Abstract
Background
Tigecycline is widely used to treat a variety of bacterial infections despite concerns regarding increased mortality in severe infections. Previous case reports have documented breakthrough bloodstream infections (BSI) during tigecycline therapy. This study aimed to investigate the incidence of, and risk factors for, breakthrough BSI during tigecycline monotherapy.
Methods
A retrospective matched case–control study was conducted in a 2700-bed tertiary referral center, involving patients who received tigecycline monotherapy. Patients with breakthrough BSI (1:1) were matched with controls without breakthrough BSI based on age, sex, and date of tigecycline therapy.
Results
Of 4505 patients treated with tigecycline, 115 (2.6%, 95% confidence interval 2.1 to 3.1%) developed breakthrough BSI. The most frequently identified pathogen in breakthrough BSI was Klebsiella pneumoniae (22.8%), followed by Candida species (17.1%), Pseudomonas aeruginosa (16.3%), and Acinetobacter baumannii (14.6%). Of the K. pneumoniae and A. baumannii isolates for which tigecycline susceptibility results were available, 50% and 23%, respectively, were tigecycline-resistant (MIC > 2 mg/L). Intraabdominal (33.9%), catheter-related (30.4%), and hepatobiliary (19.1%) infections were the main sources of breakthrough BSI. In multivariable analysis, independent risk factors for breakthrough BSI during tigecycline therapy were liver cirrhosis (adjusted odds ratio [aOR], 3.09), indwelling catheter (aOR, 3.42), previous Candida colonization (aOR, 14.95), and previous multi-drug resistant bacteria colonization (aOR, 10.30).
Conclusion
In cases where there is a high suspicion of breakthrough BSI during tigecycline therapy, meticulous management and prudent selection of empirical antibiotics are crucial due to the diverse range of causative microorganisms involved.
Similar content being viewed by others
Introduction
Tigecycline is the first glycylcycline antibiotic, with broad-spectrum activity against most gram-positive and gram-negative bacteria, including pathogens causing nosocomial infections such as methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci and extended-spectrum β-lactamase producing bacteria1. It is a semisynthetic derivative of minocycline with a modified chemical structure that allows it to avoid the two major types of tetracycline resistance: efflux pumps and ribosomal protection2. This property contributes to its broad in vitro activity against multidrug-resistant (MDR) bacteria3. Originally, tigecycline was approved for complicated skin and soft tissue infection, complicated intraabdominal infection, and community-acquired pneumonia. Although it is considered bacteriostatic, tigecycline is often used to treat MDR bacterial infections in countries like South Korea, where newer treatment options such as ceftazidime-avibactam, meropenem-vaborbactam, imipenem-cilastatin-relebactam, and cefiderocol are not yet available.
Breakthrough bloodstream infections (BSIs) are continuous or new-onset BSIs that occur after at least 48 h of appropriate antibiotic treatment4,5,6,7. Inadequate control of the infection source and use of unsuitable antibiotics due to either antimicrobial resistance of microorganisms or suboptimal concentrations of active drugs in the affected organ may account for breakthrough BSI. In two recent studies on breakthrough BSIs, cases of persistent bacteremia that continued despite appropriate antibiotic therapy for the initial bacteremia were excluded, with the focus placed on superinfection7,8.
Tigecycline is a mainly bacteriostatic antibiotic; it achieves poor serum concentrations due to a high volume of distribution9. For this reason, there have been concerns that its use in severe infections such as BSI and endocarditis may lead to a poorer prognosis. In addition, there have been case reports of breakthrough BSI during tigecycline therapy despite efficacy in abdominal and soft tissue infections and community-acquired pneumonia10,11. Since data on breakthrough BSI during tigecycline therapy are limited, we aimed to investigate the incidence and risk factors associated with the development of breakthrough BSI during tigecycline monotherapy. We focused on new-onset BSI that developed during tigecycline therapy for prior infection, aiming to guide empirical treatment when a patient becomes septic during tigecycline therapy.
