Abstract
This study aimed to explore the effect of anthocyanin-rich black sugarcane on milk production, plasma antioxidant capacity, and the storage period DPPH scavenging capacity of milk in lactating dairy cows. Sixteen lactating dairy cows were stratified and randomly assigned into two balanced dietary groups, namely Anthocyanin-rich black sugarcane (AS), and Napier grass (NG). The AS group demonstrated a significant decrease (p < 0.05) in roughage intake, total dry matter intake, and nutrient intake. Blood glucose concentration was significantly greater in the AS group compared to the NG group. Additionally, the AS group had a significantly higher superoxide dismutase inhibition rate and 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging capacity in plasma compared to the NG group. Furthermore, the DPPH scavenging capacity in the AS group was significantly elevated (p < 0.05) after 3 d of storage period. The NG group showed a significant decrease (p < 0.05) in DPPH scavenging capacity as the storage time extended. The incorporation of AS in the diet of lactating dairy cows is posited to enhance antioxidant performance, evidenced by an elevation in plasma antioxidant capacity. Additionally, AS may bestow the added benefit of preserving milk quality by augmenting the milk intrinsic DPPH scavenging capacity during storage period.
Similar content being viewed by others
Introduction
Oxidative stress (OS) is an imbalance between the production of free radicals (FR) and the ability of the body to counteract their harmful effects, leading to potential cellular damage1. Lactating dairy cows are more susceptible to oxidative stress due to the accumulation of FR, which is caused by high metabolism and this will negatively impact on their health, welfare and productivity2. Dietary antioxidants can reduce OS by maintaining redox balance, bolstering defenses, and potentially increasing milk yield in dairy ruminants3.
Anthocyanins are water-soluble pigments that serve as potent antioxidants with stronger capacity than other antioxidants4. In fact, anthocyanins exert their antioxidant effects predominantly through the neutralization of free radicals, chelation of metal ions, modulation of antioxidant enzyme activities, and regulation of cellular signal transduction pathways3. Notably, a diet with anthocyanin-rich purple Napier grass silage could increase the concentration of antioxidant enzymes and milk lactose in dairy goats5. Similarly, Ebrahimi et al.6 found that feeding dairy goats with saffron petal extract (containing 97.79 mg/100 g of anthocyanin) could increase milk protein content, milk yield, and milk antioxidative capacity. In addition, a previous study has shown that feeding dairy goats with anthocyanin-rich purple corn silage could lead to an increase in the inhibition rate of superoxide dismutase in milk7. Furthermore, Tian et al.8 have discovered that purple corn anthocyanins possess the potential to prevent oxidation during the storage period of milk. Thus, dietary supplementation of anthocyanins is an effective way to prevent oxidative stress and improve milk quality in dairy cows.
Black sugarcane (Saccharum sinensis Robx.), a highly productive tropical cultivar, is extensively cultivated in Thailand, it is notably rich in fermentable carbohydrates9. Additionally, black sugarcane rich in anthocyanin, which has been potentially used to enhance antioxidant capacity, and improving milk yield in dairy cows10. Matsuba et al.11 reported that feeding lactating dairy cows with anthocyanin-rich purple corn could increase milk yield, and blood SOD enzyme concentration. Similarly, Suong et al.12 reported that feeding goats with anthocyanin-rich black cane could increase plasma antioxidant enzymes concentration. However, to our knowledge, there is a lack of report on the impact of anthocyanin-rich diets on free radical scavenging capacity of milk during storage. We hypothesize that dietary anthocyanin-rich black sugarcane could improve plasma antioxidative capacity, and enhancing milk DPPH scavenging capacity during the storage period. Therefore, this study aimed to explore the effect of anthocyanin-rich black sugarcane on milk production, plasma antioxidant capacity, and the storage period DPPH scavenging capacity of milk in lactating dairy cows. The results of the present study may expand our understanding of how anthocyanins-rich black sugarcane enhances antioxidant capacity in lactating cows, and providing clues to prevent milk deterioration due to oxidative stress.
