738–747. https://doi.org/10.1634/theoncologist.12-6-738 (2007).
Wasserman, T. H. & Brizel, D. M. The role of amifostine as a radioprotector. Oncol. (Williston Park). 15, 1349–1354 (2001).
Hirano, S. I., Ichikawa, Y., Kurokawa, R., Takefuji, Y. & Satoh, F. A. Philosophical molecule, hydrogen may overcome senescence and intractable diseases. Med. Gas Res. 10, 47–49. https://doi.org/10.4103/2045-9912.279983 (2020).
Hirano, S., Ichikawa, Y., Sato, B., Satoh, F. & Takefuji, Y. Hydrogen is promising for medical applications. Clean. Technol. 2, 529–541. https://doi.org/10.3390/cleantechnol2040033 (2020).
Jiawen, L. et al. Endothelial Cdc42 deficiency impairs endothelial regeneration and vascular repair after inflammatory vascular injury. Respir Res. 19, 27. https://doi.org/10.1186/s12931-018-0729-8 (2018).
Sean, G. K., Lobelia, S. & Yusen, L. MAP kinase phosphatase-1, a gatekeeper of the acute innate immune response. Life Sci. 241, 117157. https://doi.org/10.1016/j.lfs.2019.117157 (2020).
Jiao, L., Rui, K. & Daolin, T. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 289, 7038–7050. https://doi.org/10.1111/febs.16059 (2022).
Morishita, R. et al. Effect of orally ingested Water Containing H2-Filled Ultrafine bubbles (UFBs) on ethanol-Induced oxidative stress in rats. Biol. Pharm. Bull. 47, 1106–1112. https://doi.org/10.1248/bpb.b24-00034 (2024).
Acknowledgements
The authors are grateful to Miyu Miyazaki at the Center for Scientific Equipment Management, Hirosaki University Graduate School of Medicine, for helping with the LC-MS/MS analysis.
Funding
This work was partially supported by a KAKENHI Grant-in-Aid for Scientific Research (B) (No. 21H02860 IK) and by a KAKENHI Grant-in-Aid for Young Scientists (No. 21K17887 MY).
Author information
Authors and Affiliations
Contributions
I.K. and M.H designed and managed the study; M.Y., Y.T., Y.S., C.K., O.I. and I.K. performed the experiments; M.Y., K.T.H., O.I. and I.K. wrote this manuscript. All authors contributed extensively to discussions regarding the work and the review of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yamaguchi, M., Htun, K.T., Tatara, Y. et al. Radio-protective effects of ultra-fine bubble hydrogen water and serum protein responses in whole-body radiation-exposed mice. Sci Rep 15, 4447 (2025). https://doi.org/10.1038/s41598-025-87963-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87963-z