Materials and methods
Study population and design
This matched case–control study was performed at the Asan Medical Center, a 2700-bed tertiary referral center in Seoul, Republic of Korea. Patients over 18 years old who received tigecycline between March 1, 2009 and July 31, 2021 were retrospectively identified. Case patients were individuals who developed breakthrough BSI after at least 48 h of tigecycline monotherapy. Breakthrough BSI was defined as new-onset BSI that arose during appropriate empirical or definitive tigecycline therapy that had been administered for more than 48 h7,8,12. When a BSI, which prompted the initiation of tigecycline therapy, persisted despite treatment, it was not classified as a breakthrough BSI. The control group was patients who received tigecycline monotherapy for at least 48 h and had no breakthrough BSI. The controls were matched 1:1 with case patients based on age, sex, and date of tigecycline therapy (within 3 months). In our hospital, tigecycline was administered with a loading dose of 100 mg, followed by a maintenance dose of 50 mg every 12 h. For patients with liver dysfunction, the maintenance dose was reduced by half based on the attending physician’s decision.
Data collection and definition
Medical records of the case and control patients were reviewed. Demographic characteristics, underlying diseases, sites of infection, antibiogram results, duration of hospital stay before tigecycline therapy, presence of indwelling catheter, microbiological data, and clinical outcomes were reviewed. The onset of BSI was defined as the day the first positive blood culture was drawn. The severity of underlying illness was based on the McCabe and Jackson classification13. The Charlson comorbidity index was used to evaluate comorbid conditions14, and the severity of illness at the time of BSI was assessed by the Acute Physiology and Chronic Health Evaluation II (APACHE II) score15. Sites of infection causing breakthrough BSI were defined according to the Centers for Disease Control and Prevention criteria16. MDR pathogens were defined as: vancomycin-resistant Enterococcus species, carbapenem-resistant Acinetobacter baumannii, carbapenem-resistant Pseudomonas aeruginosa, and carbapenem-resistant Enterobacterales. Treatment outcome of BSI was evaluated based on all-cause mortality. This information was entered into an electronic case report form, and all information was processed anonymously.
Microbiological data
Species identification and antimicrobial susceptibilities were determined using a Vitek (bioMérieux, France) or Microscan (Beckman Coulter, CA, USA), in accordance with the standard criteria of the Clinical and Laboratory Standards Institute (CLSI). Gram-negative bacterial isolates with tigecycline MIC ≤ 2 mg/L were considered susceptible to tigecycline.
Statistical analysis
Categorical variables were compared using the chi-square or Fisher’s exact test, and continuous variables were compared using the Mann–Whitney U test or Kruskal–Wallis test, as appropriate. To identify risk factors for breakthrough BSI during tigecycline therapy, all significant variables in the univariate analysis were included in a multiple logistic regression model. The final model was constructed using the backward stepwise selection procedure. All tests of significance were 2-tailed and a P value of ≤ 0.05 was considered statistically significant. Statistical analysis was performed with SPSS for Windows, version 21 (SPSS Inc, Chicago, Illinois).
Ethical approval
This study was approved by the Institutional Review Board (IRB) of Asan Medical Center (IRB No. 2022–0100). The requirement for obtaining informed consent from the patients was waived by the IRB of Asan Medical Center. To protect personal privacy, identifying information in the electronic database was encrypted. All methods were performed in accordance with the relevant guidelines and regulations and followed the principles stipulated in the Declaration of Helsinki.
Results
Microorganisms causing breakthrough BSI
During the study period, a total of 4,505 patients were treated with tigecycline, and 144 had breakthrough BSI. Of these 144 patients, 29 whose blood cultures were considered to be contaminated were excluded from the analysis, and a total of 115 patients with breakthrough BSI (2.6%, 95% confidence interval [CI], 2.1 to 3.1%) were included (Fig. 1). The contaminated blood cultures were caused by coagulase-negative staphylococci and Micrococcus species17.