Materials and methods
Animal care
The feeding trial was performed at Suranaree University of Technology (SUT) farm (Nakhon Ratchasima, Thailand) from 20 December 2023 to 30 January 2024. All animal experimental protocols were conducted in compliance with the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines. All animal manipulations were performed in accordance with the relevant guidelines and regulations of the SUT farm, and were approved following the ethics by the Animal Ethics Committee of Suranaree University of Technology issued a statement approving the experimental protocol (SUT-IACUC-0020/2023).
Treatments and management
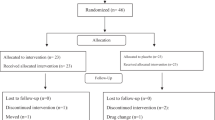
According to previous study13, a sample size of 7 (calculated as 6.83) was determined to provide power greater than 0.8 and a significance level of 0.05 for detecting a 0.7 kg/d difference in milk yield with a standard error of the mean (SEM) of 0.30. Hence, a total of 16 lactating dairy cows with similar body weight (391.84 ± 48.56 kg), day in milk (38.88 ± 15.99 d), and milk yield (11.72 ± 2.31 kg/d) were stratified and randomly assigned into two balanced dietary treatments, namely Napier grass group (NG), and anthocyanin-rich black sugarcane group (AS). All cows were administered deworming according to SUT farm feeding management code, and housed in clean individual pen (2 m × 3 m) with free access to clean water. Napier grass is a dietary staple for smallholder ruminants in subtropical areas14. Thus, Napier grass was selected as the control group. The black sugarcane and Napier grass were grown on SUT farm (Nakhon Ratchasima, Thailand). Fresh black sugarcane and Napier grass were harvested daily and processed into segments 6–8 cm lengths using the SCB-2800 cutting machine (Fermier Engineer Private Limited, Tamil Nadu, India), the commercial concentration purchased from SUT feed factory (Feed Mill, Suranaree University of Technology, Nakhon Ratchasima, Thailand). Nutrient requirement was referred to National Research Council15, concentrate was offered at 5 kg/d/head, roughage was provided ad libitum intake with 10% of refusals, the roughage was weighed and placed in empty troughs to evaluate the impact of evaporation on residual feed quality. The nutrient composition listed in Table 1. The experiment lasted 40 d, including a 10 d adapt period. All cows were fed equal amounts daily at 0800 and 1600, and the remaining feed was recorded daily during experimental period. Cows were milked in their stalls twice daily at 0500 and 1500. Milk yield was recorded daily during experimental period.
Sampling methods
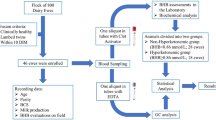
The diets were collected weekly, dried at 65 °C for 24 h, ground and passed through a 1-mm sieve, and finally stored at − 20 °C for future analysis. At the end of the experimental period, a 50 mL milk sample was collected for analysis milk composition, including protein, fat, lactose, total solid, solid not fat, and minerals, using an infrared automatic milk analyser (MilkoScan™ FT3, Foss Electric Co. Ltd., Hillerød, Denmark), blood samples were collected by jugular vein puncture, using 10 mL vacuum tubes containing heparin sodium. Plasma was harvested after centrifuged at room temperature for 15 min at 3000×g, then stored at − 80 °C until future analysis.
At the end of the experimental period, the raw milk was collected from each cow. Subsequently, the milk was subjected to high-temperature pasteurization at 75 °C for 15 s. Following pasteurization, collecting triplicate aliquots of milk from each cow for each storage period were stored in plastic tube and kept in dark at 4 °C for durations of 0, 3, and 7 d, respectively. The milk samples from each cow at corresponding time points were combined to form a composite sample, and properly preserved by being frozen at − 80 °C until analysis.