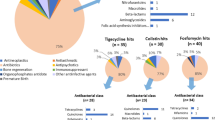
The causative pathogens of the breakthrough BSI are shown in Fig. 2. There were five cases of polymicrobial breakthrough BSI, resulting in a total of 123 breakthrough BSI isolates. Klebsiella pneumoniae (28/123, 22.8%) was the most common pathogen, followed by Candida species (21/123, 17.1%), P. aeruginosa (20/123, 16.3%), and A. baumannii (18/123, 14.6%). Gram-negative non-fermentative bacilli were isolated in 37.4% of the patients, Enterobacterales in 34.8%, and gram-positive cocci such as staphylococci and enterococci in 11.3%. Most A. baumannii isolates (94.4%) were resistant to meropenem, and about one-third of the K. pneumoniae and P. aeruginosa isolates were resistant to meropenem. Among isolates in which tigecycline susceptibility test was performed, half of K. pneumoniae isolates (8/16) were resistant to tigecycline (MIC > 2 mg/L), and 23.0% of the A. baumannii isolates (3/13) were resistant to tigecycline (Table 1).
Distribution of causative pathogens in 115 patients with breakthrough bloodstream infection during tigecycline therapy. This figure includes polymicrobial bloodstream infections in which each microorganism was counted. *Others include Bacteroides fragilis (2 cases), Enterococcus faecium (2), Klebsiella oxytoca (2), Stenotrophomonas maltophilia (2), Serratia marcescens (2), Actinomyces species (1), Alcaligenes faecalis (1), Burkholderia cepacian (1), Chryseobacterium indologenes (1), Citrobacter amalonaticus (1), Enterobacter aerogenes (1), Enterobacter asburiae (1), Enterococcus faecalis (1), Klebsiella aerogenes (1), Moraxella osloensis (1) and Morganella morganii (1).
Clinical characteristics and outcomes of patients with breakthrough BSI
The clinical features and outcomes of breakthrough BSI are shown in Table 2. The most common site of infection considered to be the cause of the breakthrough BSI was intraabdominal infection (33.9%), followed by catheter-related infection (30.4%) and hepatobiliary infection (19.1%). The median duration of tigecycline therapy before breakthrough BSI was 8 days. After the onset of BSI, 72 patients (62.6%) received intensive care unit (ICU) care, and the in-hospital crude mortality rate was 42.7%.
Risk factors for the development of breakthrough BSI during tigecycline therapy
The baseline and clinical characteristics of patients with breakthrough BSI and controls are shown in Table 3. There were no significant differences between the two groups in age, gender, prior antibiotic use, hospital stay before tigecycline therapy, and type of infection. However, the cases were more likely to have liver cirrhosis (P < 0.001), solid organ transplantation (P = 0.013), and indwelling catheters such as central venous catheters (CVC) and percutaneous drainage catheters (P = 0.012). The Charlson comorbidity index was also higher in the cases (P = 0.027), and previous colonization with Candida (P < 0.001) or MDR bacteria (P < 0.001) was also associated with breakthrough BSI. Regarding the severity of infection leading to tigecycline therapy, patients with breakthrough BSI had higher APACHE II score than controls (P < 0.001).
Significant univariate variables were included in a logistic regression model to identify independent risk factors for breakthrough BSI (Table 4). Because ICU care was highly correlated with APACH II score, we retained only APCHE II score in the model. In multivariable analysis, liver cirrhosis (adjusted odds ratio [aOR], 3.09; 95% CI, 1.13 to 8.46), indwelling catheter (aOR, 3.42; 95% CI, 1.38 to 8.48), previous Candida colonization (aOR, 14.95; 95% CI, 3.58 to 62.49), and previous MDR bacteria colonization (aOR, 10.30; 95% CI, 5.20 to 20.43) were independently associated with breakthrough BSI during tigecycline therapy.