Chemical analysis
The dry matter (DM), crude protein (CP), ash, and fat were analyzed according to Association of Official Analytical Chemists16. The DM (AOAC 930.15) content was determined using an oven at 105 °C for 24 h; CP (AOAC 984.13) content was determined by Kjeldahl system (N × 6.25); ash (AOAC 942.05) was determined by incineration at 550 °C for 4 h (Carbolite AAF1100 Ashing Furnaces, Germany); fat (AOAC 920.39) content was determined by Soxtec system (Foss Co., Ltd, Hillerød, Denmark). The acid detergent fiber (ADF) and neutral detergent fiber (NDF) were determined according to the method of Van Soest et al.17.
The feed efficiency (FE) was calculated following formula: FE = milk yield/total dry matter intake. The 3.5% fat-corrected milk (3.5 FCM) was calculated by method of Hamzaoui et al.18: 3.5% FCM = kg of milk yield × [0.432 + 0.162 × (fat %)]. Milk energy (ME) and milk energy efficiency were calculated by method of Li et al.19: ME (MJ/d) = 4.185 × Milk yield (kg/d) × [0.0929 × (Fat %) + 0.0547 × (Protein %) + 0.0395 × (Lactose%)]; Milk energy efficiency (MEE, MJ/kg) = ME (MJ/d)/DMI (kg/d).
Anthocyanin extraction and detection
Three grams of each forage sample were ground in liquid nitrogen, then placed into 50 mL plastic tube with 45 mL extract reagent (the ratio of 85:15 with 95% ethanol and 1.5 N HCl) at 4 °C for 1 h in ultrasound water bath (PS40A, Shenhuatai scientific Instrument Co. Ltd. Shenzhen, China). Subsequently, centrifuged at 10,000×g for 5 min at 4 °C (Eppendorf 5810R refrigerated centrifuge, Hamburg, Germany), and stored at − 20 °C for future analysis.
The anthocyanin composition (Table 2) including, pelargonidin (Pel), pelargonidin-3-glucoside (Pel3G), peonidin-3-glucoside (Peo3G), cyanidin (Cya), cyanidin-3-glucoside (Cya3G), malvidin (Mal), malvidin-3-glucoside (Mal3G), and delphinidin (Del) were determined using high performance liquid chromatograph tandem mass spectrometry (HPLC-MS, Agilent Technologies, Santa clara, USA) according method of Tian et al.20.
Haematological indexes analysis
A part of blood sample was transferred to SUT hospital for analysis urea nitrogen (BUN), triglyceride, glucose, and insulin.
Plasma antioxidant capacity including total antioxidant capacity (TAC, kit number: 8H20K02740), superoxide dismutase (SOD; kit number: 102555633), and glutathione peroxidase (GHS-Px; kit number: 437CC12A23) were determined using the commercial kits (Sigma-Aldrich, St. Louis, USA) according to the manufacturer’s instructions.
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging ability was determined by the method of Tian et al.20 with a minor modification. In brief, 200 µL of plasma, respectively was mixed with 1 mL of DPPH reagent (100 µmol/L, PCode: 101845869, Sigma-Aldrich, Steinheim, Germany) into a 1.5 mL centrifugal tube, vortexed for 30s, then stored in the dark at room temperature (28 °C) for 30 min, subsequently, centrifuged at 12,000×g for 10 min at 4 °C (Hermle Z323, Wehingen, Germany). Subsequently, 200 µL of supernatant was remove into a 96-well plate, and the absorbance was determined at 517 nm using a microplate reader (Epoch, BioTek Instruments, Inc. Winooski, Vermont, USA). The calculation of DPPH scavenging (%DPPHSC) was performed using the following formula as follows % DPPHSC = (Ac − As) × 100/Ac. Where, Ac is the absorbance of the control and As is the absorbance of the sample.
DPPH scavenging capacity analysis of milk
Milk samples were thawed and then centrifuged at 12,000×g for 10 min at 4 °C. The supernatant was collected and mixed with 4% acetic acid to precipitate the casein according to method of Zhao et al.21. Following this, the mixture was centrifuged again at 12,000×g for 15 min at 4 °C. The supernatant (200 µL) was removed to mix with 1 mL DPPH reagent (200 µmol/L, PCode: 101845869, Sigma-Aldrich, Steinheim, Germany) into a 1.5 mL centrifugal tube, vortexed for 30s, then stored in the dark at room temperature (28 °C) for 30 min, next, centrifuged at 12,000×g for 10 min at 4 °C (Hermle Z323, Wehingen, Germany). Subsequently, 200 µL of supernatant was remove into a 96-well plate, and the absorbance was determined at 517 nm using a microplate reader (Epoch, BioTek Instruments, Inc. Winooski, Vermont, USA). The calculation of DPPH scavenging (%DPPHSC) was performed using the following formula as follows % DPPHSC = (Ac − As) × 100/Ac. Where, Ac is the absorbance of the control and As is the absorbance of the sample.
Statistics
Data regarding body weight change, feed intake, nutrient intake, milk yield, 3.5% fat-corrected milk (FCM), milk energy (ME), milk energy efficiency (MEE), milk composition, and plasma indexes were performed T-test using SPSS (Vison 27, Chicago, IL, USA). The DPPH scavenging capacity of milk during storage period was performed one-way ANOVA with Tukey test using SPSS. The significant level was set at p < 0.05.
Results
Body weight and feed intake
No significant difference (p > 0.05) was observed in body weight between two groups (Table 3). Feed intake, which included both roughage intake and total dry matter intake, was significantly lower (p < 0.05) in the AS group compared to the NG group. Additionally, nutrient intake was significantly reduced (p < 0.001) in the AS group, with the exception of EE intake. Furthermore, nutrient intake expressed as a percentage of body weight did not differ between the two groups. However, the AS group showed a significant increase (p = 0.026) in ash intake expressed as a percentage of body weight.
Milk yield and milk composition
No significant differences (p > 0.05) were observed in milk yield, 3.5% FCM, FE, and the nutrient composition of milk, or nutrient yield of milk between the two groups (Table 4).
Blood metabolic and antioxidative parameters
The concentration of blood urea nitrogen, triglycerides, and insulin did not differ (p > 0.05) between the two groups (Table 5). Notably, blood glucose concentration in the AS group was significantly higher (p < 0.05) than in the NG group. On the other hand, the SOD inhibition rate and DPPH scavenging capacity of plasma in the AS group were significantly higher (p < 0.05) than in the NG group. The concentrations of GSH-Px and TAC in plasma showed no significant difference (p > 0.05) between the two groups.
Milk DPPH scavenging capacity during storage period
During the storage period, the AS group demonstrated elevated (p < 0.05) DPPH radical scavenging capacities at the 3 and 7 d intervals (Table 6). No significant difference (p < 0.05) in DPPH scavenging capacity was observed at 0 d between the two groups. Moreover, compared to the AS group, the NG group experienced a significant decrease (p < 0.05) in DPPH scavenging capacity as the storage time extended, while the AS group showed no significant change (p > 0.05).
Discussion
Anthocyanins, a subset of phenolic compounds, are known to impart a distinctive bitter taste to plant-based foods. The bitter taste of phenolic compounds is derived from binding to the hydrophobic regions of salivary proteins, precipitating them and inducing oral sensations of dryness, roughness, and wrinkling22. Consequently, the decrease in feed intake observed in the present study may be due to the poor palatability of the anthocyanin-rich roughage. In contrast, Tian et al.7 found that feeding dairy goats with anthocyanin-rich purple corn silage had no impact on DMI. Similarly, Suong et al.12 observed that feeding with anthocyanin-rich black cane silage had no effect on DMI and BW compared to Napier grass silage. The causes of these differences may be due to the altered palatability of the roughage by the silage23, and phenolic compounds are more tolerable to goats than to cows24. Another potential reason for the difference in feed intake could be the difference in water content between Napier grass and anthocyanin-rich black sugarcane. Estrada et al.25 demonstrated that voluntary DMI of dairy cows fed fresh grass increases as the internal water content of grass decreases. Although feed intake and nutrient intake were reduced, body weight and milk yield were not affected.