Discussion
We have investigated the incidence, causative microorganisms, and risk factors associated with breakthrough BSI during tigecycline therapy. The overall incidence of breakthrough BSI was 2.6% (95% CI, 2.1 to 3.1%), and the main causes of breakthrough BSI were intraabdominal infection and catheter-related infection. Major microorganisms were Enterobacterales, gram-negative non-fermentative bacilli such as A. baumannii and P. aeruginosa, and Candida species. Risk factors for the development of breakthrough BSI included liver cirrhosis, presence of indwelling catheter such as CVC and percutaneous drainage catheter, and previous colonization with Candida or MDR bacteria.
The main causes for which tigecycline therapy was initiated were intraabdominal, hepatobiliary, and respiratory infections, whereas the main causative foci of breakthrough BSI were intraabdominal, catheter-related, and hepatobiliary infection. In intraabdominal and hepatobiliary infections, it appears that complications such as anastomotic leak and biliary obstruction may arise during tigecycline therapy, potentially leading to breakthrough BSI due to inadequate source control. Furthermore, catheter-related infection, the second main cause of breakthrough BSI, is thought to be related to a suboptimal serum concentration of tigecycline. Indwelling catheter was an independent predictor of breakthrough BSI in our patients with tigecycline therapy. In previous studies of breakthrough BSI during various antibiotics therapy, the presence of indwelling catheter was identified as an independent risk factor for breakthrough BSI18,19. Infections that led to tigecycline therapy did not include catheter-related infection. However, when breakthrough BSI occurred, catheter-related infection was the infection source in 30.4% of cases. It is well known that maintaining a peak serum concentration of tigecycline above 2 mg/L is difficult9,20, so tigecycline is not an ideal agent for treating BSI or other endovascular infections. Thus, it is possible that many catheter-related breakthrough BSIs in our study occurred because of low serum concentration of tigecycline.
Another major cause of breakthrough BSI can be resistance or reduced susceptibility to tigecycline. The microorganism most frequently involved in our breakthrough BSIs was K. pneumoniae; 32.1% of the K. pneumoniae isolates were carbapenem-resistant, and 50% were tigecycline-resistant (MIC > 2 mg/L). A. baumannii and P. aeruginosa were also major pathogens in our breakthrough BSIs. In a previous report, breakthrough BSIs were caused by intrinsically tigecycline-resistant microorganisms (P. aeruginosa) or microorganisms with reduced susceptibility to tigecycline21.
Liver cirrhosis was also identified as a risk factor for breakthrough BSI. Tigecycline is metabolized primarily by liver glucuronidation, and significant hepatic dysfunction and hepatic failure have been reported in some patients22. For this reason, dose reduction and close monitoring are recommended for patients whose liver function has deteriorated to Child–Pugh C23. In clinical practice, tigecycline dose is often reduced in patients with only mild liver function. Hence, it is plausible that the serum level of tigecycline may have been low in patients with liver cirrhosis due to inadequate dosage, potentially leading to breakthrough BSI.
Candida species were also identified as major microorganisms causing breakthrough BSI during tigecycline therapy. Risk factors for developing Candida infection included Candida colonization, severity of illness, use of CVC, and exposure to broad-spectrum antibiotics24. The high incidence of Candida breakthrough BSI is most likely caused by an increase in selection pressure for Candida because tigecycline is active against both gram-positive and gram-negative bacteria1,23. In addition, 88.9% of the patients who developed candidemia had CVCs, and 55.6% were in an ICU at the start of tigecycline therapy. We found that previous colonization with Candida or MDR bacteria was an independent risk factor for breakthrough BSI. Of the breakthrough BSI patients with MDR gram-negative bacilli (GNB), 76.5% had previous MDR GNB colonization; of those with Candida breakthrough BSI, 27.8% had previous Candida colonization. Indwelling catheter, compromised liver function, and high Charlson comorbidity score may contribute to the development of breakthrough BSI in these patients.
The present study has several limitations. First, it was a retrospective observational study conducted in a single tertiary center, so the potential effect of unmeasured variables and residual confounding cannot be excluded. A larger prospective study involving multiple centers is needed to validate the results. Second, since our hospital’s antimicrobial susceptibility panel does not include tigecycline, we were only able to assess susceptibility to tigecycline in a limited number of breakthrough BSI isolates. Therefore, a correlation between tigecycline resistance and breakthrough BSI could not be definitively established. Third, high-dose tigecycline (200 mg as a single dose, followed by 100 mg every 12 h) is recommended in severe infections or MDR bacterial infections to address the low serum concentration of tigecycline associated with conventional dose25,26,27. If high-dose tigecycline had been routinely used in our patients, the rate and causes of breakthrough BSI might have differed. These limitations highlight the need for further research to enhance our understanding of breakthrough BSI during tigecycline therapy and its implications for patient management and treatment outcomes.
In conclusion, this study found an incidence of approximately 2.6% of breakthrough BSI during tigecycline therapy. Patients with liver cirrhosis, indwelling catheter, previous Candida colonization, and previous MDR bacterial colonization were found to be at increased risk. In addition, breakthrough BSI involves a diverse spectrum of causative microorganisms such as Candida, carbapenem-resistant gram-negative bacilli, and methicillin-resistant staphylococci. Consequently, when breakthrough BSI is suspected in patients receiving tigecycline, switching to a single antibiotic for empirical treatment is not feasible. These findings emphasize the need for careful management and appropriate empirical antibiotic therapy when breakthrough BSI is suspected in patients receiving tigecycline. Further research is warranted to develop optimal strategies for the prevention and management of breakthrough BSI during tigecycline therapy.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Freire, A. T. et al. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis 68, 140–151. https://doi.org/10.1016/j.diagmicrobio.2010.05.012 (2010).
Breedt, J. et al. Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a double-blind phase 3 comparison study with vancomycin-aztreonam. Antimicrob Agents Chemother 49(11), 4658–4666. https://doi.org/10.1128/AAC.49.11.4658-4666.2005 (2005).
Karageorgopoulos, D. E., Kelesidis, T., Kelesidis, I. & Falagas, M. E. Tigecycline for the treatment of multidrug-resistant (including carbapenem-resistant) Acinetobacter infections: a review of the scientific evidence. J Antimicrob Chemotherapy 62, 45–55 (2008).
Anderson, E. T., Young, L. S. & Hewitt, W. L. Simultaneous antibiotic levels in “breakthrough” gram-negative rod bacteremia. Am J Med 61, 493–497 (1976).
Rangaraj, G., Granwehr, B. P., Jiang, Y., Hachem, R. & Raad, I. Perils of quinolone exposure in cancer patients: breakthrough bacteremia with multidrug‐resistant organisms. Cancer 116(4), 967–973. https://doi.org/10.1002/cncr.24812 (2010).
Tsai, M. H. et al. Breakthrough bacteremia in the neonatal intensive care unit: incidence, risk factors, and attributable mortality. Am J Infect Control 43, 20–25. https://doi.org/10.1016/j.ajic.2014.09.022 (2015).
Lee, J. Y. et al. Clinical features and risk factors for development of breakthrough gram-negative bacteremia during carbapenem therapy. Antimicrob Agents Chemother 60, 6673–6678. https://doi.org/10.1128/AAC.00984-16 (2016).
Lee, Y. T. et al. Multicenter study of clinical features of breakthrough acinetobacter bacteremia during carbapenem therapy. Antimicrob Agents Chemother https://doi.org/10.1128/AAC.00931-17 (2017).
Muralidharan, G., Micalizzi, M., Speth, J., Raible, D. & Troy, S. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob Agents Chemother 49, 220–229. https://doi.org/10.1128/AAC.49.1.220-229.2005 (2005).
Parsonage, M. et al. Breakthrough bacteraemia with a susceptible Enterococcus faecalis during tigecycline monotherapy. J Antimicrob Chemother 65, 370–374. https://doi.org/10.1093/jac/dkp455 (2010).