Milk yield and milk composition are important parameters for predicting the lactating cow productive performance. Consistent with the findings of Tian et al.7, our study determined that feeding with anthocyanin-rich black sugarcane in the diet did not adversely affect milk yield or milk composition of lactating cows.
Blood metabolite measurements are essential for evaluating animal health and nutritional status. In this study, there was no significant effect of AS on concentration of BUN, triglyceride, and insulin. These observations are in agreement with findings of Suong et al.12 who indicated that anthocyanin rich black cane silage had no effect on BUN, triglyceride, and insulin levels in goats. Similar result was found in dairy goats26. In contrast, we observed that AS group showed a significantly higher (p < 0.05) blood glucose concentration. The reasons for this discrepancy are unclear and warrant further exploration.
Normally, the free radicals can be neutralized by the body own antioxidant capacity and maintaining a stable state. For example, the superoxide dismutase (SOD) converting superoxide anion (O2−·) into hydrogen peroxide (H2O2), then converted into H2O by glutathione peroxidase (GSH-Px)27. Anthocyanins have garnered widespread recognition for their exceptional antioxidant properties. Their antioxidant prowess is primarily exerted through several key mechanisms: scavenging of free radicals, chelation of metal ions, modulation of antioxidant enzyme activities, and regulation of signal transduction pathways within cells3. In this study, our observed that AS significantly elevated plasma SOD inhibition rate and DPPH scavenging capacity. These findings suggest that anthocyanins are bioavailable, capable of absorption into the bloodstream where they contribute electrons to O2−·, thereby enhancing antioxidant defense. This outcome aligns with the research by Tian et al.28 who reported that purple corn anthocyanin increased SOD inhibition rate, and upregulated the mRNA expression levels of SOD2 gene in dairy goat. However, the concentrations of GSH-Px and TAC did not exhibit a corresponding increase alongside the enhanced SOD inhibition rate and DPPH scavenging capacity. Suggesting that the impact of anthocyanin supplementation on antioxidant activity may be contingent upon the source of the anthocyanin compounds or the dosage thresholds integrated into the diets. For example, Suong et al.12 reported a significant elevation in the blood concentrations of TAC, SOD, and GSH-Px in goats, attributed to an intake of 56.79 mg/d anthocyanin from black cane silage. In another study, Tian et al.26 observed an increase in GSH-Px concentration in the blood of goats without a concurrent alteration in DPPH scavenging capacity, resulting from a intake of 1.31 mg/d anthocyanin from purple corn pigment. Accordingly, incorporating anthocyanin-rich black sugarcane as roughage in the diets of lactating dairy cows to enhance their antioxidant capacity presents significant practical benefits.
The oxidative stability of milk during storage is a critical concern for the dairy industry, as oxidation can lead to the development of off-flavors and a concomitant decline in the nutritional quality of these products29. Lipid oxidation generates numerous free FR, which in turn drive lipid peroxidation30, impacting milk quality and flavor. Ajmal et al.31 documented a progressive decline in DPPH free radical scavenging activity in immediately chilled raw milk following pasteurization, with losses of 25.39% after 3 days and a further decrease to 30.85% after 6 days. Silva et al.32 demonstrated that the incorporation of antioxidants in goat milk yoghurt positively influences its quality throughout the storage period. Milk antioxidants can quench or scavenge free radicals, thereby preventing milk oxidation and enhancing its antioxidant capacity33. Siwach et al.34 reported that fortifying milk with lycopene can prolong the shelf life of milk fat, leveraging its antioxidant properties.
The DPPH assay, which measures a sample’s antioxidant capacity by its ability to scavenge the DPPH radical, is sensitive for assessing milk’s shelf life, particularly in the first hours and days of storage35. In this study, we observed that the DPPH scavenging capacity was significantly higher (p < 0.05) in the AS group compared to the NG group after 3 d of storage. Conversely, as storage time increased, the DPPH scavenging ability of the NG group markedly declined (p < 0.05). Suggesting that AS supplementation in lactating dairy cows’ diets can curb lipid peroxidation by sustaining free radical scavenging, thus preserving milk flavor and shelf life. These findings align with those of Tian et al.36, who demonstrated that the addition of purple corn anthocyanin to milk can prevent lipid oxidation during storage. This effects is attributed to AS rich in anthocyanin, which act as hydrogen donors of lipid FRs in the process of lipid oxidation and can be transformed into more stable FR, enabling them to transform into more stable forms while effectively scavenging peroxyl radicals, preventing chain reactions, and inhibiting peroxide formation37. Similarly, Tian et al.8 reported that the addition of purple corn anthocyanin pigment to milk enhances antioxidant activity, and increase the sensory scores. Therefore, incorporating anthocyanin-rich black sugarcane as roughage in the diets of lactating dairy cows can mitigate lipid peroxidation, thereby prolonging milk shelf life through the preservation of milk’s free radical scavenging capacity. This strategy aligns with the consumer preference for natural, non-additive enhancements in food products.
Conclusion
We found that AS did not alter milk yield or milk composition, aligning with the similar body weight and milk nutrient profiles observed between the AS and NG groups. However, AS did lead to notable enhancements in certain physiological parameters, particularly those related to plasma antioxidant capacity. Plasma from the AS group exhibited higher SOD inhibition rates and DPPH scavenging capacities compared to the NG group. Moreover, the AS group milk exhibited a significant increase in DPPH scavenging capacity after 3 days of storage, suggesting a potential for AS to improve milk oxidative stability during storage. These results highlight the potential of AS to improve the antioxidant capacity of lactating dairy cows, which could have beneficial implications for better preserving milk quality and potentially contribute positively to cow health.
Data availability
The data used in this study will be shared upon a reasonable request to the corresponding author.
References
Filomeni, G., De Zio, D. & Cecconi, F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell. Death Differ. 22, 377–388. https://doi.org/10.1038/cdd.2014.150 (2015).
Salama, A. A., Hamzaoui, S., Albanell, E., Such, X. & Caja, G. Metabolic and behavior responses of lactating goats under heat stress. Small Ruminant Res. 203, 106496. https://doi.org/10.1016/j.smallrumres.2021.106496 (2021).
Tian, X. & Lu, Q. Anthocyanins in dairy cow nutrition: a review. Agriculture 12, 1806. https://doi.org/10.3390/agriculture12111806 (2022).
Rice-evans, C. A., Miller, N. J., Bolwell, P. G., Bramley, P. M. & Pridham, J. B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 22, 375–383. https://doi.org/10.3109/10715769509145649 (1995).
Onjai-Uea, N., Paengkoum, S., Taethaisong, N., Thongpea, S. & Paengkoum, P. Enhancing milk quality and antioxidant status in lactating dairy goats through the Dietary Incorporation of Purple Napier Grass Silage. Animals 14, 811. https://doi.org/10.3390/ani14050811 (2024).
Ebrahimi, S., Nasri, M. F. & Farhangfar, S. Dietary supplementation of saffron petal elicits positive effects on performance, antioxidant status, and health of dairy goats. Small Ruminant Res. 231, 107179. https://doi.org/10.1016/j.smallrumres.2023.107179 (2024).
Tian, X. et al. Purple corn (Zea mays L.) stover silage with abundant anthocyanins transferring anthocyanin composition to the milk and increasing antioxidant status of lactating dairy goats. J. Dairy Sci. 102, 413–418 (2019).
Tian, X. Z. et al. Effect of purple corn anthocyanin on antioxidant activity, volatile compound and sensory property in milk during storage and light prevention. Front. Nutr. 9, 862689. https://doi.org/10.3389/fnut.2022.862689 (2022).
Wanapat, M., Chumpawadee, S. & Paengkoum, P. Utilization of urea-treated rice straw and whole sugar cane crop as roughage sources for dairy cattle during the dry season. Asian-Australasian J. Anim. Sci. 13, 474–477. https://doi.org/10.5713/ajas.2000.474 (2000).