Peleg, A. Y. et al. Acinetobacter baumannii bloodstream infection while receiving tigecycline: a cautionary report. J Antimicrob Chemother 59, 128–131 (2007).
Rangaraj, G., Granwehr, B. P., Jiang, Y., Hachem, R. & Raad, I. Perils of quinolone exposure in cancer patients: breakthrough bacteremia with multidrug-resistant organisms. Cancer 116, 967–973. https://doi.org/10.1002/cncr.24812 (2010).
Mccabe, W. R. & Jackson, G. G. Gram-negative bacteremia: II. clinical, laboratory, and therapeutic observations. Arch Internal Med 110, 856–864. https://doi.org/10.1001/archinte.1962.03620240038007 (1962).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40, 373–383. https://doi.org/10.1016/0021-9681(87)90171-8 (1987).
Knaus, W. A., Draper, E. A., Wagner, D. P. & Zimmerman, J. E. APACHE II: a severity of disease classification system. Crit Care Med 13, 818–829 (1985).
CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 32 edn, Vol. 42 (2019).
Hall, K. K. & Lyman, J. A. Updated review of blood culture contamination. Clin Microbiol Rev 19, 788–802. https://doi.org/10.1128/CMR.00062-05 (2006).
Spanik, S. et al. Risk factors, aetiology, therapy and outcome in 123 episodes of breakthrough bacteraemia and fungaemia during antimicrobial prophylaxis and therapy in cancer patients. J Med Microb 46(6), 517–523 (1997).
Lopez Dupla, M. et al. Clinical characterization of breakthrough bacteraemia: a survey of 392 episodes. J Internal Med 258(2), 172–180 (2005).
Rodvold, K. A. et al. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J Antimicrob Chemother 58, 1221–1229 (2006).
Greer, N. D. Tigecycline (Tygacil): the first in the glycylcycline class of antibiotics. Proc (Bayl Univ Med Cent) 19, 155–161. https://doi.org/10.1080/08998280.2006.11928154 (2006).
Grosse, E. J. E. et al. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: results of 2 double-blind phase 3 comparison studies with vancomycin-aztreonam. Clin Infect Dis 41, S341–S353 (2005).
Stein, G. E. & Craig, W. A. Tigecycline: a critical analysis. Clin Infect Dis 43, 518–524 (2006).
Pappas, P. G. et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis 62, e1-50. https://doi.org/10.1093/cid/civ933 (2016).
De Pascale, G. et al. Pharmacokinetics of high-dose tigecycline in critically ill patients with severe infections. Ann Intensive Care 10, 94. https://doi.org/10.1186/s13613-020-00715-2 (2020).
De Pascale, G. et al. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care 18, R90. https://doi.org/10.1186/cc13858 (2014).
Tamma, P. D. et al. Infectious diseases society of america 2024 guidance on the treatment of antimicrobial-resistant gram-negative infections. Clin Infect Dis https://doi.org/10.1093/cid/ciae403 (2024).
Acknowledgements
We thank Kyung Ah Lee for her assistance in data collection.
Funding
This study was supported by a grant (grant number: 2025IL0026) from the Asan Institute for Life Sciences, Asan Medical Center Seoul, Korea.
Author information
Authors and Affiliations
Contributions
Study conceptation and design : EH song, YP Chong; Data collection and analysis : S Jin, SY Lim, YW Lee; Manuscript writing : S Jin, EH song, YP Chong; Manuscript editing : H Sung, MN Kim, S Bae, J Jung, MJ Kim, SH Kim, SH Choi, SO Lee, YS Kim, EH song, YP Chong. All authors meet the ICMJE authorship criteria.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jin, S., Lim, S.Y., Lee, Y.W. et al. Clinical and microbiological analysis of risk factors for breakthrough bloodstream infection during Tigecycline Therapy. Sci Rep 15, 4266 (2025). https://doi.org/10.1038/s41598-025-88048-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88048-7