Al-Aidi, R. H. et al. The impact of anthocyanin supplementation on oxidative stress reduction and improve milk production in Friesian crossbred cows. Dijlah J. Agricultural Sci. 2, 76–83 (2024).
Matsuba, T. et al. Effects of feeding purple corn (Zea mays L.) silage on productivity and blood superoxide dismutase concentration in lactating cows. J. Dairy Sci. 102, 7179–7182 (2019).
Suong, N. T. M., Paengkoum, S., Schonewille, J. T., Purba, R. A. P. & Paengkoum, P. Growth performance, blood biochemical indices, rumen bacterial community, and carcass characteristics in goats fed anthocyanin-rich black cane silage. Front. Veterinary Sci. 9, 880838 (2022).
Paengkoum, P. & Paengkoum, S. Effects of cassava chips used as non-structural carbohydrate source for lactating dairy cows fed urea-treated rice straw. KMUTT RD J. 30, 435–448 (2007).
Sejian, V., Chauhan, S. S., Devaraj, C., Malik, P. K. & Bhatta, R. Climate Change and Livestock Production: Recent Advances and Future Perspectives (Springer, 2021).
Council, N. R. Nutrient Requirements of Goats: Angora, Dairy, and meat Goats in Temperate and Tropical CountriesVol. 15 (National Academies, 1981).
Helrich, K. Association of official analytical chemists. Official methods of analysis of the Association of Official Analytical Chemists, 15th ed. Washington, DC: Arlington, VA (1990).
Van Soest, P., Robertson, J. B. & Lewis, B. A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597 (1991).
Hamzaoui, S., Salama, A., Albanell, E., Such, X. & Caja, G. Physiological responses and lactational performances of late-lactation dairy goats under heat stress conditions. J. Dairy Sci. 96, 6355–6365. https://doi.org/10.3168/jds.2013-6665 (2013).
Li, F. et al. Effect of dietary physically effective fiber on ruminal fermentation and the fatty acid profile of milk in dairy goats. J. Dairy Sci. 97, 2281–2290. https://doi.org/10.3168/jds.2013-6895 (2014).
Tian, X., Paengkoum, P., Paengkoum, S., Thongpea, S. & Chao, B. Comparison of forage yield, silage fermentative quality, anthocyanin stability, antioxidant activity, and in vitro rumen fermentation of anthocyanin-rich purple corn (Zea mays L.) stover and sticky corn stover. J. Integr. Agric. 17, 2082–2095. https://doi.org/10.1016/S2095-3119(18)61970-7 (2018).
Zhao, X. et al. Effects of different fat mixtures on milk fatty acid composition and oxidative stability of milk fat. Anim. Feed Sci. Technol. 185, 35–42. https://doi.org/10.1016/j.anifeedsci.2013.06.009 (2013).
Karolkowski, A., Belloir, C., Briand, L. & Salles, C. Non-volatile compounds involved in bitterness and astringency of pulses: a review. Molecules 28, 3298. https://doi.org/10.3390/molecules28083298 (2023).
Niderkorn, V. & Jayanegara, A. Opportunities offered by plant bioactive compounds to improve silage quality, animal health and product quality for sustainable ruminant production: a review. Agronomy 11, 86. https://doi.org/10.3390/agronomy11010086 (2021).
Waghorn, G. C. & McNabb, W. C. Consequences of plant phenolic compounds for productivity and health of ruminants. Proc. Nutr. Soc. 62, 383–392. https://doi.org/10.1079/PNS2003245 (2003).
Estrada, J. C., Delagarde, R., Faverdin, P. & Peyraud, J. Dry matter intake and eating rate of grass by dairy cows is restricted by internal, but not external water. Anim. Feed Sci. Technol. 114, 59–74. https://doi.org/10.1016/j.anifeedsci.2003.11.013 (2004).
Tian, X. Z. et al. Effects of purple corn anthocyanin on blood biochemical indexes, ruminal fluid fermentation, and Rumen Microbiota in goats. Front. Veterinary Sci. 8, 715710. https://doi.org/10.3389/fvets.2021.715710 (2021).
Majlesi, A., Yasini, S., Azimpour, S. & Mottaghian, P. Evaluation of oxidative and antioxidant status in dairy calves before and after weaning. Bulgarian J. Veterinary Med. 24, 184–190. https://doi.org/10.15547/bjvm.2270 (2021).
Tian, X. et al. Effects of anthocyanin-rich purple corn (Zea mays L.) stover silage on nutrient utilization, rumen fermentation, plasma antioxidant capacity, and mammary gland gene expression in dairy goats. J. Anim. Sci. 97, 1384–1397. https://doi.org/10.1093/jas/sky477 (2019).
Khan, I. T. et al. Antioxidant properties of milk and dairy products: a comprehensive review of the current knowledge. Lipids Health Dis. 18, 1–13 (2019).
Tian, X. Z. et al. Effect of supplementation with selenium-yeast on muscle antioxidant activity, meat quality, fatty acids and amino acids in goats. Front. Veterinary Sci. 8, 813672. https://doi.org/10.3389/fvets.2021.813672 (2022).
Ajmal, M. et al. Impact of immediate and delayed chilling of raw milk on chemical changes in lipid fraction of pasteurized milk. Lipids Health Dis. 17, 1–10. https://doi.org/10.1186/s12944-018-0843-0 (2018).
Silva, F. A. et al. The effect of Isabel grape addition on the physicochemical, microbiological and sensory characteristics of probiotic goat milk yogurt. Food Funct. 8, 2121–2132. https://doi.org/10.1039/C6FO01795A (2017).
Taylor, M. & Richardson, T. Antioxidant activity of skim milk: effect of heat and resultant sulfhydryl groups. J. Dairy Sci. 63, 1783–1795. https://doi.org/10.3168/jds.S0022-0302(80)83140-7 (1980).
Siwach, R., Tokas, J. & Seth, R. Use of lycopene as a natural antioxidant in extending the shelf-life of anhydrous cow milk fat. Food Chem. 199, 541–546. https://doi.org/10.1016/j.foodchem.2015.12.009 (2016).
Smet, K. et al. A change in antioxidative capacity as a measure of onset to oxidation in pasteurized milk. Int. Dairy J. 18, 520–530. https://doi.org/10.1016/j.idaairyj.2007.11.012 (2008).
Tian, X., Lu, Q., Paengkoum, P. & Paengkoum, S. Effect of purple corn pigment on change of anthocyanin composition and unsaturated fatty acids during milk storage. J. Dairy Sci. 103, 7808–7812. https://doi.org/10.3168/jds.2020-18409 (2020).
Narayan, M., Naidu, K. A., Ravishankar, G., Srinivas, L. & Venkataraman, L. Antioxidant effect of anthocyanin on enzymatic and non-enzymatic lipid peroxidation. Prostaglandins Leukotrienes Essent. Fat. Acids (PLEFA). 60, 1–4. https://doi.org/10.1054/plef.1998.0001 (1999).
Funding
This work was supported by (i) Suranaree University of Technology (SUT), (ii) Thailand Science Research and Innovation (TSRI), and (iii) National Science, Research and Innovation Fund (NSRF) (NRIIS Number 179295).
Author information
Authors and Affiliations
Contributions
C. B. and T. W. conducted the experiments; C. B. drafted the initial manuscript; G. W.: Performed the data analysis and revised the manuscript; S. S. contributed to the revision of the manuscript; P. L. designed the experiments, provided funding acquisition, and supervised the project. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ban, C., Wen, G., Srisaikham, S. et al. Effect of anthocyanin rich black sugarcane on milk production and antioxidant capacity in lactating dairy cows. Sci Rep 15, 3407 (2025). https://doi.org/10.1038/s41598-025-88010-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88010-7